Abstract
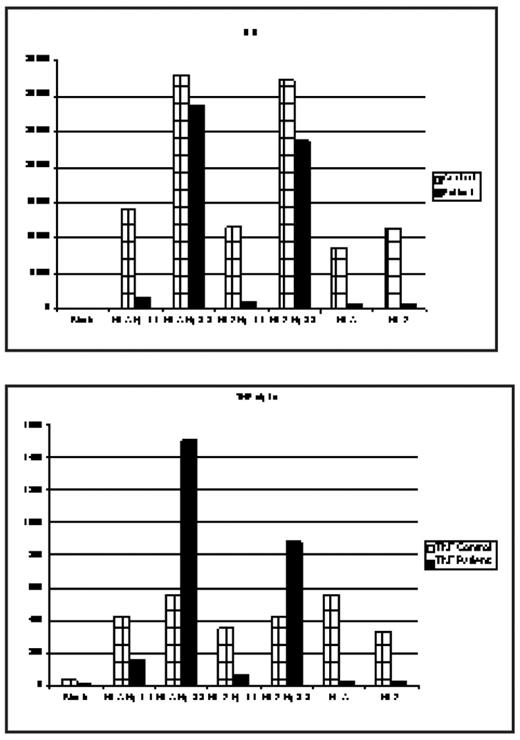
The deleterious effects of hemolysis through its end product cell free hemoglobin(Hb) as a nitric oxide (NO) scavenger is well established and has been incriminated in the pathogenesis of some complications of SCD such as pulmonary hypertension, leg ulcers, renal dysfunction, and possibly stroke. These observations have led some investigators to hypothesize that these complications form a subphenotype of SCD related predominantly to hemolysis and endothelial dysfunction. In hemolytic states Hb released from RBC complexes with Haptoglobin (Hp) and is removed from the circulation by macrophages and monocytes through binding CD163, the Hb scavenger receptor expressed on these cells. When the binding capacity of Hp is exceeded, the concentration of free Hb rises in the plasma. Hp is a polymorphic protein encoded by a gene on chromosome 16q2.2; there are two allelic variants, Hp 1 and Hp 2. Hp 2 is believed to have resulted from an intragenic duplication event, leading to an elongated Hp a-chain. Individuals homozygous for the long a2 chain express large multimeric molecules (Hp 2-2). During the past decade, a considerable body of evidence has accumulated suggesting that Hp-2 allele is a major susceptibility gene for the development of vascular complications (coronary artery restenosis and development of cardiovascular disease) especially in diabetic patients. It has been hypothesized that the Hb-Hp2 complexes have a 10-fold greater affinity for the CD 163 receptor, and the binding of Hb-Hp2 complexes generates a more powerful inflammatory response with a more prominent cytokine release. Recently, we performed a preliminary analysis of the distribution of Hp1 and Hp2 alleles among pediatric and adult SCD patients and reported a significantly higher allele frequency for Hp2 among pediatric patients, suggesting a survival advantage for carriers of Hp1 allele (Yaun et al, Blood, 2005). We now report the results of an exploratory in vitro study of cytokine release from purified mononuclear cells obtained from a normal control and an SCD patient following exposure to Hb-Hp1-1 and Hb-Hp2-2 complexes. Mononuclear cells (106/well) isolated by Ficoll-Hypaque density gradient were incubated with Hb A-Hp1-1, Hb A-Hp2-2, Hb S Hp1-1, and Hb S-Hp2-2 complexes with a 1:1 ratio (wt/wt) at a final concentration of 1 mg/ml. After 24 hr incubation at 37°C, the supernatants collected after centrifugation were used for cytokine assays by a multiplex bead method. A blank (medium only), Hb A and Hb S without Hp were also incubated with mononuclear cells. Multiplex bead assays showed that cytokine release (GM-CSF, IL-1b, IL-6, IL-10, and TNFa) was much higher (3–12 fold) from both the control and SCD mononuclear cells upon exposure to Hb-Hp2-2 complexes, but much less or no effect by Hb-Hp1-1. The fold induction of TNFa and IL-1b was much higher in SCD cells than in control cells. There was no significant difference between Hb A and Hb S in terms of cytokine release when they were complexed with either Hp1-1 or Hp2-2, suggesting that the cytokine release was predominantly related to Hp type but not to Hb. Pure Hb A and Hb S increased cytokines over the control (blank) but to a significantly smaller extent than Hb (A or S) Hp-2-2 complexes.
These preliminary results are confirmatory of a deleterious effect of the Hp2-2 genotype through a more pronounced inflammatory response and are suggestive of a potential novel mechanism whereby hemolysis could result in adverse outcomes related to Hp polymorphisms. If confirmed in larger studies and through phenotypic associations, attenuation of this response via anti-inflammatory modalities may provide a therapeutic strategy.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author