Abstract
CD44, the leukocyte adhesion receptor for hyaluronan, has been considered a therapeutic target on the basis of the robust anti-inflammatory effect of CD44-specific antibodies in animal models of immune-mediated diseases. However, CD44 deficiency does not provide substantial protection against inflammation. Using intravital video microscopy in a murine model of rheumatoid arthritis, we show that CD44 deficiency and anti-CD44 antibody treatment exert disparate effects on leukocyte recruitment in inflamed joints. Leukocyte rolling, which is increased in CD44-deficient mice, is promptly abrogated in anti–CD44-treated wild-type mice. CD44-specific antibodies also trigger platelet deposition on granulocytes and subsequent depletion of this leukocyte subset in the circulation. These in vivo effects require CD44 cross-linking and are reproducible with an antibody against Gr-1, a molecule that, like CD44, is highly expressed on granulocytes. Anticoagulant pretreatment, which prevents platelet deposition, mitigates both granulocyte depletion and the suppressive effect of CD44-specific antibody on joint swelling. Our observations suggest that cross-linking of prominent cell surface molecules, such as CD44 or Gr-1, can initiate a rapid self-elimination program in granulocytes through engagement of the coagulation system. We conclude that the robust anti-inflammatory effect of CD44-specific antibodies in arthritis is primarily the result of their ability to trigger granulocyte depletion.
Introduction
CD44 is a widely expressed transmembrane glycoprotein with adhesive and signaling functions.1 On leukocytes, CD44 supports their rolling under flow by binding to the extracellular matrix glycosaminoglycan hyaluronan (HA) when HA is expressed on the surface of activated or inflamed vascular endothelium.2 In addition to being a principal receptor for HA,3 CD44 has recently been reported to serve as a ligand for other adhesion molecules, including leukocyte (L)–selectin4 and endothelial (E)–selectin.5 Beyond its role in cell rolling, CD44 may cooperate with a leukocyte integrin in mediating firm adhesion to endothelium,6 a step required for leukocyte exit from the circulation.7 In vivo studies using CD44-specific monoclonal antibody (mAb) treatment reported robust anti-inflammatory effects in animal models of several immune-mediated human diseases, including rheumatoid arthritis (RA),8-10 type I diabetes,11 multiple sclerosis,12 and asthma,13 leading to consideration of CD44 as a therapeutic target. The efficacy of anti-CD44 treatment was attributed to the ability of mAbs to neutralize the HA-binding function of the receptor14 and/or to induce its proteolytic removal (shedding) from the cell surface.8,13 On the basis of these reports, it was expected that CD44 gene knockout (KO) mice could be protected from inflammation. This was not exactly the case, however. For example, CD44 KO mice of the DBA/1 background showed moderate resistance15 or no resistance10 to collagen-induced arthritis, yet administration of CD44-specific mAbs to wild-type (WT) DBA/1 mice resulted in a prompt resolution of joint inflammation.8,10
To understand the mechanistic basis of this discrepancy, we sought to determine by intravital video microscopy (IVM) how leukocyte recruitment (rolling/adhesion) in the blood vessels of inflamed joints was affected by CD44 deficiency vs anti-CD44 treatment. We chose proteoglycan (PG)–induced arthritis (PGIA) in BALB/c mice16 as a disease model because the anti-inflammatory effect of anti-CD44 treatment has been repeatedly demonstrated in PGIA8,9 and because CD44-deficient mice are available in the PGIA-susceptible BALB/c background.17 In addition, IVM has been performed on the mouse ankle joint,18 a site that is affected with inflammation in mice with PGIA as commonly as in patients with RA. Here we demonstrate that the recruitment of leukocytes in the vessels of inflamed synovium of anti-CD44 antibody-treated WT mice is fundamentally different from their recruitment in CD44 KO mice. We have also found that in vivo ligation of CD44 with bivalent antibodies can elicit unexpected reactions in leukocytes in a CD44 function-independent manner.
Methods
Mice
Female BALB/c mice were obtained from the National Cancer Institute. CD44-deficient BALB/c mice were generated by backcrossing with CD44 KO DBA/1 mice.15,17 WT/CD44 KO chimeras (expressing or lacking CD44 in hematopoietic cells only) were created by bone marrow (BM) transplantation after lethal irradiation according to a protocol described by Khan et al.19 In brief, WT and CD44 KO mice were irradiated twice with 4.5 Gy (Gammacell; Atomic Energy of Canada, Mississauga, ON) 4 hours apart and then injected intravenously with 2.5 × 107 BM cells from nonirradiated CD44 KO and WT donors, respectively. Irradiated WT mice reconstituted with WT BM, and irradiated CD44 KO mice injected with CD44 KO BM, served as nonchimeric controls for the chimeras. BM for transplantation was harvested from the femurs and tibias of donor mice by flushing sterile phosphate-buffered saline (PBS) through the marrow cavities. BM reconstruction in the recipients was tested 6 weeks after transplantation by measuring CD44 expression on peripheral blood leukocytes (PBLs) using flow cytometry17 on a FACSCalibur instrument (BD Biosciences, San Jose, CA). The presence or absence of CD44 on the endothelium of reconstituted mice was confirmed on the basis of the binding or the lack of binding of fluorescence-labeled anti-CD44 mAb to the vascular intima. CD44 fluorescence in the ear blood vessels of anesthetized mice was visualized by IVM as described elsewhere20 after intravenous administration of 4 to 8 μg anti-CD44 mAb in 50 μL PBS/mouse.
Induction and assessment of PGIA
Female WT, CD44-deficient, or chimeric BALB/c mice were immunized intraperitoneally 3 times with human cartilage PG in a synthetic adjuvant as described.16,18 Most of the WT mice developed arthritis between days 9 and 15 (CD44 KO mice between days 20 and 28) after the last PG injection. The degree of inflammation in each paw was assessed visually and scored on a scale of 0 to 4. For uniformity, mice that reached ankle arthritis scores of approximately 3 (moderately severe inflammation) within 7 days after the onset of PGIA, were used. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Rush University Medical Center.
