Abstract
Background: Systemic mastocytosis (SM) and hypereosinophilic syndrome (HES) are JAK2V617F-negative orphan diseases without effective long-term therapeutic options. Both of these diseases share clinical and laboratory features with FIP1L1-PDGFRA-positive chronic eosinophilic leukemia (CEL). SM is associated with KITD816V or other KIT mutations. HES is currently not molecularly characterized. The exquisite sensitivity of FIP1L1-PDGFRA to inhibition by imatinib mesylate (IM) underlies the efficacy of this agent in treating FIP1L1-PDGFRA-positive CEL. In contrast, KITD816V is resistant to inhibition by IM, and is only moderately sensitive to dasatinib (cellular IC50=micromolar concentrations), which may explain the relatively disappointing clinical responses in SM with these agents. TG101348 is an orally bioavailable JAK2-selective inhibitor that is currently being tested in a Phase 1 clinical trial for the treatment of myelofibrosis. The aim of the current study was to evaluate TG101348 (relative to the tyrosine kinase inhibitors IM, dasatinib, and sorafenib) for its ability to:
inhibit growth of leukemic cell lines that carry KITD816V and FIP1L1-PDGFRA; and
inhibit the in vitro growth of eosinophil colonies derived from progenitor cells from HES patients.
Methods: The following cell lines were used for in vitro experiments: HMC-1, a mast cell leukemia line (KITD816V-positive); EOL-1, a CEL derived line (FIP1L1-PDFRA-positive), Ba/F3 T674I, a Ba/F3 line that express the IM-resistant FIP1L1-PDFRAT674I mutation, and HEL, a human erythroleukemia line (JAK2V617F-positive). Cell proliferation assays were performed in triplicate using the XTT assay (Leukemia. 2007 21:1658). Drug concentrations in these experiments ranged from 2.9 × 10−12 M to 10−4 M. JAK Inhibitor I (Calbiochem) is a non-selective JAK inhibitor tool compound. Eosinophil colonies were obtained by plating PBMCs from healthy controls or HES patients in methylcellulose, in the presence of IL-3, IL-5, and GM-CSF. Effects of IM and TG101348 on eosinophil colony growth were studied in parallel experiments.
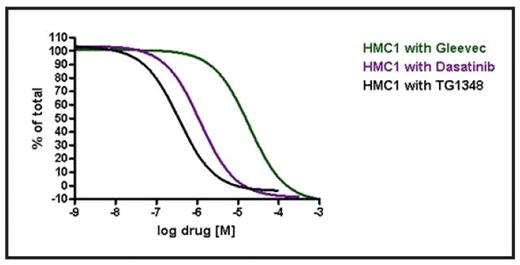
Results: Consistent with prior observations, the HMC-1 cell line was resistant to inhibition by IM (IC50>10 mM), but was moderately sensitive to dasatinib (Table). In contrast, TG101348 inhibited HMC-1 growth at nanomolar concentrations, similar to its effect on HEL cells that harbor the JAK2V617F mutation. TG101348 inhibited HMC-1 growth 53-fold and 4-fold more potently than IM and dasatinib, respectively (Figure). Sorafenib, a multikinase inhibitor that reportedly has activity against KIT kinase (enzyme IC50=68 nM), had only a limited effect on HMC-1 growth (IC50=4000 nM). In contrast to HMC-1, EOL-1 growth was inhibited by IM, dasatinib, sorafenib, and TG101348 at picomolar concentrations (Table). The T674I mutation (in the context of FIP1L1-PDGFRA) corresponds to the T315I mutation in BCR-ABL – it occurs infrequently in CEL and is usually found in the context of transformation to acute leukemia. Growth of Ba/F3 cells harboring FIP1L1-PDFGRA-T674I has been shown to be inhibited by sorafenib (confirmed in this report; Table) and nilotinib, at nanomolar concentrations. TG101348 is less potent at inhibiting growth of these cells, with cellular IC50 of ~2 mM – pharmacokinetic data from the ongoing Phase I study of TG101348 shows that this concentration is achievable in plasma with once daily dosing.
| Drug . | Cellular IC50 . | . | . | . |
|---|---|---|---|---|
| . | HMC-1 (nM) . | EOL-1 (pM) . | Ba/F3 T674I (nM) . | HEL (nM) . |
| Imatinib | 18800 | 4 | 10700 | - |
| Dasatinib | 1300 | <1 | 30900 | - |
| Sorafenib | 4000 | 2 | 16 | - |
| Jak Inhibitor I | 926 | 30 | 4300 | ? |
| TG101348 | 355 | <1 | 1950 | 300 |
| Drug . | Cellular IC50 . | . | . | . |
|---|---|---|---|---|
| . | HMC-1 (nM) . | EOL-1 (pM) . | Ba/F3 T674I (nM) . | HEL (nM) . |
| Imatinib | 18800 | 4 | 10700 | - |
| Dasatinib | 1300 | <1 | 30900 | - |
| Sorafenib | 4000 | 2 | 16 | - |
| Jak Inhibitor I | 926 | 30 | 4300 | ? |
| TG101348 | 355 | <1 | 1950 | 300 |
Conclusions: The JAK2-selective inhibitor TG101348 is a potent inhibitor of KITD816V and FIP1L1-PDGFRA, warranting clinical trials using this drug in SM and FIP1L1- PDGFRA-positive CEL. Data regarding TG101348 effects on signaling intermediates in leukemic cell lines and on eosinophil colony growth will be presented at the meeting – data from the latter experiments may indicate a therapeutic role for TG10134 in the treatment of patients with HES.
Disclosures: Shorr:TargeGen: Employment, Equity Ownership. Tefferi:TargeGen: Research Funding. Pardanani:TargeGen: Research Funding.
Author notes
Corresponding author