Abstract
Specific inhibitors of PI3K isoforms are currently evaluated for their therapeutic potential in leukemia. We found that BCR/ABL+ human leukemic cells express PI3Kδ and therefore explored its impact on leukemia development. Using PI3Kδ-deficient mice, we define a dual role of PI3Kδ in leukemia. We observed a growth-promoting effect in tumor cells and an essential function in natural killer (NK) cell–mediated tumor surveillance: Abelson-transformed PI3Kδ-deficient cells induced leukemia in RAG2-deficient mice with an increased latency, indicating that PI3Kδ accelerated leukemia progression in vivo. However, the absence of PI3Kδ also affected NK cell–mediated tumor surveillance. PI3Kδ-deficient NK cells failed to lyse a large variety of target cells because of defective degranulation, as also documented by capacitance recordings. Accordingly, transplanted leukemic cells killed PI3Kδ-deficient animals more rapidly. As a net effect, no difference in disease latency in vivo was detected if both leukemic cells and NK cells lack PI3Kδ. Other tumor models confirmed that PI3Kδ-deficient mice succumbed more rapidly when challenged with T- or B-lymphoid leukemic or B16 melanoma cells. Thus, the action of PI3Kδ in the NK compartment is as relevant to survival of the mice as the delayed tumor progression. This dual function must be taken into account when using PI3Kδ inhibitors as antileukemic agents in clinical trials.
Introduction
The 8 mammalian phosphoinositide 3-kinase (PI3K) isoforms have been classified based on their lipid specificity and structure. Class I PI3Ks form heterodimers consisting of a catalytic subunit (PI3Kα, β, γ, δ) and a regulatory subunit (p85α, p85β, p55α, p55γ, and p50α for the class IA forms; PI3Kα, β, δ; and p101 and p84 for PI3Kγ, the class IB enzyme): upon stimulation, the heterodimers are recruited to the plasma membrane, where they generate the lipid second messenger PIP3 (phosphatidylinositol 3,4,5-trisphosphate) by phosphorylating phosphatidylinositol-4,5-bisphosphate (PIP2). PIP3 provides a signal recognized by pleckstrin homology domains, which are present in a large number of downstream targets (including protein kinases such as Akt/protein kinase B and Bruton tyrosine kinase, guanine nucleotide exchange factors, GTPase-activating proteins, and adaptor molecules). Class IA catalytic subunits (PI3Kα, β, δ) signal downstream of cytokine and tyrosine kinase receptors (eg, B-cell receptors) through binding of their Src-homology domain 2 (SH2)–containing regulatory subunits to phosphotyrosine residues in YxxM-containing motifs. The class IB PI3Kγ is the only isoform which transmits signals downstream of heterotrimeric G protein–coupled receptors
A constitutive activation of PI3Ks and its downstream target Akt has been found in human cancer and is linked to cell survival and proliferation.1 Gain-of-function mutations of PI3Kα are among the most frequent mutations identified so far and have been linked to cancer progression in colon, breast, brain, and lung. The role of the other isoforms is less clear, although the expression of PI3Kβ, γ, and δ has also been shown to transform chicken embryo fibroblasts.2
The inhibitors LY294002 and wortmannin have been widely used to study the PI3K pathway. While these compounds are popular pharmacologic tools, their significance is limited by their lack of selectivity, which is predicted to cause global effects on all PI3K isoforms. In addition, there are known off-target effects on other signaling molecules (eg, inhibition of PLA2).3 Accordingly, gene targeting was critical to specifically delineate the roles of individual PI3K isoforms in health and disease.4
The catalytic subunits PI3Kα and PI3Kβ are widely expressed and gene deletion results in embryonic lethality.5,6 In contrast, PI3Kγ and PI3Kδ display a tissue specific expression: PI3Kδ is predominantly found in hematopoietic cells, and the phenotype of PI3Kδ−/− animals is dominated by defects in B-cell development and function.7-10 It was also shown that both PI3Kγ and PI3Kδ are required for T-cell development and thymocyte survival.11 Very recently, the importance of PI3Kδ in natural killer (NK) cells has also been appreciated.12,13
The expression pattern of PI3Kδ and its effects in B-lymphoid cells have raised hopes that specific inhibitors of PI3Kδ may suppress the proliferation and survival of transformed hematopoietic cells. Indeed, high levels of PI3Kδ have been found in leukemic cells from patients with acute myeloid leukemia (AML).14 Here, we explored the foundations of this conceptual framework by examining how the absence of PI3Kδ affects leukemia formation in Abelson-induced B-cell transformation. This model is of clinical relevance in humans since the Abl kinase is constitutively activated by a chromosomal translocation (t9;22). The resulting abnormal chromosome is referred to as Philadelphia chromosome: it encodes a fusion protein, BCR/ABL, which has constitutive kinase activity and is associated with chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL). Our experiments revealed a dual role of PI3Kδ in Abelson-driven leukemia progression: the lack of PI3Kδ inhibited disease progression by a direct, cell-autonomous effect in the tumor cells. However, in vivo, this effect was overridden, because PI3Kδ deficiency significantly accelerated leukemia formation by disabling NK-cell function. The absence of PI3Kδ severely impaired the ability of NK cells to undergo degranulation and to kill leukemic cells.
Methods
Statistical analysis
P values were calculated using an unpaired 2-tailed t test (mean values represent data ± SEM; all experiments were performed in triplicate, and 3 independent experiments were evaluated). Differences in Kaplan-Meier plots were analyzed using the log-rank test by GraphPad Prism software (San Diego, CA).
Mice and disease models
All mice were on a 129Sv/C57BL/6 background. To ensure appropriate controls, experiments were performed with littermates. PI3Kδ−/− mice9 and RAG2−/− mice15 were described previously. Two disease models were used: newborn mice were infected by intraperitoneal injection of 100 μL replication-incompetent ecotropic retrovirus encoding for v-abl.16 For transplantation experiments, either 106 cells from independently derived PI3Kδ+/− and PI3Kδ−/− leukemic cell lines, or 5 × 105 EL4 (subclone) or 5 × 105 Eμ-myc–derived leukemic cells (in 100 μL phosphate-buffered saline [PBS]) were injected via tail vein into sublethally irradiated recipient mice. Eμ-myc cells (provided by C. Schuster, MUW, Vienna, Austria) were derived from Eμ-myc transgenic mice.17 Injected mice were checked daily for onset of disease. Sick mice were killed and analyzed for organ weights, white blood cell counts, and the presence of leukemic cells in bone marrow, spleen, and blood. NK-cell depletion was performed using the NK1.1 antibody, which was produced using the hybridoma cell line PK136 (No. HB-191; ATCC, Manassas, VA). Briefly, cell culture supernatant was harvested after serum deprivation and precipitated using saturated ammonium sulfate solution. The pellet was resuspended in buffer (20 mM sodium phosphate; pH 7.0), and antibodies were purified by HiTrap Protein G-HP affinity columns (GE Healthcare, Little Chalfont, United Kingdom). Eluted antibody was desalted using PD-10 columns (GE Healthcare). The purity of the antibody solution was determined by denaturing SDS-PAGE. A total of 150 μg purified antibody was injected intraperitoneally 3 days before transplantation of leukemic cells and subsequently every third day.
B16 melanoma cells (5 × 105 in 0.1 mL PBS) were injected via tail vein into sublethally irradiated recipient mice. After 3 weeks, metastatic lung infiltration was evaluated. All animal experiments were carried out with 8- to 20-week-old littermate control mice in accordance with Austrian legal regulations.
Cell culture
Details are provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
FACS and cell-cycle analysis
Cells were analyzed by fluorescence-activated cell sorting (FACS) using the BD FACS-Canto II FACS device and BD FACS Diva software (Becton Dickinson, Vienna, Austria) as described by Hoelbl et al.19 The following antibodies were used: (1) BD Biosciences-Pharmingen: CD19-FITC, CD43-PE, B220-PerCP, DX5-APC, CD3-PE, Ly49D (4E5), CD244 (2B4), CD107a-FITC, Mac-1–PerCP, CD27-PE, CD16, IFN-γ–PE, and H-2Dd–PE; (2) eBioscience (San Diego, CA): Ly49H(3D10), NKG2D-PE, and NKp46; (3) NK-cell ligands on tumor target cells: PanRae-1 and Mult-1 (R&D Systems, Minneapolis, MN).
To obtain cell-cycle profiles, cells were harvested in 0.5 mL hypotonic lysis solution (50 μg/mL propidium iodide in 0.1% sodium citrate, 0.1% Triton X-100, 100 μg/mL RNAse) and analyzed by FACS.
Generation of NK-cell cultures
Freshly isolated splenocytes were incubated with DX5-coupled magnetic-activated cell sorting (MACS) beads (Miltenyi Biotec, Auburn, CA) and subjected to positive selection. NK-cell preparations were pooled from at least 5 animals for each genotype and expanded in vitro using medium containing 5000 U/mL recombinant human IL-2 for 10 days.20,21 Purity of NK-cell cultures was determined by FACS.
Since high amounts of IL-2 during NK-cell expansion might obscure NK-cell defects,22 NK cells were starved by deprivation of IL-2 for 12 hours prior to all experiments.