Abs and reagents
The hybridomas producing CD44-specific mAbs IM7 and KM8121 were purchased from ATCC (Manassas, VA), and the mAbs purified on protein G-sepharose (Pierce Chemical, Rockford, IL). IM7 mAb was fragmented and purified using ImmunoPure F(ab′)2 and Fab preparation kits (Pierce Chemical) according to the manufacturer's instructions. Purified anti–Gr-1 mAb (clone RB6-8C5), mAbs against P-selectin (clone RB40-34), and P-selectin glycoprotein ligand-1 (PSGL-1; clone 4RA10), phycoerythrin (PE)-conjugated anti-CD41 (platelet-specific) mAb, PE-conjugated IM7, and rat IgG isotypes (IgG1, IgG2a, and IgG2b) were obtained from BD Biosciences. Alexa Fluor 488-conjugated anti–Gr-1 mAb and purified goat antirat IgG (H + L) were from Invitrogen (Carlsbad, CA). Recombinant mouse (rm) P-selectin-Fc fusion protein was from R&D Systems (Minneapolis, MN). Purified rat IgG and sodium heparin were purchased from Sigma-Aldrich (St Louis, MO). Reagents used for in vivo treatment were diluted in PBS and sterilized by passing through sterile 0.22 μm pore-size syringe filters.
IVM
IVM on the mouse ankle joint has been described in detail previously.18 In brief, the ankle synovium of the anesthetized mouse was surgically exposed and placed under the water-immersion objective (40×/0.8 NA) of an intravital epifluorescence microscope (Nikon Eclipse E600FN). To ensure constant body temperature and maintain a liquid bridge between the tissue and the microscope objective, the ankle synovial tissue was continuously superfused with warm (37°C) PBS. Blood leukocytes were visualized by intravenous injection of rhodamine 6G (R6G; Invitrogen), a fluorescent mitochondrial dye.22 R6G-labeled leukocytes in the synovial postcapillary venules were video recorded for 1 minute with a digital video camera (CoolSnap; RS Photometrics, Tucson, AZ) before and after intravenous administration of sterile reagents at different doses and time points as described in “Ab treatment protocols.” Double-color videos were created on the same microscope using a dual band-pass emission filter that allowed for simultaneous recording of green (anti–Gr-1-Alexa Fluor 488 mAb)18 and red (anti–CD41-PE mAb) fluorescence. The video images were acquired and analyzed using MetaMorph software (Molecular Devices, Sunnyvale, CA). The parameters used to characterize the adhesion behavior of leukocytes in the synovial vessels have been described previously.17,18 Briefly, rolling cell frequency was expressed as the number of rolling cells/minutes/mm vessel perimeter. Because the perimeter/circumference of the venules, along which the cells rolled, differed in size among vessels, we used the perimeter (dπ, where d denotes diameter) to normalize the rolling cell frequency for vessel size. The velocity of rolling was expressed in micrometers per second. Adherent (immobile) cells were counted along a 100-μm–long segment of each venule, and the numbers corrected for 0.1 mm2 vessel surface (dπl, where l denotes vessel length).
Ab treatment protocols
Anesthetized mice received intravenous injections of purified whole or fragmented mAbs against CD44 or Gr-1, or nonspecific rat IgG (200 or 40 μg/animal) before IVM, and the changes in leukocyte recruitment were recorded for 30 minutes. In one set of experiments, leukocyte recruitment was video-recorded 2, 4, or 24 hours after anti-CD44 treatment. For intervention studies, reagents (listed in Table 1) were administered intravenously 25 minutes before injection of anti-CD44 mAb. IVM records were made before pretreatment (0 minutes) and immediately before (25 minutes) and 5 minutes after anti-CD44 mAb injection. Platelet depletion was carried out using polyclonal rat IgG antibodies (pAb) against mouse platelet GPIbα (CD42b) according to the manufacturer's instruction (Emfret Analytics, Eibelstadt, Germany). Administration of antiplatelet polyclonal Ab (pAb) intravenously at a dose of 100 μg/mouse resulted in a reduction of circulating platelet counts less than 15% of normal in less than 30 minutes. Animals were subjected to treatment with anti-CD44 mAb 25 minutes after injection of platelet-depleting pAb, and videos recorded as described in “IVM.”
Determination of PBL and platelet counts
Total PBL and neutrophil counts were determined in Turk-stained suspensions and Wright-Giemsa–stained smears, respectively, of blood samples drawn before and at various time points after the treatments, up to 24 hours. Cell surface densities of CD44 and Gr-1, and platelet counts in heparinized blood samples were determined by flow cytometry. Red blood cells were removed by hypotonic lysis before incubation with antibodies against leukocyte CD44, Gr-1, or with nonspecific rat IgG isotype controls. The blood samples collected for platelet counts were diluted with Tris-buffered saline (containing 20 U/mL heparin) and Tyrode's buffer (containing 0.5% bovine serum albumin), pelleted by centrifugation at 900g, and processed for CD41 labeling and flow cytometry without hemolysis.
Assessment of short-term treatment effects on joint inflammation (paw swelling)
Separate groups of mice with PGIA were subjected to treatment with mAb to CD44 (IM7) or control rat IgG (200 μg each, single dose). Alternatively, arthritic animals were pretreated with reagents (which were found to antagonize leukocyte-platelet interactions) or PBS 25 minutes before receiving anti-CD44 mAb. Joint thickness (ankles and wrists) was measured with a digital microcaliper (World Precision Instruments, Sarasota, FL) before and 24 hours after the anti-CD44 or rat IgG treatment, and the results expressed as changes in cumulative joint thickness (mm) at 24 hours relative to 0 hours.
Statistical analysis
Results are expressed as the mean plus or minus SE, ratios of the means, or median and range. Data were analyzed using SPSS (Chicago, IL). The Student t test was used for analysis of 1 or 2 groups, and one-way analysis of variance was used for comparison of multiple groups. Differences were considered significant when P was .05 or less.
