Abstract
Introduction: Immunotherapy is most likely to work in a setting of low tumor burden, making vaccine strategies attractive as maintenance therapy post autologous peripheral blood stem cell transplantation (PBSCT) for multiple myeloma (MM). However, it is difficult to ascertain the effects of the vaccines from delayed responses to the transplant. A novel immunotherapeutic, APC8020 (Mylovenge), was studied as consolidation therapy after for MM post PBSCT (Mayo vaccine trial). Mylovenge is prepared from autologous antigen presenting cells, including dendritic cells, partially purified from an unmobilized leukapheresis product by gradient density isolation and then incubated for two days with autologous serum containing M protein obtained pretransplant. We report long term results of the Mayo vaccine trial compared retrospectively to a consecutive cohort of MM patients who underwent autologous stem cell transplant at Mayo Clinic during the same time period.
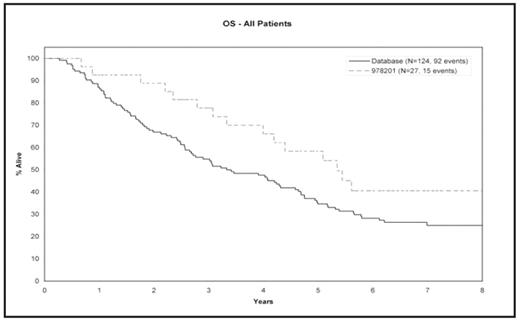
Methods: The Mayo vaccine trial patients had transplants between July of 1998 and May of 2001. Using these cutoff dates we analyzed 151 total MM patients, 27 from the vaccine trial (9 newly diagnosed and 18 relapsed) and 124 from database (DB) (50 newly diagnosed patients and 74 relapsed). The median (range) of follow-up for alive patients in vaccine trial is 6.5 years (2.9 – 8), and in the DB is 7.1 years (6 – 8). Two-sided Fisher’s exact tests and stratified log rank tests (with newly diagnosed or relapsed as the stratum) were used to compare baseline patient characteristics and time to event distributions (overall survival – OS, progression free survival – PFS, and time to progression – TTP) between the two groups.
Results: The median (range) of age in vaccine trial and DB was 56 (30–69) and 57 (36–71) years respectively. There were no significant differences in the known prognostic factors including PCLI, B2M, and CRP. The median (95% confidence interval - CI) TTP for the vaccine trial and DB patients was 1.5 years (1.3 – 2.4) and 1.6 years (1.3 – 1.8); stratified log rank p value = 0.46. The median (95% CI) PFS for the vaccine trial and DB patients was 1.5 years (1.3 – 2.4) and 1.5 years (1.1 – 1.8), stratified logrank p value = 0.30. The median (95% CI) OS for the vaccine trial and DB patients was 5.3 years (4.0 -N/A), and 3.4 years (2.7 – 4.6); stratified logrank p value = 0.02.
Conclusions: Despite the fact that no difference was seen in TTP or PFS, post-transplant vaccine therapy was associated with prolonged survival. This is similar to what has been reported in other tumor systems including prostate cancer and glioblastoma multiforme. This approach warrants further investigation, including preclinical studies aimed at optimizing the immune response and randomized trials to define the role of vaccine therapy in myeloma.
Disclosures: Lacy:Celgene: Research Funding. Kumar:Celgene: Research Funding; Millennium : Research Funding; Genzyme: Research Funding. Rajkumar:Celgene: Research Funding.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal