Abstract
Introduction: Mantle cell lymphoma (MCL) is considered to be an incurable disease with a poor prognosis, but the prognosis can be significantly different among the patients. The new prognostic index MIPI (MCL International Prognostic Index) has been proposed recently (Hoster ASH 2006, Blood 2008). Three prognostic groups with different survival (low-risk, intermediate-risk and high-risk) can been identified, based on four variables: WBC count, ECOG performance status, LDH and age.
Aim: To validate MIPI on an independent unselected cohort of newly diagnosed patients with MCL in the Czech Lymphoma Study Group (CLSG) registry.
Methods and patients: Out of 293 patients with MCL diagnosed and registered in the period 1999–2007, 149 patients had central pathology review and confirmation of MCL diagnosis and were eligible for the analysis. The age median was 65 year (24–86), 63% were male (M:F ratio 1,7:1). Most of patients were diagnosed in advanced Ann Arbor stage IV (82%), limited stages I+II formed only 10,5%. The bone marrow was involved in 75% of cases. B-symptoms were present in 45% patients, LDH level elevated in 51%, poor performance status (ECOG 2–4) in 21% and the median leukocyte count was 7,9 ×109/L. A chemotherapy was used as a first line treatment in 144 patients, the combination with rituximab (R) in 106 ones (73%). The most used regimens were hyperCVAD/MTX-HDaraC (30x), R-CHOP (30x), CHOP (19x), R-FC (13x), then R-maxiCHOP/HDaraC (12x), R-CHOP/HDaraC (9x), COP (8x) and others. A consolidation of the first remission with high-dose chemotherapy and autologous stem cell transplantation was used in 12 patients, and an allogeneic transplantation in 2 patients. A first-line radiotherapy was used in 14 patients. Median follow-up is 31 months.
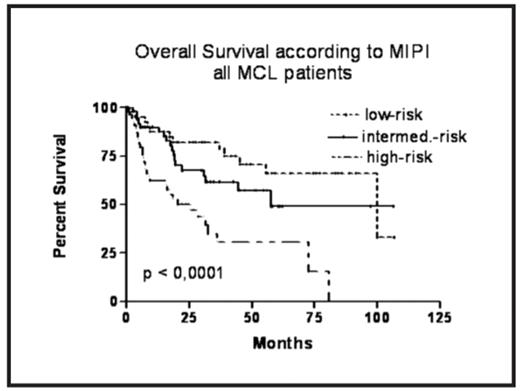
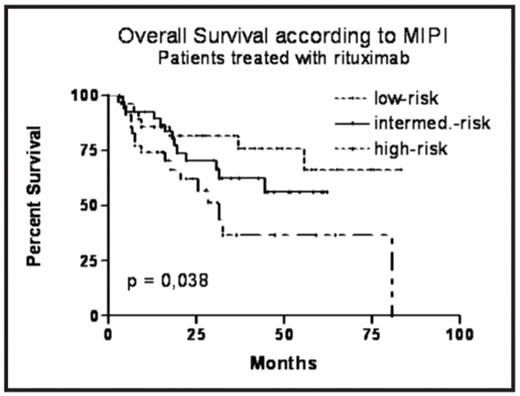
Results: Median overall survival (OS) in the whole group of confirmed MCL patients was 58 months, median progression-free survival (PFS) was 24 months. The MIPI index can be calculated for 148 patients, 28% of them belong to low-risk (LR), 35% to intermediate-risk (IR) and 37% to high risk (HR) group. All clinical stages were included. Our comparison of survival curves according to MIPI risk groups confirms a different prognosis – the median OS in the LR group was not reached, in the IR group is the median OS 58 months, and in the HR group 25 months (p < 0,0001). The 3-year OS probability for LR, IR and HR group is 82%, 62% and 31%, resp. Similarly, median PFS in the LR, IR and HR group is 45, 24 and 13 months, resp. (p < 0,0001). The analysis of rituximab-treated subgroup was performed as well, with a significant difference between the three groups regarding to OS and PFS. The 3y OS probability for LR, IR and HR group is 82%, 63% and 37%, the median OS for LR and IR was not reached, for HR is 31 months (p<0.05). The median PFS in LR group was not reached (with 3y PFS probability 70%), in IR and HR group the median is 27 and 17 months, resp. (p<0.01).
Conclusion: Our retrospective analysis confirms a validity of the MIPI prognostic model even in a non-selected population of patients with MCL. This prognostic index seems to be valid also in the era of rituximab.
Disclosures: Pytlik:Roche: Honoraria. Belada:Roche: Consultancy; Amgen: Consultancy; Bayer-Schering-Pharma: Consultancy. Papajik:Roche: Honoraria; Amgen: Honoraria; Bayer-Schering-Pharma: Honoraria. Kubackova:Roche: Research Funding; Bayer-Schering-Pharma: Membership on an entity’s Board of Directors or advisory committees; Wyeth: Membership on an entity’s Board of Directors or advisory committees. Trneny:Biogen: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding.
Supported by the grant IGA MZ CR NR/9453-3. On behalf of CLSG.
Author notes
Corresponding author