Results
Leukocyte rolling and adhesion in the blood vessels of inflamed joints in untreated CD44-deficient and anti-CD44 (IM7) mAb-treated WT mice
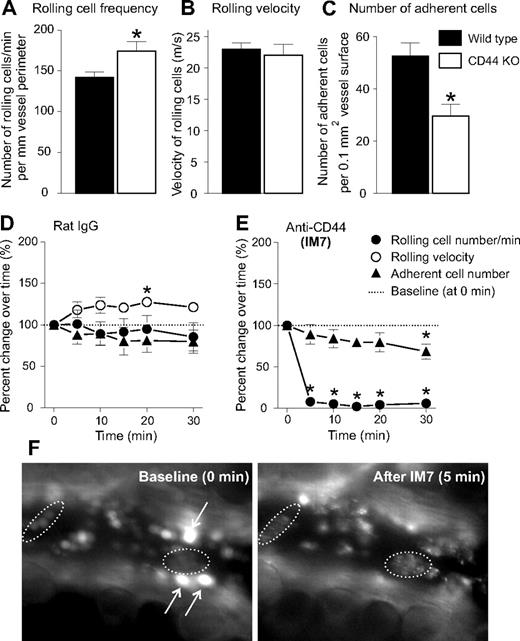
To assess the impact of CD44 deficiency on leukocyte recruitment in the inflamed ankle joint, we induced PGIA in CD44 KO and WT BALB/c mice by intraperitoneal immunization with human cartilage PG in adjuvant. The synovium of arthritic joints of both genotypes of mice was subjected to IVM after labeling of leukocytes in the circulation by intravenous injection of R6G.18 We found that, although the overall frequency of leukocyte-endothelial interactions was similar in WT and CD44 KO mice, the number of rolling cells was significantly higher, and the number of firm adherent leukocytes significantly lower in the synovial vessels of CD44 KO mice than in the vessels of WT counterparts (Figure 1A-C; Videos S1,S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results are consistent with earlier reports,17,19 demonstrating that, in the absence of CD44, the ratio of rolling and firm adhesion interactions shifted in favor of rolling.
Leukocyte-endothelium interactions in arthritic mouse ankle synovium are differentially affected by CD44 deficiency and anti-CD44 (mAb IM7) treatment. In comparison with WT mice (■), CD44 KO mice (□) showed (A) significantly elevated rolling cell number, (B) comparable rolling velocity, and (C) significantly reduced number of firm adherent cells (n = 22 mice/genotype, *P < .05). (D-F) Immediate effects of (D) rat IgG (control) and (E) anti-CD44 (IM7) treatment on the (●) frequency of rolling leukocytes, (○) rolling velocity, and (▲) adherent cell number, in the synovial venules. IVM videos were recorded before (0 minutes, baseline) and at the indicated time points after intravenous injection of rat IgG or IM7 (both at 200 μg). Results are expressed as percentage change (mean ± SE) relative to baseline (100%; n = 8 mice/group, *P < .05). IM7 treatment had reduced the number of rolling cells so drastically that the rolling velocity could not be determined. (F) IVM snapshots of fluorescent leukocytes before (0 minutes, baseline; left) and 5 minutes (right) after injection of IM7. Arrows point to bright rolling cells that appear before (left), but not after (right) IM7 injection. Dotted lines encircle adherent cells that underwent a change in morphology, ie, exhibited a “hairy” (“disheveled”) appearance after IM7 administration.
Leukocyte-endothelium interactions in arthritic mouse ankle synovium are differentially affected by CD44 deficiency and anti-CD44 (mAb IM7) treatment. In comparison with WT mice (■), CD44 KO mice (□) showed (A) significantly elevated rolling cell number, (B) comparable rolling velocity, and (C) significantly reduced number of firm adherent cells (n = 22 mice/genotype, *P < .05). (D-F) Immediate effects of (D) rat IgG (control) and (E) anti-CD44 (IM7) treatment on the (●) frequency of rolling leukocytes, (○) rolling velocity, and (▲) adherent cell number, in the synovial venules. IVM videos were recorded before (0 minutes, baseline) and at the indicated time points after intravenous injection of rat IgG or IM7 (both at 200 μg). Results are expressed as percentage change (mean ± SE) relative to baseline (100%; n = 8 mice/group, *P < .05). IM7 treatment had reduced the number of rolling cells so drastically that the rolling velocity could not be determined. (F) IVM snapshots of fluorescent leukocytes before (0 minutes, baseline; left) and 5 minutes (right) after injection of IM7. Arrows point to bright rolling cells that appear before (left), but not after (right) IM7 injection. Dotted lines encircle adherent cells that underwent a change in morphology, ie, exhibited a “hairy” (“disheveled”) appearance after IM7 administration.
On the basis of the robust suppression of arthritis observed in arthritic WT mice treated with a CD44-specific mAb (clone IM7),8,9 we anticipated that anti-CD44 (IM7) treatment of WT mice would reproduce the effect of CD44 deficiency, but it would do so with a greater efficiency and within a relatively short period of time after administration. To investigate this, we monitored the changes in leukocyte-endothelium interactions in the arthritic ankle joints of WT mice for 30 minutes after intravenous injection of IM7. Because leukocyte-vessel interactions are dynamic in nature, some fluctuation in the rolling and adhesion interactions is expected to occur over time in untreated mice or in those injected with control substances.18 This was indeed the case in control mice treated with normal rat IgG (200 μg/mouse intravenously), where the number of interacting (both rolling and firm adherent) leukocytes slightly decreased, and rolling velocity increased over time compared with “baseline” (0 minutes; before rat IgG injection; Figure 1D). Similar minor fluctuations were observed in a limited number of mice after injection with purified rat IgG1, IgG2a, or IgG2b isotypes (data not shown). The CD44-specific rat mAb IM7 was administered at 200 μg, a dose that was found to rapidly reduce joint swelling in PGIA.9 After injection of IM7, there was a sudden drop in the rolling interactions; essentially, no rolling cells could be seen between 5 and 30 minutes (Figure 1E). IM7 also significantly reduced the number of adherent cells, although this reduction in firm adhesion was not much greater than the reduction observed after rat IgG treatment (compare Figure 1D,E). Concomitant with the disappearance of rolling cells, leukocytes adhering to endothelium exhibited peculiar changes in morphology after IM7 administration (Figure 1F). As demonstrated in Video S3, rolling ceased, whereas adherent leukocytes began to appear “hairy” (or “disheveled”) less than 1 minute after injection of IM7. Rolling cells reappeared slowly, whereas only a few adherent cells were seen 2 to 4 hours after IM7 injection (data not shown); at 24 hours, the rolling cell frequency was still reduced by approximately 60% and firm adhesion by 50%, relative to average pretreatment levels.
Leukocyte rolling and adhesion after treatment with an anti-CD44 mAb (KM81), which blocks CD44-HA interactions
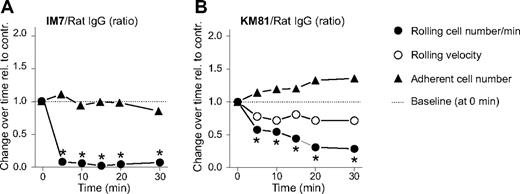
The IM7 mAb, which recognizes an epitope proximal to the N-terminal HA-binding domain of CD44,8 has only a moderate influence on the binding of HA to cell surface CD44.9,21 This casts doubt on the ability of IM7 to block CD44-mediated rolling of leukocytes on endothelium in an entirely HA-dependent manner. Therefore, we also tested the effect of KM81, another CD44-specific rat mAb with an epitope within the HA-binding domain.21 Because KM81 had been shown to directly inhibit the rolling interaction between leukocyte CD44 and endothelial HA,14,23 this mAb was expected to exert a stronger effect than IM7 on leukocyte rolling. Similar to IM7 (Figure 2A), KM81 (at 200 μg) significantly reduced the frequency of cells that rolled on synovial endothelium (Figure 2B). However, the rolling inhibitory effect of KM81 was weaker and developed more slowly than in the case of IM7 (compare Figure 2A,B). The number of firm adherent leukocytes was not significantly affected by KM81 (Figure 2B); but less than 10 minutes after administration of this mAb, adherent cells began to show changes in morphology similar to those seen immediately after IM7 injection. Overall, the changes after KM81 treatment, although smaller in magnitude, were similar to those after IM7 administration. This observation suggested that a quantitative difference in the affinity of these mAbs to CD44 (in favor of IM7), rather than in their influence on HA binding, accounted for the slight difference in their in vivo effects.
Comparison of the effects of anti-CD44 mAbs IM7 and KM81 on leukocyte-endothelium interactions in the inflamed synovium of WT mice. Changes in leukocyte rolling and adhesion are shown after intravenous administration of anti-CD44 mAbs (A) IM7 and (B) KM81 (both at 200 μg) relative to changes after control rat IgG injection (200 μg). Symbols are the same as in Figure 1. The results are expressed as the ratio of changes observed after specific versus control IgG treatment at consecutive time points (n = 8 mice/group, *P < .05).
Comparison of the effects of anti-CD44 mAbs IM7 and KM81 on leukocyte-endothelium interactions in the inflamed synovium of WT mice. Changes in leukocyte rolling and adhesion are shown after intravenous administration of anti-CD44 mAbs (A) IM7 and (B) KM81 (both at 200 μg) relative to changes after control rat IgG injection (200 μg). Symbols are the same as in Figure 1. The results are expressed as the ratio of changes observed after specific versus control IgG treatment at consecutive time points (n = 8 mice/group, *P < .05).
IM7-mediated inhibition of rolling is dependent on leukocyte, not endothelial, CD44, and requires CD44 cross-linking
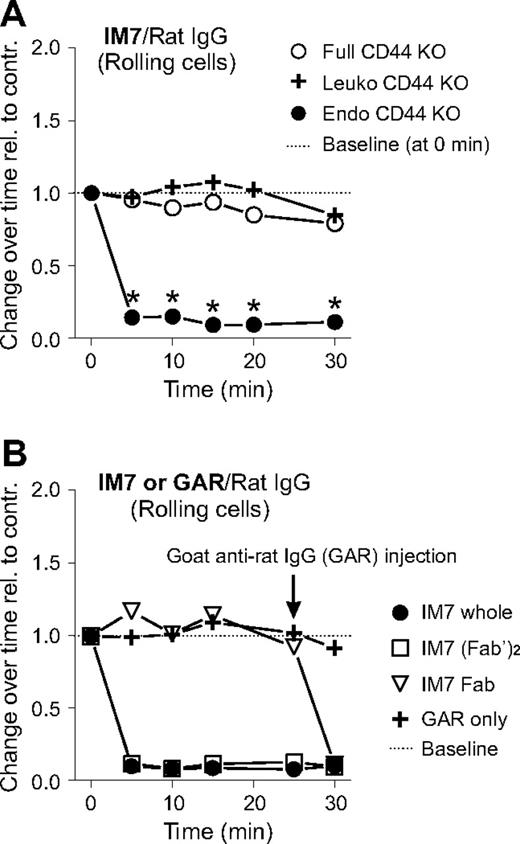
Because CD44 is expressed on both leukocytes and endothelial cells, it was of interest to determine whether binding of the anti-CD44 mAb (in this case, IM7) to leukocytes or endothelium, or to both, accounted for the disruption of rolling interactions in vivo. To this end, we generated CD44 chimeras by BM transfer between WT and CD44 KO mice. WT mice reconstituted with CD44 KO BM did not express CD44 on leukocytes (“Leuko CD44 KO”). Conversely, CD44 KO mice that received WT BM did not express CD44 on endothelium (“Endo CD44 KO”),19 but CD44 was present on their leukocytes. As expected, administration of IM7 to CD44 KO mice (“Full CD44 KO”) did not change the rolling interactions of leukocytes with endothelium in the arthritic joints (Figure 3A). Rolling remained unaltered by IM7 treatment in chimeric mice lacking CD44 on leukocytes (Figure 3A; Leuko CD44 KO); ie, rolling in Leuko CD44 KO mice was similar to that in Full CD44 KO mice. However, rolling was severely impaired in IM7-treated chimeras expressing CD44 on leukocytes but not in those expressing it on endothelium (Figure 3A; Endo CD44 KO). In addition to the disappearance of rolling cells, adherent leukocytes in Endo CD44 KO chimeras displayed changes in morphology (not shown) similar to those seen in WT mice after IM7 injection (Figure 1F). Together, these results indicated that IM7 binding to leukocyte, but not to endothelial, CD44 was responsible for both the disruption of leukocyte rolling and the morphologic alteration of adherent cells.