Cytotoxicity assays, semiquantitative aggregation assay, and degranulation assay
[51Cr]-release assay was used to monitor target cell lysis as described by Wei et al.23 A total of 104 target cells/well were mixed with NK-effector cells in triplicates and incubated for 4 hours at 37°C in IL-2–free medium in 96-well round-bottom microplates. Tumor target cells used included: YAC-1, RMA, RMA-S, RMA-Rae-1, B16, an NK-responsive EL4 subclone, Eμ-myc–transformed cells, Daudi and Jurkat cells, and v-abl–transformed PI3Kδ+/− and PI3Kδ−/− cells. Table S1 and Figure S2E depict surface receptor expression of NK-cell ligands of these tumor target cells. Redirected antibody-dependent cellular cytotoxicity (R-ADCC) was performed as described by Caraux et al24 and Zompi et al.25 Briefly, IL-2–expanded NK cells were preincubated with the antibodies (20 μg/mL) directed against activating NK-cell receptors. These included CD16, NKG2D, NKp46, Ly49D, Ly49H, CD244, NK1.1, and isotype-matched control rat anti-mouse I-A antibodies. Incubation was performed for 20 minutes at 37°C before the 4-hour [51Cr]-release assay in which FcR+-Daudi cells were used as target cells. Daudi cells are per se not recognized by NK cells in the absence of activating antibodies. In addition, NK-cell degranulation was quantified by FACS using surface expression of CD107a.26 Stimuli used to induce degranulation included coincubation with various tumor target cells and activating antibodies (analog to R-ADCC). Ionomycin (A23187; Sigma-Aldrich, St Louis, MO) treatment (5 μM) was used as a positive control, and isotype-matched rat anti-mouse I-A was used as a negative control. Degranulation was analyzed 4 hours after coincubation with target cells and 12 hours after antibody-induced stimulation.
Aggregate formation was tested in a semiquantitative NK-cell aggregation assay as described by Stoekl et al27 and Caraux et al.24 Aggregate formation efficiency was determined at various time points as indicated.
IC87114 was provided by ICOS (Bothell, WA),28 and used at 5 μM. DMSO controls were performed.
Confocal imaging
NK cells were handled according to standard protocols. Details are provided in Document S1.
Immunoblotting
Human leukemic cells were derived from the bone marrow of patients with CML (n = 7) or BCR/ABL+ ALL (n = 6). Aspirated bone marrow cells were collected in heparin-containing syringes and subjected to isolation of mononuclear cells (MNCs) using Ficoll. All patients gave informed consent in accordance with the Declaration of Helsinki before bone marrow puncture. MNCs were frozen in 90% FCS plus 10% DMSO and stored in liquid nitrogen. For immunoblots thawed human cells or 107 cells from established murine cell lines were lysed, processed as described previously,16,19 and probed either with antibodies specific for PI3Kδ (sc-7176), β (sc-7175), α (sc-7174; all from Santa Cruz Biotechnology, Santa Cruz, CA), and PI3Kγ29 (a generous gift from R. Wetzker, Jena, Germany) which were used as described.30 Anti-pAkt (S473) and anti-Akt antibodies were from Cell Signaling Technology (Danvers, MA).
RNA isolation and RT-PCR analysis
RNA isolation and reverse transcriptase–polymerase chain reaction (RT-PCR) analysis are described in Document S1. Capacitance measurements are also described in detail in Document S1.
Results
PI3Kδ expression in human and murine leukemic cells
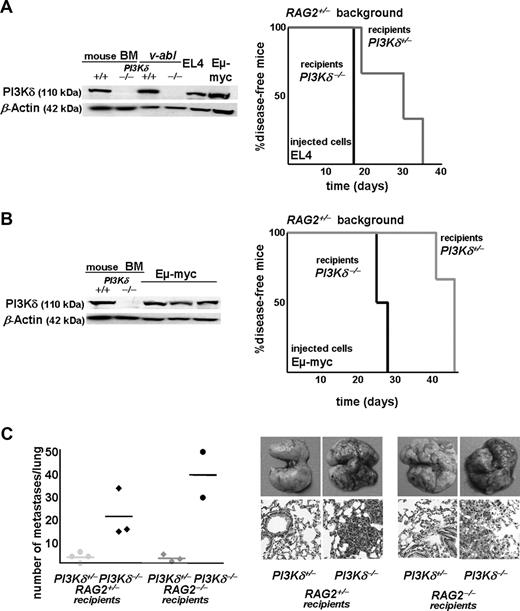
PI3Kδ was expressed in all leukemic cell samples derived from patients with BCR/ABL+ CML and ALL (Figure 1A). PI3Kδ inhibitors are considered novel therapeutic options for the treatment of hematopoietic malignancies. Hence, we studied the impact of PI3Kδ for Abelson-induced leukemia progression. Murine PI3Kδ+/− and PI3Kδ−/− bone marrow cells were infected in vitro with a replication-incompetent ecotropic form of the Abelson virus (Ab-MuLV; v-abl). This procedure renders the cells independent of growth factors and allows them to form stable cell lines. Immortalized cell lines of each genotype were obtained from each bone marrow preparation. These cell lines consisted of B220+CD19+CD43+ cells (Figure 1B). Bone marrow cells express all 4 catalytic PI3K isoforms (α, β, γ, and δ). This pattern changed in the transformed cells that showed a predominant expression of PI3Kα, γ, and δ. As expected, PI3Kδ mRNA and protein was absent in the cell lines derived from PI3Kδ−/− bone marrow (Figure 1C). Moreover, v-abl–transformed cells lost the expression of PI3Kβ—this was detectable both at the mRNA and protein levels (Figure 1C) and confirmed in human patient samples (E.Z., unpublished observations, December 2007). However, the lack of PI3Kδ did not translate into changes in the phosphorylation of Akt, a major downstream signaling molecule (Figure 1D). Similarly, we failed to detect any alterations in the proliferative or apoptotic response of the PI3Kδ−/− compared with PI3Kδ+/− cell lines. Cell-cycle distribution, growth characteristics, apoptotic responses to UV irradiation, and serum withdrawal were comparable in several individually derived cell lines (Figure 1E; data not shown). These experiments supported the conclusion that PI3Kδ was not a prerequisite for the transformation of B cells by the Abelson oncogene and that lack of PI3Kδ did not impair cell proliferation nor alter apoptotic responses of Abelson-transformed cells in vitro.
PI3Kδ protein expression in BCR/ABL+ leukemia. (A) Immunoblotting for PI3Kδ in cell extracts from 6 patients with BCR/ABL+ ALL and 7 patients with CML (right panel, top and bottom). All samples express PI3Kδ. Healthy human bone marrow and naive murine PI3Kδ+/− and PI3Kδ−/− bone marrow preparations served as controls. (B) Typical example of FACS-based analysis of CD19 and CD43 surface expression of v-abl—transformed murine PI3Kδ+/− and PI3Kδ−/− cell lines (n = 6 for each genotype analyzed). (C) Representative RT-PCR analysis of PI3Kα, β, γ, and δ isoform expression in freshly isolated bone marrow cells and in v-abl–transformed PI3Kδ+/+, PI3Kδ+/−, and PI3Kδ−/− cell lines (left panel). Western blot analysis of PI3Kα, β, δ, and PI3Kγ in stable v-abl–transformed cell lines (right panel). MACS-purified pro-B cells derived from PI3Kδ+/− and PI3Kδ−/− bone marrow served as positive and negative controls, respectively. (D) Western blot analysis of phosphorylated Akt in v-abl–transformed PI3Kδ+/− and PI3Kδ−/− cell lines. NIH3T3 cells were used as control (ctr). (E) Cell-cycle profiles of v-abl–transformed PI3Kδ+/− and PI3Kδ−/− cell lines.
PI3Kδ protein expression in BCR/ABL+ leukemia. (A) Immunoblotting for PI3Kδ in cell extracts from 6 patients with BCR/ABL+ ALL and 7 patients with CML (right panel, top and bottom). All samples express PI3Kδ. Healthy human bone marrow and naive murine PI3Kδ+/− and PI3Kδ−/− bone marrow preparations served as controls. (B) Typical example of FACS-based analysis of CD19 and CD43 surface expression of v-abl—transformed murine PI3Kδ+/− and PI3Kδ−/− cell lines (n = 6 for each genotype analyzed). (C) Representative RT-PCR analysis of PI3Kα, β, γ, and δ isoform expression in freshly isolated bone marrow cells and in v-abl–transformed PI3Kδ+/+, PI3Kδ+/−, and PI3Kδ−/− cell lines (left panel). Western blot analysis of PI3Kα, β, δ, and PI3Kγ in stable v-abl–transformed cell lines (right panel). MACS-purified pro-B cells derived from PI3Kδ+/− and PI3Kδ−/− bone marrow served as positive and negative controls, respectively. (D) Western blot analysis of phosphorylated Akt in v-abl–transformed PI3Kδ+/− and PI3Kδ−/− cell lines. NIH3T3 cells were used as control (ctr). (E) Cell-cycle profiles of v-abl–transformed PI3Kδ+/− and PI3Kδ−/− cell lines.
Abelson-transformed PI3Kδ−/− cells induce leukemia in mice with an increased latency
To study the behavior of the Abelson-transformed cell lines in vivo, we injected 106 cells of 3 individually derived PI3Kδ+/− and PI3Kδ−/− cell lines via tail vein into RAG2−/− animals (n = 8 for PI3Kδ+/− and n = 6 for PI3Kδ−/−). As depicted in Figure 2A, mice that had received PI3Kδ+/− leukemic cells succumbed to disease with a significantly faster time course than mice that had received PI3Kδ−/− leukemic cells (median survival, 13 days vs 20 days; P = .005). The phenotype of the disease induced by PI3Kδ−/− or PI3Kδ+/− leukemic cells was comparable (Figure 2B,C). Based on the fact that no significant differences between PI3Kδ+/− and PI3Kδ−/− leukemic cells were detectable in vitro but became apparent in vivo, we reasoned that an altered interaction with the immune system might account for this discrepancy. Abelson-transformed cells are mainly recognized and eradicated by NK cells, which are the only lymphoid compartment present in RAG2−/− mice. We therefore tested whether the increased disease latency upon injection of PI3Kδ−/− leukemic cells resulted from an altered interaction with NK cells. Indeed, PI3Kδ−/− leukemic cells were significantly better lysed by wild-type (wt) NK cells than PI3Kδ+/− cells (Figure 2D). NK cells recognize tumor targets via distinct surface receptors; the importance of the NKG2D receptor in tumor surveillance has recently been highlighted by the generation of NKG2D-deficient animals.31 Interestingly, PI3Kδ−/− leukemic cells showed significantly higher expression of the NKG2D ligand Rae-1 (Figure 2E).
