Abstract
HIF is a transcription factor that regulates gene expression in response to decreases in cellular oxygenation (hypoxia). Genes activated by HIF are involved in glycolysis, glucose transport, angiogenesis, cell proliferation, cell migration, cell metabolism, and cell survival. Many of these genes confer protection against the consequences of oxygen deprivation while others enhance resistance to chemotherapy or radiotherapy. Clinically, evidence of elevated HIF protein correlates with poor prognosis in lung, breast, colorectal, brain, pancreatic, ovarian, renal, and bladder cancers. Our preliminary tissue micro array (TMA) data suggested that HIF is frequently stabilized in lymphoma (
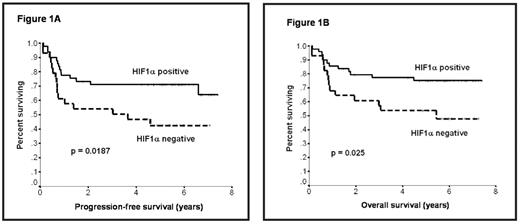
In patients treated with CHOP, there was no difference in outcome between HIF pos and HIF neg subsets. In multivariate analysis in the R-CHOP treated group, controlling for the International Prognostic Index (IPI), HIF pos expression remained a significant independent predictor for OS (p=0.03), while the IPI was of borderline significance (p=0.051). Comparison with other biomarkers showed that HIF did not correlate with expression of bcl-2, CD10, MUM-1, or FOXP1; while a high correlation was detected with bcl-6 and HIF (among HIF pos R-CHOP patients, 94% were bcl-6 pos and 6% were bcl-6 neg; p=0.004). We conclude that expression of HIF-1α is an important and heretofore undiscovered independent favorable prognostic factor for PFS and OS in DLBCL patients treated with R-CHOP, but not in patients treated with CHOP. These data suggest there may be an important biological interaction between CD20 monoclonal antibody-based therapy and HIF or downstream genes regulated by HIF. Further study of this observation is warranted.
Survival according to HIF-1α status in patients treated with R-CHOP.
Disclosures: No relevant conflicts of interest to declare.
Author notes
Corresponding author