Abrogation of cell rolling by IM7 is dependent on leukocyte CD44 and requires CD44 cross-linking. (A) The graph shows the changes in the frequency of rolling leukocytes in synovial venules of CD44 KO mice (○, full CD44 KO), or of chimeric mice lacking CD44 in leukocytes ( , Leuko CD44 KO) or in endothelial cells (●, Endo CD44 KO) after IM7 (200 μg) injection. Results are expressed as ratios of changes in leukocyte rolling frequency over time in mice treated with IM7 relative to rat IgG-treated controls (n = 8 mice/treatment group, *P < .05). (B) Comparison of the effects of (●) whole IM7 antibody and its (□) F(ab′)2 or (▽) Fab fragments on leukocyte rolling in WT mice. IVM records were made before and up to 30 minutes after injection of antibodies (200 μg each). For secondary cross-linking of CD44, goat anti–rat IgG (GAR; 100 μg) was administered intravenously at 25 minutes.
, Leuko CD44 KO) or in endothelial cells (●, Endo CD44 KO) after IM7 (200 μg) injection. Results are expressed as ratios of changes in leukocyte rolling frequency over time in mice treated with IM7 relative to rat IgG-treated controls (n = 8 mice/treatment group, *P < .05). (B) Comparison of the effects of (●) whole IM7 antibody and its (□) F(ab′)2 or (▽) Fab fragments on leukocyte rolling in WT mice. IVM records were made before and up to 30 minutes after injection of antibodies (200 μg each). For secondary cross-linking of CD44, goat anti–rat IgG (GAR; 100 μg) was administered intravenously at 25 minutes.  , GAR alone served as a negative control. Results are expressed as ratios of changes in cell rolling, as described for panel A (n = 5 mice/group).
, GAR alone served as a negative control. Results are expressed as ratios of changes in cell rolling, as described for panel A (n = 5 mice/group).
Abrogation of cell rolling by IM7 is dependent on leukocyte CD44 and requires CD44 cross-linking. (A) The graph shows the changes in the frequency of rolling leukocytes in synovial venules of CD44 KO mice (○, full CD44 KO), or of chimeric mice lacking CD44 in leukocytes ( , Leuko CD44 KO) or in endothelial cells (●, Endo CD44 KO) after IM7 (200 μg) injection. Results are expressed as ratios of changes in leukocyte rolling frequency over time in mice treated with IM7 relative to rat IgG-treated controls (n = 8 mice/treatment group, *P < .05). (B) Comparison of the effects of (●) whole IM7 antibody and its (□) F(ab′)2 or (▽) Fab fragments on leukocyte rolling in WT mice. IVM records were made before and up to 30 minutes after injection of antibodies (200 μg each). For secondary cross-linking of CD44, goat anti–rat IgG (GAR; 100 μg) was administered intravenously at 25 minutes.
, Leuko CD44 KO) or in endothelial cells (●, Endo CD44 KO) after IM7 (200 μg) injection. Results are expressed as ratios of changes in leukocyte rolling frequency over time in mice treated with IM7 relative to rat IgG-treated controls (n = 8 mice/treatment group, *P < .05). (B) Comparison of the effects of (●) whole IM7 antibody and its (□) F(ab′)2 or (▽) Fab fragments on leukocyte rolling in WT mice. IVM records were made before and up to 30 minutes after injection of antibodies (200 μg each). For secondary cross-linking of CD44, goat anti–rat IgG (GAR; 100 μg) was administered intravenously at 25 minutes.  , GAR alone served as a negative control. Results are expressed as ratios of changes in cell rolling, as described for panel A (n = 5 mice/group).
, GAR alone served as a negative control. Results are expressed as ratios of changes in cell rolling, as described for panel A (n = 5 mice/group).
We then asked whether cross-linking of CD44 and Fc receptors or individual CD44 molecules by IM7 on the surface of leukocytes was involved in the reaction of the cells to antibody treatment. As shown in Figure 3B, injection of IM7(Fab′)2 had the same disruptive effect as the whole mAb on leukocyte rolling, whereas rolling was not disturbed by the IM7 Fab. However, injection of goat anti–rat IgG 25 minutes after IM7 Fab administration resulted in a prompt disruption of leukocyte rolling (Figure 3B) and also in morphologic changes (not shown) that were indistinguishable from those induced by whole IM7. The results of this experiment indicated that the reaction of leukocytes to IM7 treatment was independent of Fc receptor but required cross-linking of individual CD44 molecules on the cell surface.
Depletion of circulating granulocytes by anti-CD44 mAbs
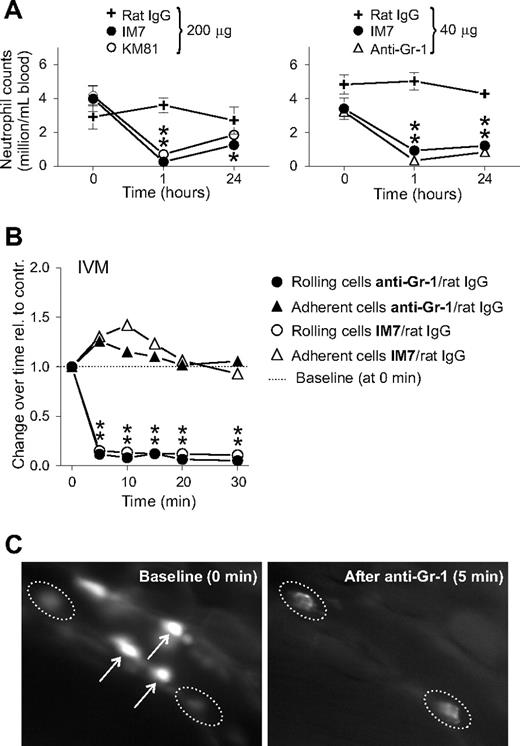
Because anti-CD44 (both IM7 and KM81) treatment significantly reduced the frequency of leukocyte rolling interactions in the vascular bed of inflamed joints (Figure 2), we expected the number of circulating (noninteracting) leukocytes to increase after treatment. Therefore, we determined total PBL and differential counts before (0 hours), as well as 1 and 24 hours after IM7 and KM81 injection (200 μg each). Intriguingly, both anti-CD44 mAbs drastically reduced the PBL counts at 1 hour, resulting almost exclusively from the disappearance of neutrophils (> 90% loss) from the circulation (Figure 4A, left). Some recovery in circulating neutrophils was noted at 24 hours, but the counts remained significantly suppressed in IM7-treated mice.
mAbs against CD44 (IM7 and KM81) and Gr-1 (RB6-8C5) deplete granulocytes in the circulation and exert similar effects on the rolling/adhesion behavior of leukocytes. (A) Peripheral blood neutrophil counts in WT mice before and 1 and 24 hours after intravenous administration of: (top left) 200 μg ( ) control rat IgG, (●) IM7, or (○) KM81; and (top right) 40 μg (
) control rat IgG, (●) IM7, or (○) KM81; and (top right) 40 μg ( ) rat IgG, (●) IM7, or (▲) anti–Gr-1 (n = 9 mice/group, *P < .05). (B) Comparison of the effects of IM7 and anti–Gr-1 mAbs (both at 40 μg) on the rolling and adhesion of leukocytes in the inflamed synovial vessels. The results are expressed as ratios of changes observed after treatment with specific mAbs vs control rat IgG (n = 8 mice/group, *P < .05). (C) IVM snapshots of leukocytes in a synovial venule before (0 minutes, baseline, left panel) and 5 minutes after intravenous injection of 40 μg anti–Gr-1 mAb (right panel). Arrows indicate bright rolling cells (left panel) that disappeared after anti–Gr-1 injection (right panel), and dotted lines encircle adherent cells that underwent changes in morphology (became hairy/disheveled in appearance) after anti–Gr-1 treatment.
) rat IgG, (●) IM7, or (▲) anti–Gr-1 (n = 9 mice/group, *P < .05). (B) Comparison of the effects of IM7 and anti–Gr-1 mAbs (both at 40 μg) on the rolling and adhesion of leukocytes in the inflamed synovial vessels. The results are expressed as ratios of changes observed after treatment with specific mAbs vs control rat IgG (n = 8 mice/group, *P < .05). (C) IVM snapshots of leukocytes in a synovial venule before (0 minutes, baseline, left panel) and 5 minutes after intravenous injection of 40 μg anti–Gr-1 mAb (right panel). Arrows indicate bright rolling cells (left panel) that disappeared after anti–Gr-1 injection (right panel), and dotted lines encircle adherent cells that underwent changes in morphology (became hairy/disheveled in appearance) after anti–Gr-1 treatment.
mAbs against CD44 (IM7 and KM81) and Gr-1 (RB6-8C5) deplete granulocytes in the circulation and exert similar effects on the rolling/adhesion behavior of leukocytes. (A) Peripheral blood neutrophil counts in WT mice before and 1 and 24 hours after intravenous administration of: (top left) 200 μg ( ) control rat IgG, (●) IM7, or (○) KM81; and (top right) 40 μg (
) control rat IgG, (●) IM7, or (○) KM81; and (top right) 40 μg ( ) rat IgG, (●) IM7, or (▲) anti–Gr-1 (n = 9 mice/group, *P < .05). (B) Comparison of the effects of IM7 and anti–Gr-1 mAbs (both at 40 μg) on the rolling and adhesion of leukocytes in the inflamed synovial vessels. The results are expressed as ratios of changes observed after treatment with specific mAbs vs control rat IgG (n = 8 mice/group, *P < .05). (C) IVM snapshots of leukocytes in a synovial venule before (0 minutes, baseline, left panel) and 5 minutes after intravenous injection of 40 μg anti–Gr-1 mAb (right panel). Arrows indicate bright rolling cells (left panel) that disappeared after anti–Gr-1 injection (right panel), and dotted lines encircle adherent cells that underwent changes in morphology (became hairy/disheveled in appearance) after anti–Gr-1 treatment.
) rat IgG, (●) IM7, or (▲) anti–Gr-1 (n = 9 mice/group, *P < .05). (B) Comparison of the effects of IM7 and anti–Gr-1 mAbs (both at 40 μg) on the rolling and adhesion of leukocytes in the inflamed synovial vessels. The results are expressed as ratios of changes observed after treatment with specific mAbs vs control rat IgG (n = 8 mice/group, *P < .05). (C) IVM snapshots of leukocytes in a synovial venule before (0 minutes, baseline, left panel) and 5 minutes after intravenous injection of 40 μg anti–Gr-1 mAb (right panel). Arrows indicate bright rolling cells (left panel) that disappeared after anti–Gr-1 injection (right panel), and dotted lines encircle adherent cells that underwent changes in morphology (became hairy/disheveled in appearance) after anti–Gr-1 treatment.
A rat mAb (clone RB6-8C5) used for neutrophil depletion in mice24,25 recognizes the myeloid differentiation marker Gr-1/Ly-6G, an antigen highly expressed on mature murine granulocytes.26 The granulocyte-depleting property of anti-CD44 mAbs prompted us to compare IM7 with the anti–Gr-1 mAb with respect to their relative potency in neutrophil elimination. As shown in Figure 4A (right), IM7 was nearly as effective as the anti–Gr-1 mAb in depleting neutrophils in the circulation after intravenous administration at a moderate dose (40 μg each) to arthritic mice. Gr-1 is referred to as a lineage-specific marker on granulocytes,26 but its function is not known at present. It is assumed that the anti–Gr-1 mAb depletes circulating granulocytes by inducing their sequestration in the spleen and liver, from which they never return to the circulation.25 Because the initial response of leukocytes to IM7 treatment was perceived as a rapid loss of rolling capacity, we next asked whether in vivo binding of anti–Gr-1 mAb could also disrupt the rolling of granulocytes. Intravenous administration of the anti–Gr-1 mAb to arthritic mice (either WT or CD44 KO) was followed by exactly the same reactions as injection of IM7 to WT mice, ie, rapid disappearance of rolling cells (Figure 4B), and the disheveled appearance of adherent cells in the synovial vessels (Figure 4C; Videos S4,S5).
Deposition of platelets on anti-CD44- and anti–Gr-1 mAb-covered leukocytes in vivo
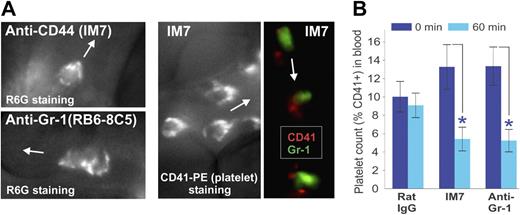
Close inspection of the morphology of leukocytes in the IVM movies recorded shortly after injection of the dye R6G, followed by IM7 (or anti–Gr-1) mAb administration, revealed progressive capture of fluorescent “particles” by the adherent leukocytes, contributing to their hairy/disheveled appearance. The surface topography of R6G-stained cells in IM7-treated (Figure 5A top left) and anti–Gr-1-treated (Figure 5A bottom left) mice was strikingly similar to the appearance of leukocytes stained in vivo with a fluorescent platelet marker (CD41)–specific mAb in IM7-treated mice (Figure 5A middle). This indicated that the particles deposited on the surface of adherent leukocytes after injection of IM7 (Figures 1F, 5A; Video S3), KM81 (not shown) or anti–Gr-1 (Figures 4C, 5A; Videos S4,S5) were indeed platelets. IVM records after coinjection of anti–CD41-PE and anti–Gr-1–Alexa 488 mAbs into IM7-treated mice revealed a close proximity of the red and green labels on leukocytes, indicating that the platelets preferentially bound to granulocytes (Figure 5A right). A drop in platelet counts in the circulation 1 hour after injection of IM7 or anti–Gr-1 (Figure 5B), coinciding with the disappearance of granulocytes (Figure 4A), also suggested that granulocytes and platelets were retained/removed together from the circulation.
Platelets are deposited on the surface of leukocytes after anti-CD44 or anti–Gr-1 treatment. (A) Surface topography of adherent leukocytes in (top left) IM7-treated and (bottom left) anti–Gr-1–treated WT mice after in vivo staining with R6G suggests deposition of particles, which proved to be platelets as indicated by (middle panel) platelet-specific CD41-PE mAb staining in vivo. The right panel shows colocalization of CD41 (platelets, red) and Gr-1 (granulocytes, green) fluorescence within adherent cells in the blood vessel of an IM7-treated mouse, indicating preferential deposition of platelets on granulocytes. Arrows indicate the direction of blood flow in the synovial venules. (B) Treatment of WT mice with IM7 or anti–Gr-1 mAb results in a significant reduction of platelet counts in the circulation 1 hour after treatment (0 minutes, dark blue bars; 60 minutes, light blue bars; n = 8 mice/group; *P < .05).
Platelets are deposited on the surface of leukocytes after anti-CD44 or anti–Gr-1 treatment. (A) Surface topography of adherent leukocytes in (top left) IM7-treated and (bottom left) anti–Gr-1–treated WT mice after in vivo staining with R6G suggests deposition of particles, which proved to be platelets as indicated by (middle panel) platelet-specific CD41-PE mAb staining in vivo. The right panel shows colocalization of CD41 (platelets, red) and Gr-1 (granulocytes, green) fluorescence within adherent cells in the blood vessel of an IM7-treated mouse, indicating preferential deposition of platelets on granulocytes. Arrows indicate the direction of blood flow in the synovial venules. (B) Treatment of WT mice with IM7 or anti–Gr-1 mAb results in a significant reduction of platelet counts in the circulation 1 hour after treatment (0 minutes, dark blue bars; 60 minutes, light blue bars; n = 8 mice/group; *P < .05).
Effects of pretreatment with antibodies against PSGL-1 or P-selectin or platelets, soluble P-selectin, and heparin on IM7-induced leukocyte/platelet responses
To assess the contribution of cell surface molecules to the reactions of leukocytes and platelets to anti-CD44 mAb injection, we used a panel of antibodies and reagents that were expected to antagonize one or more of these responses to IM7. Mice were pretreated with one of the antibodies/reagents (at doses indicated in the footnotes of Table 1) 25 minutes before IM7 injection (200 μg). Leukocyte rolling frequencies and leukocyte-platelet binding were monitored by IVM, and neutrophil counts were determined at 0, 25, and 30 minutes. As shown in Table 1, injection of mAb against PSGL-1 strongly reduced leukocyte rolling before IM7 injection (25 minutes) but had no impact on the IM7-induced rolling disruption, platelet binding, or neutrophil depletion (+IM7; 5 minutes) compared with control pretreatment with PBS or rat IgG. Anti–P-selectin mAb exhibited a minor inhibitory effect on rolling and moderate inhibition of IM7-induced platelet deposition, but it appeared to induce some platelet binding and neutrophil loss on its own (Table 1). Because an interaction between platelet P-selectin and leukocyte PSGL-1 had been implicated in neutrophil-platelet binding,27 these results were somewhat disappointing. In an alternative attempt to interfere with the PSGL-1–P-selectin interaction, we injected mice with rmP-selectin–Fc fusion protein before IM7 treatment. Surprisingly, P-selectin mimicked all the in vivo effects of IM7 (disruption of rolling, induction of platelet deposition, and neutrophil loss) (Table 1; Videos S6,S7), which did not change after IM7 injection. In mice pretreated with platelet-depleting pAb, the IM7-triggered events were strongly antagonized but not completely inhibited (Table 1; Figure 6A left and middle). The similar consequences of anti-CD44, anti–Gr-1, and soluble P-selectin treatments suggested the involvement of a common mechanism. A procoagulant state was reported in mutant mice with high amounts of P-selectin in the circulation because of shedding on deletion of its cytoplasmic tail, as well as in WT mice infused with rmP-selectin.28 In another study, anti–Gr-1 treatment induced severe intravascular coagulation in mice after priming with high amounts of tumor necrosis factor-α (TNF-α).29 These reports suggested that the coagulation system could contribute to the responses of leukocytes not only to P-selectin and anti–Gr-1 mAb, but also to IM7. Indeed, heparin (40 U/mouse) strongly antagonized all the effects of IM7 (Table 1; Figure 6A right). Furthermore, heparin showed similar antagonistic effects when injected into mice before anti–Gr-1 or rmP-selectin treatment (data not shown).