Abelson-transformed PI3Kδ−/− cells induce leukemia in mice with an increased latency. (A) Kaplan-Meier plot of RAG2−/− mice after transplantation of 106 transformed cells (3 independently derived cell lines per genotype were injected into n = 8 for PI3Kδ+/− and n = 6 for PI3Kδ−/−). Mice that had been injected with transformed PI3Kδ−/− cells developed leukemia significantly later as determined by a log-rank test (median survival, 13 vs 20 days; P = .005). (B) H&E stains of blood smears (top), spleens (middle), and livers (bottom) from RAG2−/− mice after injection of v-abl–transformed cells (magnification, ×100, Zeiss AxioImager 21 [Jena, Germany], 10× objective, NA 0.25, air; camera: Pixelink Color, 1600 × 1200; software: PixelINK Capture 3.0). (C) Spleen, bone marrow, and blood were analyzed for infiltration with CD19+CD43+ leukemic cells by FACS. Tissue infiltration was slightly lower in mice that had received PI3Kδ−/− cells without reaching statistical significance (data represent means ± SEM). (D) [51Cr]-release assay using IL-2–expanded wt NK cells as effectors and Abelson-transformed leukemic cells as targets. PI3Kδ−/− leukemic cells were significantly better eradicated by wt NK cells than PI3Kδ+/− leukemic target cells at any effector-target cell ratio tested (for ratios 20:1, 10:1, 5:1, and 2:1, P = .02, P = .02, P = .003, and P = .006 as determined in an unpaired 2-tailed t test). (E) Quantification of pan–Rae-1, Mult-1, and MHC I expression by FACS of in vitro–derived Abelson-transformed cell lines. PI3Kδ−/− leukemic cells showed a significantly higher surface expression of pan-Rae1 when compared with PI3Kδ+/− leukemic cells (mean fluorescence intensity [MFI] of 1082 ± 199 vs 639 ± 259; P = .035 in an unpaired 2-tailed t test; n = 4 for each genotype). n.s., indicates P > .05; *P < .05; and **P < .01.
Abelson-transformed PI3Kδ−/− cells induce leukemia in mice with an increased latency. (A) Kaplan-Meier plot of RAG2−/− mice after transplantation of 106 transformed cells (3 independently derived cell lines per genotype were injected into n = 8 for PI3Kδ+/− and n = 6 for PI3Kδ−/−). Mice that had been injected with transformed PI3Kδ−/− cells developed leukemia significantly later as determined by a log-rank test (median survival, 13 vs 20 days; P = .005). (B) H&E stains of blood smears (top), spleens (middle), and livers (bottom) from RAG2−/− mice after injection of v-abl–transformed cells (magnification, ×100, Zeiss AxioImager 21 [Jena, Germany], 10× objective, NA 0.25, air; camera: Pixelink Color, 1600 × 1200; software: PixelINK Capture 3.0). (C) Spleen, bone marrow, and blood were analyzed for infiltration with CD19+CD43+ leukemic cells by FACS. Tissue infiltration was slightly lower in mice that had received PI3Kδ−/− cells without reaching statistical significance (data represent means ± SEM). (D) [51Cr]-release assay using IL-2–expanded wt NK cells as effectors and Abelson-transformed leukemic cells as targets. PI3Kδ−/− leukemic cells were significantly better eradicated by wt NK cells than PI3Kδ+/− leukemic target cells at any effector-target cell ratio tested (for ratios 20:1, 10:1, 5:1, and 2:1, P = .02, P = .02, P = .003, and P = .006 as determined in an unpaired 2-tailed t test). (E) Quantification of pan–Rae-1, Mult-1, and MHC I expression by FACS of in vitro–derived Abelson-transformed cell lines. PI3Kδ−/− leukemic cells showed a significantly higher surface expression of pan-Rae1 when compared with PI3Kδ+/− leukemic cells (mean fluorescence intensity [MFI] of 1082 ± 199 vs 639 ± 259; P = .035 in an unpaired 2-tailed t test; n = 4 for each genotype). n.s., indicates P > .05; *P < .05; and **P < .01.
Impaired lysis of leukemic cells by PI3Kδ−/− NK cells in vivo
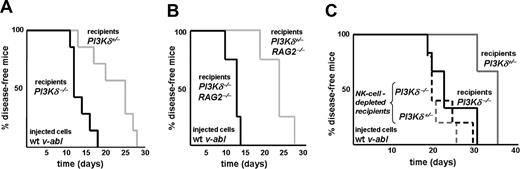
The experiments summarized in Figure 2 documented that loss of PI3Kδ delays tumor progression in vivo. Based on these findings, specific inhibitors of PI3Kδ ought to be useful in a clinical setting. However, PI3Kδ is also present in immune cells which are capable of eliminating tumor cells. An inhibitor of PI3Kδ thus may have an impact on immunologic surveillance. This was separately examined by injecting 3 independently derived wt Abelson-transformed leukemic cell lines into PI3Kδ+/− and PI3Kδ−/− recipient mice (n = 7 for each group). Whereas PI3Kδ−/− recipient mice succumbed to the disease rapidly (median survival, 12 days), PI3Kδ+/− animals survived significantly longer (median survival, 25 days; P = .003; Figure 3A). This effect was attributable to differences in NK cell–mediated tumor surveillance: experiments in RAG2−/−PI3Kδ−/− and RAG2−/−PI3Kδ+/− recipient animals recapitulated the results obtained with PI3Kδ+/− and PI3Kδ−/− mice (Figure 3B). Leukemia development occurred with a significantly shortened latency in RAG2−/−PI3Kδ−/− animals when compared with RAG2−/−PI3Kδ+/− recipient mice (median survival, 12 days vs 23 days; P = .007). Antibody-mediated NK-cell depletion further confirmed the central role of NK cells (Figure 3C): injection of wt leukemic cell lines resulted in a significant difference in disease latency between PI3Kδ+/− and PI3Kδ−/− recipient mice (median survival, 35 days for PI3Kδ+/− vs 22 days for PI3Kδ−/− mice; P = .03) that was completely abolished by NK-cell depletion (median survival, 19 days; P = .61). These experiments verified the key role of PI3Kδ for NK cell–mediated lysis of leukemic tumor targets in vivo.
Mice deficient for PI3Kδ develop leukemia significantly faster. (A) Kaplan-Meier plot of PI3Kδ+/− and PI3Kδ−/− recipient animals after injection of wt v-abl–transformed cells. PI3Kδ−/− recipient mice developed disease significantly earlier when compared with their PI3Kδ+/− littermate controls (log-rank test; P = .003; n = 7 for each group; 3 independently derived cell lines were injected). (B) Kaplan-Meier plot of PI3Kδ+/− and PI3Kδ−/− recipients on a RAG2−/− background after injection of wt v-abl–transformed cells. Again, PI3Kδ−/− recipients succumbed significantly earlier to disease than PI3Kδ+/− littermate controls (log-rank test; P = .007; n = 4 for each group). (C) Kaplan-Meier plot of 10 PI3Kδ+/− and 10 PI3Kδ−/− recipient animals after injection of wt v-abl–transformed cells. A total of 5 animals of each group received anti-NK1.1 antibody to deplete NK cells (dashed lines). In the absence of NK-cell depletion, PI3Kδ−/− recipient mice again developed disease significantly earlier when compared with their PI3Kδ+/− littermate controls (solid lines; log-rank test; P = .03). This difference was lost upon NK-cell depletion (dashed lines; log-rank test; P = .61; n = 5 for each group).
Mice deficient for PI3Kδ develop leukemia significantly faster. (A) Kaplan-Meier plot of PI3Kδ+/− and PI3Kδ−/− recipient animals after injection of wt v-abl–transformed cells. PI3Kδ−/− recipient mice developed disease significantly earlier when compared with their PI3Kδ+/− littermate controls (log-rank test; P = .003; n = 7 for each group; 3 independently derived cell lines were injected). (B) Kaplan-Meier plot of PI3Kδ+/− and PI3Kδ−/− recipients on a RAG2−/− background after injection of wt v-abl–transformed cells. Again, PI3Kδ−/− recipients succumbed significantly earlier to disease than PI3Kδ+/− littermate controls (log-rank test; P = .007; n = 4 for each group). (C) Kaplan-Meier plot of 10 PI3Kδ+/− and 10 PI3Kδ−/− recipient animals after injection of wt v-abl–transformed cells. A total of 5 animals of each group received anti-NK1.1 antibody to deplete NK cells (dashed lines). In the absence of NK-cell depletion, PI3Kδ−/− recipient mice again developed disease significantly earlier when compared with their PI3Kδ+/− littermate controls (solid lines; log-rank test; P = .03). This difference was lost upon NK-cell depletion (dashed lines; log-rank test; P = .61; n = 5 for each group).
Cell-intrinsic roles of PI3Kδ in leukemic cell and NK cell: the net effect
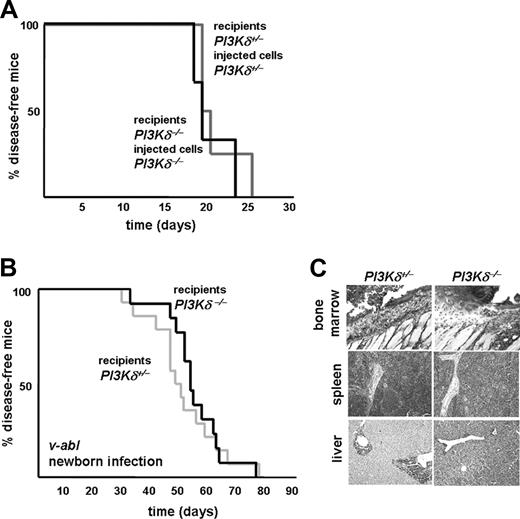
It was difficult to predict the net effect, which would result in vivo from the absence of PI3Kδ in both the leukemic cells and the NK cells. The impaired ability of PI3Kδ−/− cells to form leukemia might be counteracted by the reduced ability of PI3Kδ−/− NK cells to combat leukemia. Hence, disease latency may remain unaffected because of the opposite effects of PI3Kδ deficiency in the tumor cells (favoring increased latency) and NK cells (promoting decreased latency). This question appears of particular relevance in view of the development of PI3Kδ kinase inhibitors. To test whether impaired transformation or reduced immunologic surveillance would prevail, we injected Abelson-transformed PI3Kδ+/− leukemic cells into PI3Kδ+/− recipient animals. Similarly, we injected PI3Kδ−/− leukemic cells into PI3Kδ−/− littermate animals. A total of 3 independently derived Abelson-transformed leukemic cell lines of each genotype were used, and the experiments are summarized in Figure 4A. No significant difference was detected (mean survival time, 19 vs 19.5 days; P = .95). In addition, we used a second disease model that closely mimics leukemia development in humans. Newborn PI3Kδ+/− and PI3Kδ−/− mice were challenged with a single exposure to Ab-MuLV. This procedure induces the development of a slowly evolving mono- or oligoclonal leukemia; the leukemic cells progressively infiltrate bone marrow, spleen, liver, and lymph nodes. Again, as depicted in Figure 4B, we failed to see any significant alterations in latency or incidence in this disease model (mean survival time, 49 vs 53 days; P = .7). Regardless of the expression of PI3Kδ, all mice died of leukemia within 80 days. The phenotype of the disease was comparable (Figure 4C).
Net effect of PI3Kδ deficiency for leukemia development. (A) Kaplan-Meier plot of PI3Kδ+/− recipients after injection of PI3Kδ+/−v-abl–transformed cells and of PI3Kδ−/− recipients after injection of PI3Kδ−/−v-abl–transformed cells. Matching genotypes of recipient animals with the genotype of the injected cell line abolished any differences in leukemia onset and progression (log-rank test; P = .95). (B) Accordingly, upon v-abl infection of newborn PI3Kδ+/− and PI3Kδ−/− recipients, no significant difference in survival kinetics was detected (log-rank test; P = 0.7). No differences were observed between PI3Kδ+/+ and PI3Kδ+/− control animals, as verified in an independent experiment (data not shown). (C) H&E-stained histologic sections of infiltrated bone marrow (top row), spleen (middle row), and liver (bottom row) of PI3Kδ+/− and PI3Kδ−/− animals that had been challenged with v-abl at the newborn age (magnification, ×100, Zeiss AxioImager 21, 10× objective, NA 0.25, air, camera: Pixelink Color, 1600 × 1200; software: PixelINK Capture 3.0).
Net effect of PI3Kδ deficiency for leukemia development. (A) Kaplan-Meier plot of PI3Kδ+/− recipients after injection of PI3Kδ+/−v-abl–transformed cells and of PI3Kδ−/− recipients after injection of PI3Kδ−/−v-abl–transformed cells. Matching genotypes of recipient animals with the genotype of the injected cell line abolished any differences in leukemia onset and progression (log-rank test; P = .95). (B) Accordingly, upon v-abl infection of newborn PI3Kδ+/− and PI3Kδ−/− recipients, no significant difference in survival kinetics was detected (log-rank test; P = 0.7). No differences were observed between PI3Kδ+/+ and PI3Kδ+/− control animals, as verified in an independent experiment (data not shown). (C) H&E-stained histologic sections of infiltrated bone marrow (top row), spleen (middle row), and liver (bottom row) of PI3Kδ+/− and PI3Kδ−/− animals that had been challenged with v-abl at the newborn age (magnification, ×100, Zeiss AxioImager 21, 10× objective, NA 0.25, air, camera: Pixelink Color, 1600 × 1200; software: PixelINK Capture 3.0).
PI3Kδ−/− NK cells have an impaired cytolytic ability
Abelson-transformed leukemic cells are mainly subject to surveillance by NK cells as shown previously.21,32 To understand why the deletion of PI3Kδ impaired NK-cell function and consequently the clearance of leukemic cells in vivo, we isolated NK/NKT cells for further characterization. We did not detect any obvious differences in NK-cell numbers and functional subsets (Figure S1) and in the expression of cell-surface markers on primary and IL-2–expanded NK cells regardless of the genotype (Table S2). Differences were evident in cytotoxicity assays against target cells (Figure 5A). Regardless of the nature of the target cells, PI3Kδ−/− NK cells were significantly less capable to lyse these when compared with PI3Kδ+/− NK cells. We used target cells recognized by various different pathways, including Abelson-transformed cells (see Table S3 for a detailed summary of [51Cr]- and FACS-based quantification). These experiments pointed at a common defect unrelated to a distinct activation pathway. The presence of a general defect was further supported by experiments using R-ADCC. Preincubation with activating antibodies stimulated NK-cell cytotoxicity toward Daudi cells that are per se not recognized by NK cells. Regardless of the receptor stimulated, PI3Kδ−/− NK cells were never considerably activated (Figure S2; Table S3), which indicates that PI3Kδ is a key element of a common lytic pathway.
PI3Kδ−/− NK cells fail to efficiently lyse leukemic target cells. (A) The cytolytic function of IL-2–expanded PI3Kδ+/− and PI3Kδ−/− NK cells was assessed by [51Cr]-release assay using various cell lines as target cells as indicated. PI3Kδ−/− NK cells showed consistently impaired specific lysis when compared with PI3Kδ+/− NK cells, regardless of the target cell line used. The complete table of all target lines including statistics is provided in Table S3. (B) Top panels: typical confocal images of CD107a+ granules of PI3Kδ+/− and PI3Kδ−/− NK cells are given. Bottom panel: quantification of granule numbers. Confocal images of CD107a+ granules were acquired in the z-stack mode on a Zeiss LSM510 confocal microscope with a 100× oil-immersion objective (NA 1.3) with the 488-nm line of the Ar laser and a LP 505-nm emission filter. About 24 slices were acquired with a distance of 0.3 μm between the slices. Images were imported into ImageJ software, and maximum projection images were calculated. Stained vesicles were selected by auto-thresholding, followed by quantification and analysis of all selected regions. Horizontal lines indicate data mean. (C) A FACS-based degranulation assay measuring surface expression of the late endosomal marker CD107a was performed. FACS histograms illustrate the percentage of PI3Kδ+/− (top row) and PI3Kδ−/− (bottom row) NK cells expressing CD107a under basal conditions (left panels) and after stimulation by coincubation with v-abl–transformed target cells. Significantly more PI3Kδ+/− NK cells were stimulated to express CD107a on their surface compared with PI3Kδ−/− NK cells (data mean ± SEM). (D) Bar graphs depicting different stimuli used to induce degranulation in PI3Kδ+/− and PI3Kδ−/− NK cells: none of the target cells or specific antibodies induced degranulation of PI3Kδ−/− NK cells. (E) Electrophysiologic capacitance measurements were performed and confirmed the defect of PI3Kδ−/− NK cells in Ca2+-dependent exocytosis/degranulation. Electrophysiologic capacitance measurement relies on recording the passive current, which has to be injected into the cell to keep a constant voltage and this corresponds to the size (surface area) of a cell: the bigger the cell, the more current has to be injected. In vitro–expanded murine NK cells are round cells (means of absolute capacitance of unstimulated PI3Kδ+/− NK cells, 12.4 ± 5.4 pF; of PI3Kδ−/− NK cells, 13.73 ± 5.9 pF). Two typical examples of the exocytotic response under the superfusion with calcimycin are illustrated (left panel). The mean increase in relative capacitance after superfusion of PI3Kδ+/− and PI3Kδ−/− NK cells was compared (mean increase in relative capacitance for PI3Kδ+/− NK cells: 21% ± 2%; for PI3Kδ−/−NK cells: 8.5% ± 1%; P < .001; n = 10 for each genotype; the experiment was repeated with 3 independent NK-cell preparations). The increase in relative capacitance was obtained by comparing the peak increase in relative capacitance after superfusion with calcimycin with resting cell capacitance under superfusion with control solution (right panel). Error bars represent SEM. n.s. indicates P > .05; *P < .05; and **P < .01.
PI3Kδ−/− NK cells fail to efficiently lyse leukemic target cells. (A) The cytolytic function of IL-2–expanded PI3Kδ+/− and PI3Kδ−/− NK cells was assessed by [51Cr]-release assay using various cell lines as target cells as indicated. PI3Kδ−/− NK cells showed consistently impaired specific lysis when compared with PI3Kδ+/− NK cells, regardless of the target cell line used. The complete table of all target lines including statistics is provided in Table S3. (B) Top panels: typical confocal images of CD107a+ granules of PI3Kδ+/− and PI3Kδ−/− NK cells are given. Bottom panel: quantification of granule numbers. Confocal images of CD107a+ granules were acquired in the z-stack mode on a Zeiss LSM510 confocal microscope with a 100× oil-immersion objective (NA 1.3) with the 488-nm line of the Ar laser and a LP 505-nm emission filter. About 24 slices were acquired with a distance of 0.3 μm between the slices. Images were imported into ImageJ software, and maximum projection images were calculated. Stained vesicles were selected by auto-thresholding, followed by quantification and analysis of all selected regions. Horizontal lines indicate data mean. (C) A FACS-based degranulation assay measuring surface expression of the late endosomal marker CD107a was performed. FACS histograms illustrate the percentage of PI3Kδ+/− (top row) and PI3Kδ−/− (bottom row) NK cells expressing CD107a under basal conditions (left panels) and after stimulation by coincubation with v-abl–transformed target cells. Significantly more PI3Kδ+/− NK cells were stimulated to express CD107a on their surface compared with PI3Kδ−/− NK cells (data mean ± SEM). (D) Bar graphs depicting different stimuli used to induce degranulation in PI3Kδ+/− and PI3Kδ−/− NK cells: none of the target cells or specific antibodies induced degranulation of PI3Kδ−/− NK cells. (E) Electrophysiologic capacitance measurements were performed and confirmed the defect of PI3Kδ−/− NK cells in Ca2+-dependent exocytosis/degranulation. Electrophysiologic capacitance measurement relies on recording the passive current, which has to be injected into the cell to keep a constant voltage and this corresponds to the size (surface area) of a cell: the bigger the cell, the more current has to be injected. In vitro–expanded murine NK cells are round cells (means of absolute capacitance of unstimulated PI3Kδ+/− NK cells, 12.4 ± 5.4 pF; of PI3Kδ−/− NK cells, 13.73 ± 5.9 pF). Two typical examples of the exocytotic response under the superfusion with calcimycin are illustrated (left panel). The mean increase in relative capacitance after superfusion of PI3Kδ+/− and PI3Kδ−/− NK cells was compared (mean increase in relative capacitance for PI3Kδ+/− NK cells: 21% ± 2%; for PI3Kδ−/−NK cells: 8.5% ± 1%; P < .001; n = 10 for each genotype; the experiment was repeated with 3 independent NK-cell preparations). The increase in relative capacitance was obtained by comparing the peak increase in relative capacitance after superfusion with calcimycin with resting cell capacitance under superfusion with control solution (right panel). Error bars represent SEM. n.s. indicates P > .05; *P < .05; and **P < .01.
One major mechanism in NK cell–mediated tumor clearance is the lytic granule secretory pathway. To efficiently kill target cells, the NK-cell releases the content of its lytic granules by fusion with the plasma membrane. During this process, CD107a (LAMP-1), a component of lysosomal membranes, is integrated into the plasma membrane (degranulation). We first verified the presence of CD107a+ lytic granules by confocal microscopy in PI3Kδ−/− NK cells. We failed to detect any significant differences in number, shape, or size of CD107a+ granules (Figure 5B). Similarly, effector molecules such as perforin and granzymes were expressed at comparable amounts (Figure S1C). However, FACS-based degranulation assays revealed that coincubation of PI3Kδ+/− and PI3Kδ−/− NK cells with wt v-abl target cells resulted in a 4-fold increase of CD107a expression on the surface of PI3Kδ+/− NK cells compared with PI3Kδ−/− NK cells (Figure 5C right panels). Similar results were obtained with various other experimental stimuli (Figure 5D), including coincubation with 7 target cell lines and stimulation of 6 distinct activating receptors by specific antibodies.
It is evident from Figure 5D that the response to the different stimuli was variable; however, PI3Kδ−/− NK cells were always less responsive. The notable exception was challenge with Jurkat cells (compare bars 3 and 4 in the right panel of Figure 5D); these, however, are killed by a FAS-dependent pathway rather than by NK-cell granule exocytosis.33 Degranulation is ultimately triggered by a rise in intracellular Ca2+. It is also evident from Figure 5D that the defect in PI3Kδ−/− NK cells affected a very late step because the difference was readily detected upon challenging cells with the calcium ionophore ionomycin (compare bars 3 and 4 in the left panel of Figure 5D). This observation again indicates that PI3Kδ is a key molecule involved in the ultimate common degranulation trigger regardless of the upstream stimulus (ie, at a very distal level independent from any target cell contact or activating receptor). This conjecture was rigorously tested by directly monitoring degranulation in real time: the fusion of the membrane of the lytic granules with the outer cell membrane leads to a subtle increase in cell size that can be measured by recording NK-cell capacitance in a whole-cell patch clamp configuration. Single NK cells were superfused with a Ca2+ ionophore (ionomycin), which triggers degranulation, and the increase in cell capacitance was recorded. Two typical examples of the exocytotic response under the superfusion with ionomycin are illustrated for PI3Kδ+/− and PI3Kδ−/− NK cells in Figure 5E. In PI3Kδ+/− NK cells, superfusion with ionomycin resulted in a mean increase in relative capacitance of 21% plus or minus 2% compared with 8.5% plus or minus 1% for PI3Kδ−/− NK cells (P < .001). Hence, PI3Kδ+/− NK cells showed a significantly higher increase in cell-surface area compared with PI3Kδ−/− NK cells. These results prove a central role for PI3Kδ at a very distal step in the process of degranulation.
It is conceivable that additional upstream defects in PI3Kδ−/− NK cells impair the ability of cells to engage their targets. Accordingly, we assessed the ability of NK cells to form aggregates with target cells. Both, PI3Kδ+/− and PI3Kδ−/− NK cells form aggregates initially at comparable numbers (Figure 6A top panel). However, after 4 hours, aggregates formed by PI3Kδ−/− NK cells were apparently less stable because of the decline in numbers (Figure 6B); this effect was even more pronounced after 12 hours (Figure 6A bottom panels, 6B). These results were confirmed with a selective inhibitor of PI3Kδ: aggregate formation by PI3Kδ+/− NK cells was not impaired in the presence of a selective PI3Kδ inhibitor, but degranulation and killing of target cells was reduced (Figure 6C). This pharmacologic approach provided independent confirmation for our conclusion that PI3Kδ was indispensable for degranulation and killing of target cells.
PI3Kδ is dispensable for the initial formation of aggregates with leukemic target cells, but is indispensable for the maintenance of aggregates and for degranulation. (A) Initial aggregate formation (top panels) and maintainance of aggregates (bottom panels) of PI3Kδ+/− and PI3Kδ−/− NK cells with wt v-abl target cells was analyzed by light microscopy (magnification: top panel, ×100; bottom panel, ×430; one example for each genotype is depicted, obtained with a Zeiss Axiovert 135, 10× Ph1 [NA 0.25], camera: Cool Snap HQ [Ottobrunn, Germany], software: Metamorph 4.0 [Visitron, Puchheim, Germany]), (B) quantified by FACS, and observed over time. Error bars represent SEM (*P < .05; **P < .01). (C) A selective inhibitor of PI3Kδ was used to describe the requirement for PI3Kδ for killing, aggregate formation, and degranulation of NK cells when incubated with leukemic target cells. As normalized to PI3Kδ+/− NK cells, PI3Kδ inhibition severely impaired killing (61.3% of PI3Kδ+/− NK cells) and degranulation (37.7% of PI3Kδ+/− NK cells), whereas initial aggregate formation was not affected (115% of PI3Kδ+/− NK cells).
PI3Kδ is dispensable for the initial formation of aggregates with leukemic target cells, but is indispensable for the maintenance of aggregates and for degranulation. (A) Initial aggregate formation (top panels) and maintainance of aggregates (bottom panels) of PI3Kδ+/− and PI3Kδ−/− NK cells with wt v-abl target cells was analyzed by light microscopy (magnification: top panel, ×100; bottom panel, ×430; one example for each genotype is depicted, obtained with a Zeiss Axiovert 135, 10× Ph1 [NA 0.25], camera: Cool Snap HQ [Ottobrunn, Germany], software: Metamorph 4.0 [Visitron, Puchheim, Germany]), (B) quantified by FACS, and observed over time. Error bars represent SEM (*P < .05; **P < .01). (C) A selective inhibitor of PI3Kδ was used to describe the requirement for PI3Kδ for killing, aggregate formation, and degranulation of NK cells when incubated with leukemic target cells. As normalized to PI3Kδ+/− NK cells, PI3Kδ inhibition severely impaired killing (61.3% of PI3Kδ+/− NK cells) and degranulation (37.7% of PI3Kδ+/− NK cells), whereas initial aggregate formation was not affected (115% of PI3Kδ+/− NK cells).
PI3Kδ−/− mice are highly susceptible to tumor development
Additional tumor models were chosen that expressed significant levels of PI3Kδ, because these qualified as suitable candidates for a PI3Kδ-targeted therapy (Figure 7; data not shown). A total of 5 × 105 cells of the murine T lymphoma cell line EL4 were injected into PI3Kδ+/− and PI3Kδ−/− recipient mice. These subsequently developed leukemia with spleno- and hepatomegaly. The PI3Kδ−/− recipient mice succumbed to the disease rapidly (median survival, 17 days on the RAG2+/− background; 16.5 days on RAG2−/− background). In contrast, the PI3Kδ+/− animals survived the leukemic challenge significantly longer (median survival, 30 days and 20 days on the RAG2+/− [P = .045] and RAG2−/− [P = .039] background, respectively) (Figure 7A; data not shown). Similarly, a cell line derived from an Eμ-myc transgenic mouse, which represents a model for human Burkitt lymphoma, was injected intravenously into PI3Kδ+/−, PI3Kδ−/−, RAG2−/−PI3Kδ+/−, and RAG2−/−PI3Kδ −/− mice. The onset of disease was rapid in PI3Kδ−/− animals, with a median survival of 25.5 and 13 days on RAG2+/− (Figure 7B) and RAG2−/− (not shown) backgrounds, respectively. Again, the PI3Kδ+/− animals survived significantly longer (median survival of 45 days on RAG2+/− background; P = .039; Figure 7B; median survival of 31 days on RAG2−/− background; P = .022; data not shown). Finally, we challenged our concept in an independent, nonhematologic tumor model by injecting the melanoma cell line B16 intravenously in PI3Kδ+/−, PI3Kδ−/−, RAG2−/−PI3Kδ+/−, and RAG2−/−PI3Kδ−/− littermate controls (Figure 7C). B16 cells have been reported to express PI3Kδ34 and are known to be under the immunologic control of NK cell–mediated clearance.35 At 3 weeks after the injection of the B16 cells, the metastatic infiltrates in lungs of recipient mice were compared. At this time point, the PI3Kδ−/− animals were severely affected and showed significant infiltration of tumor cells in the lungs, regardless of whether they were on a wt or a RAG2−/− background. Despite the low animal numbers, the differences clearly met the criteria of being statistically significant (P = .005 for wt and P = .015 for RAG2−/− background; Figure 7C). The difference is also emphasized by hematoxylin and eosin (H&E) stains of sections of the affected lungs. Thus, PI3Kδ regulates NK cell–mediated tumor surveillance regardless of the origin of the tumor cell.
PI3Kδ−/− mice in additional hematologic and nonhematologic tumor models. (A) PI3Kδ protein expression in murine leukemic cell lines. Naive murine PI3Kδ+/− and PI3Kδ−/− bone marrow preparations served as controls. Kaplan-Meier plot after injection of EL4 cells intravenously into PI3Kδ+/−RAG2+/− and PI3Kδ−/−RAG2+/− recipients (log-rank test; P = .045). (B) PI3Kδ protein is expressed in Eμ-myc–derived leukemic cells. Upon injection of Eμ-myc–derived cells, PI3Kδ−/−RAG2+/− recipients succumbed to disease significantly faster than PI3Kδ+/−RAG2+/− littermate controls (log-rank test; P = .039). (C) B16 melanoma cells were injected intravenously and numbers of metastatic infiltrates per lung were counted under the binocular microscope. RAG2+/−PI3Kδ−/− recipient mice showed a significantly higher number of metastatic infiltrates than RAG2+/−PI3Kδ+/− (P = .005), and this was also true on the RAG2−/− background (P = .015). Horizontal lines indicate data mean. Thus, increased tumor formation in PI3Kδ−/− mice is not restricted to lymphoid malignancies. Formation of lung metastasis in littermate controls (top panels: photographs, digital camera, Canon EOS 300D; bottom panels: H&E-stained histologic sections of lungs; magnification, ×20 Zeiss AxioImager 71; see Figure 2B).
PI3Kδ−/− mice in additional hematologic and nonhematologic tumor models. (A) PI3Kδ protein expression in murine leukemic cell lines. Naive murine PI3Kδ+/− and PI3Kδ−/− bone marrow preparations served as controls. Kaplan-Meier plot after injection of EL4 cells intravenously into PI3Kδ+/−RAG2+/− and PI3Kδ−/−RAG2+/− recipients (log-rank test; P = .045). (B) PI3Kδ protein is expressed in Eμ-myc–derived leukemic cells. Upon injection of Eμ-myc–derived cells, PI3Kδ−/−RAG2+/− recipients succumbed to disease significantly faster than PI3Kδ+/−RAG2+/− littermate controls (log-rank test; P = .039). (C) B16 melanoma cells were injected intravenously and numbers of metastatic infiltrates per lung were counted under the binocular microscope. RAG2+/−PI3Kδ−/− recipient mice showed a significantly higher number of metastatic infiltrates than RAG2+/−PI3Kδ+/− (P = .005), and this was also true on the RAG2−/− background (P = .015). Horizontal lines indicate data mean. Thus, increased tumor formation in PI3Kδ−/− mice is not restricted to lymphoid malignancies. Formation of lung metastasis in littermate controls (top panels: photographs, digital camera, Canon EOS 300D; bottom panels: H&E-stained histologic sections of lungs; magnification, ×20 Zeiss AxioImager 71; see Figure 2B).
Discussion
It has been appreciated in the past that signaling via PI3K was important for growth and survival of transformed cells.1 The PI3Kδ isoform has recently been established as important subform for leukemia progression in AML.14 We therefore investigated whether PI3Kδ is also expressed in human BCR/ABL-transformed cells and found a consistent expression in both BCR/ABL+ CML and ALL leukemic cells. Thus, PI3Kδ inhibition would also qualify as potential treatment option for these disease entities. Our data show that in v-abl–induced murine leukemia, the presence of PI3Kδ indeed accelerated leukemia progression in vivo. Hence, the absence of PI3Kδ obviously cannot be compensated for by any other of the isoforms expressed. We noted a striking difference between the in vitro and the in vivo situation: in vitro, absence of PI3Kδ did not impair transformation, immortalization, and growth of tumor cells. In contrast in vivo, the absence of PI3Kδ clearly impaired leukemia progression. Our observations are consistent with the interpretation that differences in NK cell–mediated clearance are responsible for the differences in disease latency. wt NK cells lyse PI3Kδ−/− leukemic cells significantly better than their PI3Kδ+/− leukemic counterparts. Leukemia surveillance is accomplished—at least in part—by NK cells.21,32 NK cells recognize tumor cells via receptors on their cell surface (reviewed by Lanier36 ). The NKG2D receptor plays a key role in mediating tumor cell lysis by binding to Rae1 on the target cell. Indeed, Rae1 is expressed at significantly higher levels on PI3Kδ−/− cells. Regardless of the underlying mechanism, these previous experiments and our current observations would conceptually validate PI3Ks, specifically the PI3Kδ isoform, as a target in leukemia. However, our current experiments and previous reports highlight the role of the immune system in determining the natural course of a tumor disease.21,32,37-39 Here, we observed that PI3Kδ deficiency resulted in a major impairment of NK-cell function. Because NK cells are required to eliminate leukemic cells of B-cell origin, the clearance of tumor cells was hampered; this shaped the course of the disease, obviating any benefit arising from the lack of PI3Kδ in the tumor cells. This fact is highlighted by the experiments in RAG2−/−PI3Kδ−/− mice. These animals rely on NK cells as their sole means of eliminating tumors: as predicted, the de-creased latency in leukemia development was recapitulated in RAG2−/−PI3Kδ−/− mice. Conversely, antibody-mediated NK-cell depletion abolished any difference in survival between PI3Kδ+/− and PI3Kδ−/− mice. In summary, these data unequivocally document the importance of NK cells for immune-surveillance of v-abl–induced leukemia and the absolute requirement for PI3Kδ in this process.
Phosphatidyl inositol phosphates are known regulators of exocytosis. Individual lipids apparently regulate distinct steps in different vesicle populations; priming of neurotransmitter vesicles, for instance, is contingent on the formation of phosphatidyl inositol-3-phosphate (Ptdns3P).40 Very recently, Garcon et al showed that PI3Kδ is the main PI3K isoform responsible for accumulation of PIP3 at the immune synapse.41 We now show that even though PI3Kδ is dispensable for the initial formation of the synapse, it is required to maintain the integrity of the synapse over time. In line with Jiang et al,42-44 who proposed that PI3Ks trigger cytotoxicity through sequential activation of the small G-protein Rac1, the p21-activated kinase 1 (PAK1), MEK, and ERK1/2, we here show that efficient degranulation of NK cells is absolutely dependent on PI3Kδ. This conclusion is based on 2 independent sets of experiments, namely the externalization of the granule membrane constituent CD107a and the change in membrane capacitance associated with exocytosis. The first approach allowed verifying the general nature of the secretion defect (ie, target cells elicited less externalization of granule content). The measurements of membrane capacitance clearly identified a deficit in a late step. Membrane capacitance is proportional to the surface area of a given cell and thus affords real-time recordings of fusion events on a single-cell level.45,46 PI3Kδ+/− and PI3Kδ−/− NK cells did not differ in membrane capacitance under basal conditions. However, during Ca2+-triggered exocytosis, the surface area of PI3Kδ+/− NK cells increased on average by 21%; in contrast, there was only a modest increase (on average by about 8%) in PI3Kδ−/− NK cells. A general defect on exocytosis is furthermore suggested by the defect in IFN-γ secretion that might also contribute to the reduced cytolytic capacity of PI3Kδ-deficient NK cells (Figure S2). Degranulation in other cell types seems also to depend on PI3Kδ. We have evidence that CD8+ cells also require PI3Kδ for degranulation (E.Z., O.S., unpublished observations, August 2007). Others have recently reported an impairment of exocytosis in mast cells upon PI3Kδ inhibition or deficiency.47
Our observations define a new key role for PI3Kδ in NK-cell function and tumor surveillance. The general importance of our findings is underscored by the fact that comparable results were obtained upon transplantation of Eμ-myc–derived leukemic cells and a subclone of the T-lymphoma cell line EL4. These leukemia models were chosen since both transformed cell lines express significant amounts of PI3Kδ and therefore also qualify as potential targets for PI3Kδ inhibitors. Besides hematopoietic cells, melanoma cells express PI3Kδ.34 Consistent with our concept, surveillance of the murine melanoma cell line B16 was also impaired in PI3Kδ-deficient animals.
The repercussion of impaired NK cell–mediated tumor surveillance is worth considering: inhibition of PI3Kδ has been proposed to be an interesting option in the treatment of leukemia and our data do—in part—support this concept. However, our data clearly identify the possible source of a confounding effect. At the current stage, inhibition of PI3Kδ may still be a viable strategy because the dose-range required to block NK-cell functions is not necessarily the same as that causing inhibition of leukemia progression. In addition, PI3Kδ inhibitors might still represent a useful therapeutic option in patients where the leukemic cells have escaped detection by the immune system. However, our data clearly send a cautionary message: when evaluating the overall benefit of PI3Kδ inhibitors, the planning of clinical studies will have to consider both the inhibitory effects on NK cells and on tumor cells.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank K. H. Hilber, X. König, and B. Nürnberg for help with capacitance measurements and discussions. We are grateful to the staff of the Biomedical Research Institute (MUW) for taking care of mice and to J. N. Ihle for providing PI3Kδ−/− mice. Selective PI3Kδ inhibitor IC87114 was generously provided by ICOS (Bothell, WA).
This work was supported by the Austrian Nationalbank (OeNP-11132), the Austrian Science Fund (SFB-28-10, SFB-F18-6), the Gen-AU Project DRAGON, and the Austrian Academy of Sciences (DOC-fForte fellowship to O.S.).
Authorship
Contribution: E.Z., O.S., C.S., E.M.P., E.E., W.W., D.S., E.W., W.F.P., and V.S. designed and performed research; E.Z., O.S., C.S., and V.S. analyzed data; D.S., C.B., J.A.S., W.F.P., P.V., R.P., M.F., and V.S. provided vital new reagents and analytical tools; and E.Z., M.F., and V.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Veronika Sexl, Institute of Pharmacology, Währingerstrasse 13A, A-1090 Wien, Austria; e-mail: veronika.sexl@meduniwien.ac.at.


![Figure 2. Abelson-transformed PI3Kδ−/− cells induce leukemia in mice with an increased latency. (A) Kaplan-Meier plot of RAG2−/− mice after transplantation of 106 transformed cells (3 independently derived cell lines per genotype were injected into n = 8 for PI3Kδ+/− and n = 6 for PI3Kδ−/−). Mice that had been injected with transformed PI3Kδ−/− cells developed leukemia significantly later as determined by a log-rank test (median survival, 13 vs 20 days; P = .005). (B) H&E stains of blood smears (top), spleens (middle), and livers (bottom) from RAG2−/− mice after injection of v-abl–transformed cells (magnification, ×100, Zeiss AxioImager 21 [Jena, Germany], 10× objective, NA 0.25, air; camera: Pixelink Color, 1600 × 1200; software: PixelINK Capture 3.0). (C) Spleen, bone marrow, and blood were analyzed for infiltration with CD19+CD43+ leukemic cells by FACS. Tissue infiltration was slightly lower in mice that had received PI3Kδ−/− cells without reaching statistical significance (data represent means ± SEM). (D) [51Cr]-release assay using IL-2–expanded wt NK cells as effectors and Abelson-transformed leukemic cells as targets. PI3Kδ−/− leukemic cells were significantly better eradicated by wt NK cells than PI3Kδ+/− leukemic target cells at any effector-target cell ratio tested (for ratios 20:1, 10:1, 5:1, and 2:1, P = .02, P = .02, P = .003, and P = .006 as determined in an unpaired 2-tailed t test). (E) Quantification of pan–Rae-1, Mult-1, and MHC I expression by FACS of in vitro–derived Abelson-transformed cell lines. PI3Kδ−/− leukemic cells showed a significantly higher surface expression of pan-Rae1 when compared with PI3Kδ+/− leukemic cells (mean fluorescence intensity [MFI] of 1082 ± 199 vs 639 ± 259; P = .035 in an unpaired 2-tailed t test; n = 4 for each genotype). n.s., indicates P > .05; *P < .05; and **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-02-139105/7/m_zh80200825700002.jpeg?Expires=1767833276&Signature=QMCLzxA-AGM-sCDmKjAm0~8SLDY6d7F2zzfLV6IFUo4MbcHyPiuoxUsodAyhB75iEOqJrL6C5Gt6WlakD5Z2w5RQetZFUJfALf5dkKyQCodvRnFcdXo3xa3o554IYIay0iDmvp-4p9RNDKJ2EbGZRkbnVbK3RUswRJzCEksFhNF3LtCq9M2awC2LysTrz2QJ6UoM2CAVP3HiosYMlDKxYwpiGtDzMtnep5CmpydY2mTPGJXvy8cM~gH2cvbcgyR8kNE~Ks3q7pAndm9P3WiLoz9TwASVsO0tCK96rC9iTQ88fjf6POg2sBUj683-F06834bUL-aAQdIqWTw14h52nw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. PI3Kδ−/− NK cells fail to efficiently lyse leukemic target cells. (A) The cytolytic function of IL-2–expanded PI3Kδ+/− and PI3Kδ−/− NK cells was assessed by [51Cr]-release assay using various cell lines as target cells as indicated. PI3Kδ−/− NK cells showed consistently impaired specific lysis when compared with PI3Kδ+/− NK cells, regardless of the target cell line used. The complete table of all target lines including statistics is provided in Table S3. (B) Top panels: typical confocal images of CD107a+ granules of PI3Kδ+/− and PI3Kδ−/− NK cells are given. Bottom panel: quantification of granule numbers. Confocal images of CD107a+ granules were acquired in the z-stack mode on a Zeiss LSM510 confocal microscope with a 100× oil-immersion objective (NA 1.3) with the 488-nm line of the Ar laser and a LP 505-nm emission filter. About 24 slices were acquired with a distance of 0.3 μm between the slices. Images were imported into ImageJ software, and maximum projection images were calculated. Stained vesicles were selected by auto-thresholding, followed by quantification and analysis of all selected regions. Horizontal lines indicate data mean. (C) A FACS-based degranulation assay measuring surface expression of the late endosomal marker CD107a was performed. FACS histograms illustrate the percentage of PI3Kδ+/− (top row) and PI3Kδ−/− (bottom row) NK cells expressing CD107a under basal conditions (left panels) and after stimulation by coincubation with v-abl–transformed target cells. Significantly more PI3Kδ+/− NK cells were stimulated to express CD107a on their surface compared with PI3Kδ−/− NK cells (data mean ± SEM). (D) Bar graphs depicting different stimuli used to induce degranulation in PI3Kδ+/− and PI3Kδ−/− NK cells: none of the target cells or specific antibodies induced degranulation of PI3Kδ−/− NK cells. (E) Electrophysiologic capacitance measurements were performed and confirmed the defect of PI3Kδ−/− NK cells in Ca2+-dependent exocytosis/degranulation. Electrophysiologic capacitance measurement relies on recording the passive current, which has to be injected into the cell to keep a constant voltage and this corresponds to the size (surface area) of a cell: the bigger the cell, the more current has to be injected. In vitro–expanded murine NK cells are round cells (means of absolute capacitance of unstimulated PI3Kδ+/− NK cells, 12.4 ± 5.4 pF; of PI3Kδ−/− NK cells, 13.73 ± 5.9 pF). Two typical examples of the exocytotic response under the superfusion with calcimycin are illustrated (left panel). The mean increase in relative capacitance after superfusion of PI3Kδ+/− and PI3Kδ−/− NK cells was compared (mean increase in relative capacitance for PI3Kδ+/− NK cells: 21% ± 2%; for PI3Kδ−/−NK cells: 8.5% ± 1%; P < .001; n = 10 for each genotype; the experiment was repeated with 3 independent NK-cell preparations). The increase in relative capacitance was obtained by comparing the peak increase in relative capacitance after superfusion with calcimycin with resting cell capacitance under superfusion with control solution (right panel). Error bars represent SEM. n.s. indicates P > .05; *P < .05; and **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-02-139105/7/m_zh80200825700005.jpeg?Expires=1767833276&Signature=ZhpBdUVSiyBNuBwqsJeFp0ZHSFTAZpiQURGHp5oyzKHgrV8LDYko1SxhBERlQs8kzcoUXLft4thfvG6iOhsnriLG~8edSP2TD~SIzjpiCdP97uMuwmNcvZPMO5ClIQ86a1aviOmwfaR9bFUCPdR8nc6ScSkJdOxVgHiJkczJlH8t-gsEwj5hI0tlPKk9nwTRIqOTUnOY6j4evIXHxM7fzbK-Sjeo0OVo60V5S26T5g4ts8fegKs99LUwKK2-filkdFgqeCEeoJFmq5-r8lmzF1KNIt0bja5jMf3mwEydEOyKYbXsAxl5gjm9gmHKVnbo2ClkbNGoFePHYjQ-xaTsgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. PI3Kδ is dispensable for the initial formation of aggregates with leukemic target cells, but is indispensable for the maintenance of aggregates and for degranulation. (A) Initial aggregate formation (top panels) and maintainance of aggregates (bottom panels) of PI3Kδ+/− and PI3Kδ−/− NK cells with wt v-abl target cells was analyzed by light microscopy (magnification: top panel, ×100; bottom panel, ×430; one example for each genotype is depicted, obtained with a Zeiss Axiovert 135, 10× Ph1 [NA 0.25], camera: Cool Snap HQ [Ottobrunn, Germany], software: Metamorph 4.0 [Visitron, Puchheim, Germany]), (B) quantified by FACS, and observed over time. Error bars represent SEM (*P < .05; **P < .01). (C) A selective inhibitor of PI3Kδ was used to describe the requirement for PI3Kδ for killing, aggregate formation, and degranulation of NK cells when incubated with leukemic target cells. As normalized to PI3Kδ+/− NK cells, PI3Kδ inhibition severely impaired killing (61.3% of PI3Kδ+/− NK cells) and degranulation (37.7% of PI3Kδ+/− NK cells), whereas initial aggregate formation was not affected (115% of PI3Kδ+/− NK cells).