Abstract
Title: Primary Therapy with Bortezomib—The Role of Induction, Maintenance, and Re-Induction in Patients with High Risk Myeloma. Update of Results from E2A02. Angela Dispenzieri, MD1, Susanna Jacobus, PhD2, David Vesole, MD3 and Philip R. Greipp, MD1. 1Hematology, Mayo Clinic, Rochester, MN, United States, 55905 and 2Biostatistics, Eastern Cooperative Oncology Group, Boston, MA, United States, 02115. 3Saint Vincent’s Hospital, New York, NY 10010
Background: Single agent bortezomib as upfront therapy in patients with multiple myeloma (MM) results in response rates of 38% and the drug has been touted to be especially effective in patients with high risk disease. We sought to explore response rate, the role of maintenance and reinduction in a cohort of patients with high-risk MM.
Methods: Patients with newly diagnosed high-risk myeloma (beta-2 microglobulin [B2M] >= 5.5., plasma cell labeling index [PCLI] >= 1, or deletion 13q) and adequate organ and functional status were eligible. Patients were treated with bortezomib 1.3 mg/m2 day 1, 4, 8, and 11 every 21 days for 8 cycles as induction. After induction, patients were scheduled to receive bortezomib 1.3 mg/m2 every other week indefinitely. Elective peripheral stem cell mobilization (growth factor alone) was allowed after 4 cycles of bortezomib. Patients relapsing on maintenance schedule had full induction schedule resumed. Responses as defined by the EBMT criteria were determined after each cycle and needed to be confirmed after at least 6 weeks.
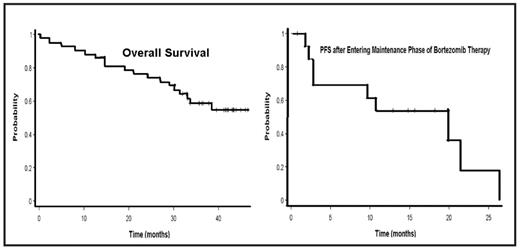
Results: Between March 15, 2004 and March 10, 2005, 44 patients enrolled on study. Among the 42 eligible treated patients, median age was 63; 51% were male. All patients had high risk disease: deletion 13q (6/41); PCLI >=1% (17/33); t(4:14) (4/27) and B2M >= 5.5 (34/43). Nineteen patients completed induction, i.e. 8 cycles of bortezomib. The most common causes for discontinuing therapy were: progressive disease (n=17), AE (n=7), alternate therapy (n=4), death (n=1), and other (n=11). Forty-eight percent of patients achieved a partial response (PR) or better with induction (0 complete responses, 2 very good PR, 17 PR, 3 minimal responses, 5 no response, 9 progressive disease, and 6 inevaluable or missing). Response rates across risk groups did not differ with a PR or better for patients with deletion 13q (2/6); PCLI >=1% (8/17); t(4:14) (2/4) and B2M >= 5.5 (15/33). One patient’s response was upgraded to CR during maintenance. The rate of painful peripheral neuropathy during all courses of therapy was: grade 1, n=25; grade 2, n=2; and grade 3, n=2. With a median follow-up of 41.6 months (95%CI 36.6–43), 43% of patients have died. Median overall survival (OS) has not been reached (Figure), and the 1- and 2-year OS rates were 88.1% (95%CI 73.7–94.9%) and 76.2% (95%CI 60.3–86.4%) respectively. The median progression free survival (PFS) was 16.3 months (95%CI 6.2–26.6) and the 1- and 2-PFS were 76.7% (95%CI 58.8–87.6%) and 38.3% (95%CI 20.6–55.9%), respectively. Fifteen patients went on to maintenance. The distribution by risk group for patients entering maintenance was not different across groups: deletion 13q, 3/6; high PCLI, 6/17; high B2M, 11/33; t(4;14), 1/4; and p53, 0/2. For those entering maintenance, the median PFS was 19.8 months (Figure), and the 1 year PFS rate was 75% (95%CI 40.8–91.2%). Of the 7 patients progressing on maintenance who received reinduction with bortezomib, no patient responded. Only 2 remain on therapy.
Conclusions: In high risk patients, upfront bortezomib results in response rates comparable to those reported for unselected cohorts of newly diagnosed myeloma patients. For patients responding to therapy, maintenance therapy is feasible. Reinduction was unsuccessful in high risk patients failing maintenance.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author