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-02-139105/7/m_zh80200825700006.jpeg?Expires=1767833276&Signature=UlQP3aP01vvdBmxsMWVxLHNZKZAGCewUBlcZdidEFhEeOW0P8y041IBMEzw-h6NdvtSca41iYnIA72QPKfgP-teF9WJ2vMgIhAgQ~dBLj2kN8~xaZ93fRAndayb-jKxrEqKfeVkQyhazBt94-OrZaa2y7u-91xAOlWVplYJPAjpfC7MCI5F8D2a6FV6C5XIP8X68F~77K55NrsijhhD6zNOte1ACmbHONkf-il-REJ47PLWSphcmhgImZ5yJ4KmjwhE5raHyOo5AK1V87kV4MZvgdRIWlRC-U8j4tpiWc9Gr7dZF0ANmSD1YTX-spP2BxPgGnLAm8iWGAwDOI7r75A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 2. Abelson-transformed PI3Kδ−/− cells induce leukemia in mice with an increased latency. (A) Kaplan-Meier plot of RAG2−/− mice after transplantation of 106 transformed cells (3 independently derived cell lines per genotype were injected into n = 8 for PI3Kδ+/− and n = 6 for PI3Kδ−/−). Mice that had been injected with transformed PI3Kδ−/− cells developed leukemia significantly later as determined by a log-rank test (median survival, 13 vs 20 days; P = .005). (B) H&E stains of blood smears (top), spleens (middle), and livers (bottom) from RAG2−/− mice after injection of v-abl–transformed cells (magnification, ×100, Zeiss AxioImager 21 [Jena, Germany], 10× objective, NA 0.25, air; camera: Pixelink Color, 1600 × 1200; software: PixelINK Capture 3.0). (C) Spleen, bone marrow, and blood were analyzed for infiltration with CD19+CD43+ leukemic cells by FACS. Tissue infiltration was slightly lower in mice that had received PI3Kδ−/− cells without reaching statistical significance (data represent means ± SEM). (D) [51Cr]-release assay using IL-2–expanded wt NK cells as effectors and Abelson-transformed leukemic cells as targets. PI3Kδ−/− leukemic cells were significantly better eradicated by wt NK cells than PI3Kδ+/− leukemic target cells at any effector-target cell ratio tested (for ratios 20:1, 10:1, 5:1, and 2:1, P = .02, P = .02, P = .003, and P = .006 as determined in an unpaired 2-tailed t test). (E) Quantification of pan–Rae-1, Mult-1, and MHC I expression by FACS of in vitro–derived Abelson-transformed cell lines. PI3Kδ−/− leukemic cells showed a significantly higher surface expression of pan-Rae1 when compared with PI3Kδ+/− leukemic cells (mean fluorescence intensity [MFI] of 1082 ± 199 vs 639 ± 259; P = .035 in an unpaired 2-tailed t test; n = 4 for each genotype). n.s., indicates P > .05; *P < .05; and **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-02-139105/7/m_zh80200825700002.jpeg?Expires=1767836211&Signature=hlunGk3sZnSdjQFDMaNj40KFkTFT2D7pbPW~D3-3wp3GXI~s3lJIWHUaBYTxa1~6bHt6A9-PZ5ZdSaj4b7fFRPdD8r2PKF1uUFvIFKKfgSSG87j5ZMDr97aCSjzYk44OqibeiQzHXLqHTxyqVwXmot~KWG9Lt5ZnSjNM06hvUgH3pBTDPeR5Fc16IZKxrUX5iVk6FGFdiV2-PML109TwEXR1QL~vimEIsXrgdxKwh8TPdgYhlVgxCzMKMBAhvMIV2zaY28CkCfytjwDE27ubaYL5JDHptFL7xksKpq0Vm~ALhFqzPkCei1vzrnjfWNZILFjs4I8FZlYC12j79uva5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 5. PI3Kδ−/− NK cells fail to efficiently lyse leukemic target cells. (A) The cytolytic function of IL-2–expanded PI3Kδ+/− and PI3Kδ−/− NK cells was assessed by [51Cr]-release assay using various cell lines as target cells as indicated. PI3Kδ−/− NK cells showed consistently impaired specific lysis when compared with PI3Kδ+/− NK cells, regardless of the target cell line used. The complete table of all target lines including statistics is provided in Table S3. (B) Top panels: typical confocal images of CD107a+ granules of PI3Kδ+/− and PI3Kδ−/− NK cells are given. Bottom panel: quantification of granule numbers. Confocal images of CD107a+ granules were acquired in the z-stack mode on a Zeiss LSM510 confocal microscope with a 100× oil-immersion objective (NA 1.3) with the 488-nm line of the Ar laser and a LP 505-nm emission filter. About 24 slices were acquired with a distance of 0.3 μm between the slices. Images were imported into ImageJ software, and maximum projection images were calculated. Stained vesicles were selected by auto-thresholding, followed by quantification and analysis of all selected regions. Horizontal lines indicate data mean. (C) A FACS-based degranulation assay measuring surface expression of the late endosomal marker CD107a was performed. FACS histograms illustrate the percentage of PI3Kδ+/− (top row) and PI3Kδ−/− (bottom row) NK cells expressing CD107a under basal conditions (left panels) and after stimulation by coincubation with v-abl–transformed target cells. Significantly more PI3Kδ+/− NK cells were stimulated to express CD107a on their surface compared with PI3Kδ−/− NK cells (data mean ± SEM). (D) Bar graphs depicting different stimuli used to induce degranulation in PI3Kδ+/− and PI3Kδ−/− NK cells: none of the target cells or specific antibodies induced degranulation of PI3Kδ−/− NK cells. (E) Electrophysiologic capacitance measurements were performed and confirmed the defect of PI3Kδ−/− NK cells in Ca2+-dependent exocytosis/degranulation. Electrophysiologic capacitance measurement relies on recording the passive current, which has to be injected into the cell to keep a constant voltage and this corresponds to the size (surface area) of a cell: the bigger the cell, the more current has to be injected. In vitro–expanded murine NK cells are round cells (means of absolute capacitance of unstimulated PI3Kδ+/− NK cells, 12.4 ± 5.4 pF; of PI3Kδ−/− NK cells, 13.73 ± 5.9 pF). Two typical examples of the exocytotic response under the superfusion with calcimycin are illustrated (left panel). The mean increase in relative capacitance after superfusion of PI3Kδ+/− and PI3Kδ−/− NK cells was compared (mean increase in relative capacitance for PI3Kδ+/− NK cells: 21% ± 2%; for PI3Kδ−/−NK cells: 8.5% ± 1%; P < .001; n = 10 for each genotype; the experiment was repeated with 3 independent NK-cell preparations). The increase in relative capacitance was obtained by comparing the peak increase in relative capacitance after superfusion with calcimycin with resting cell capacitance under superfusion with control solution (right panel). Error bars represent SEM. n.s. indicates P > .05; *P < .05; and **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-02-139105/7/m_zh80200825700005.jpeg?Expires=1767836211&Signature=gub2h2CIYwVb1PiyKZpHeuokBhMgwoyhlBiegzQZbIR2AQXfwIMdsfLjWDWRLO73zFHIc5bswUzoK0K6v9X~PxN5Co3lbaUL~1NnKSqWRep6PdKuG4JShAAlMKvrFy0Gm6Ue-uPgbVj~IlSBYt8HupXmw6EAcbQPOU~TogA1uxgXTS25S0v9teksDEdA155FFmu7rajhnItJHfB~bk74kQtzkdfsoNHpw2iw-EMDzSHmaWQi6ASn20GVY8Ur7KqiRYG0fu2IKGbfhPzu6Q8mon4OWOXdO5u97etV9cBRdJCpwzO1HH~buJmS~GJAtrhC2FiFAnd90TduGmkyY0XsfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. PI3Kδ is dispensable for the initial formation of aggregates with leukemic target cells, but is indispensable for the maintenance of aggregates and for degranulation. (A) Initial aggregate formation (top panels) and maintainance of aggregates (bottom panels) of PI3Kδ+/− and PI3Kδ−/− NK cells with wt v-abl target cells was analyzed by light microscopy (magnification: top panel, ×100; bottom panel, ×430; one example for each genotype is depicted, obtained with a Zeiss Axiovert 135, 10× Ph1 [NA 0.25], camera: Cool Snap HQ [Ottobrunn, Germany], software: Metamorph 4.0 [Visitron, Puchheim, Germany]), (B) quantified by FACS, and observed over time. Error bars represent SEM (*P < .05; **P < .01). (C) A selective inhibitor of PI3Kδ was used to describe the requirement for PI3Kδ for killing, aggregate formation, and degranulation of NK cells when incubated with leukemic target cells. As normalized to PI3Kδ+/− NK cells, PI3Kδ inhibition severely impaired killing (61.3% of PI3Kδ+/− NK cells) and degranulation (37.7% of PI3Kδ+/− NK cells), whereas initial aggregate formation was not affected (115% of PI3Kδ+/− NK cells).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/12/10.1182_blood-2008-02-139105/7/m_zh80200825700006.jpeg?Expires=1767836211&Signature=P7tL94gMSrWPujwX~6flOmd0pZVUQncDiJenSkknRsFL8be87ukOp~egr9qQFPqaQQrDtLSMT805syoCDEof4Lgi~Ek0PzwY2brh6z3nWtTS-2ZsU0atkAXAUtbZHcM0Ld-Y3RU7CddutbZYhrq3SUbGKoU~sPz838CLzfcIl5j-bmdUWRo2t3tPpXJjj~~eoLlSHx8mWNetblb9VUFSfRR5YzTLzffqvpOfZoWLKdyuLa-caltTFlY~TPHjQ4YFbM8xU477m9JnHc7FoQIVdUY8iwtmb2sLKvZo3B6nMXN8A5oMN2lDyZatYcx-uW7vwSQYElb~jOr~g118WEtLDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)