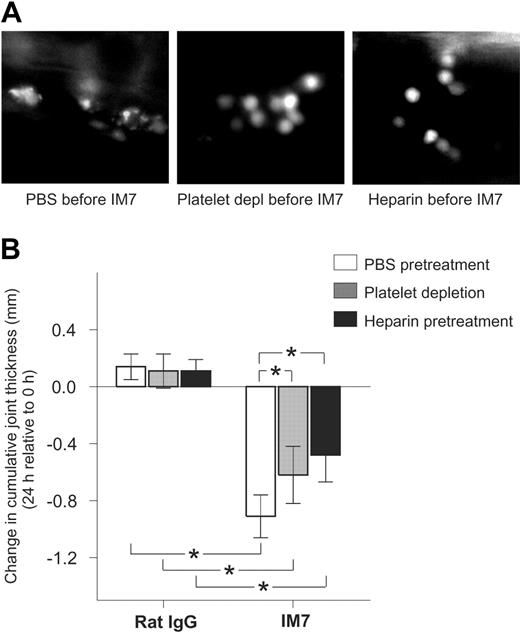
Inhibition of platelet deposition on leukocytes by administration of platelet-depleting antibodies or heparin reduces the anti-inflammatory effect of IM7 treatment in PGIA. (A) Deposition of platelets, seen as bright particles on leukocyte surfaces, in the synovial venule of a mouse pretreated with PBS (left) before IM7 injection, is inhibited in animals that received platelet-depleting pAb (middle) or heparin (right) 25 minutes before IM7 administration. (B) In control rat IgG-injected arthritic mice, platelet-depleting antibodies (▩) and heparin (■) do not have an effect on the 24-hour progression of joint swelling compared with PBS (□), but both platelet depletion and heparin significantly reduce the therapeutic effect (ie, suppression of joint swelling) of IM7. Mice were injected with PBS or received platelet-depleting pAb (100 μg) or heparin (40 U) 25 minutes before administration of rat IgG or IM7 (200 μg each). Joint thickness was measured at 0 and 24 hours. Results are expressed as changes (in mm) in cumulative (sum of 4 paws) joint thickness in 24 hours (n = 6 mice/group; *P < .05).
Inhibition of platelet deposition on leukocytes by administration of platelet-depleting antibodies or heparin reduces the anti-inflammatory effect of IM7 treatment in PGIA. (A) Deposition of platelets, seen as bright particles on leukocyte surfaces, in the synovial venule of a mouse pretreated with PBS (left) before IM7 injection, is inhibited in animals that received platelet-depleting pAb (middle) or heparin (right) 25 minutes before IM7 administration. (B) In control rat IgG-injected arthritic mice, platelet-depleting antibodies (▩) and heparin (■) do not have an effect on the 24-hour progression of joint swelling compared with PBS (□), but both platelet depletion and heparin significantly reduce the therapeutic effect (ie, suppression of joint swelling) of IM7. Mice were injected with PBS or received platelet-depleting pAb (100 μg) or heparin (40 U) 25 minutes before administration of rat IgG or IM7 (200 μg each). Joint thickness was measured at 0 and 24 hours. Results are expressed as changes (in mm) in cumulative (sum of 4 paws) joint thickness in 24 hours (n = 6 mice/group; *P < .05).
The therapeutic effects of IM7 in PGIA are mitigated, but not completely abolished, by platelet depletion and heparin pretreatment
Because administration of platelet-depleting antibodies and heparin strongly antagonized the immediate effects of IM7 on leukocytes, we sought to determine whether these reagents also reduced the anti-inflammatory action of IM7 in PGIA.8,9 Mice, at the early progressive phase of arthritis, were injected with PBS, a single dose (100 μg) of platelet-depleting pAb, or heparin (40 U), followed 25 minutes later with injection of rat IgG (control) or IM7 (both at 200 μg). As reported before,9 this high dose of IM7 resulted in significant reduction of joint swelling, compared with rat IgG, in 24 hours (Figure 6B). Although the platelet-depleting pAb and heparin had no impact on joint swelling in the control animals, both strongly reduced the therapeutic effect of IM7. However, joint swelling still remained significantly suppressed in IM7-treated mice even after platelet depletion and heparin treatment (Figure 6B).
Discussion
Several in vivo studies have implicated CD44 as an adhesion molecule that can mediate the rolling interactions of leukocytes with endothelium-expressed HA at sites of inflammation.14,30 However, in disease models investigated thus far, the lack of CD44 provided much less potent protection against inflammation than anti-CD44 treatment. This study was undertaken to understand the mechanistic basis of the discrepancy between the effects of CD44 deficiency and anti-CD44 treatment on leukocyte recruitment in experimentally induced inflammatory arthritis, a disease in which most of the conflicting results have been reported.8,10,15,31,32 We have found that the initial phases of leukocyte recruitment (rolling and adhesion) in the inflamed synovial vessels of mice are altered when CD44 is absent. The increased propensity of CD44 KO leukocytes to roll rather than adhere in this model is in line with previous reports,19,20 although at present it is unclear to what extent the lack of HA recognition contributes to this alteration of leukocyte behavior in CD44 KO mice. Neutrophils from WT mice do not show significant attachment to, or rolling on, immobilized HA,19 but CD44-mediated attachment can be induced in these cells on exposure to preformed complexes of HA and HA-binding proteins, such as TNF-α–induced protein-633 (G.H. and K.M., unpublished data, March 5, 2008). Both HA30 and TNF-α–induced protein-6 are produced abundantly at inflammatory sites.34 It is likely that HA-rich complexes, which support CD44-mediated leukocyte rolling/adhesion, are formed under the conditions of inflammation, but they cannot be used in CD44 KO mice. CD44 KO neutrophils show impairment in transendothelial migration toward a chemotactic stimulus applied in vivo.19 Reduced adhesion may also contribute to the delayed onset and slightly reduced severity of arthritis in CD44 KO mice with collagen-induced arthritis or PGIA.15,35 In contrast to the variable degree of protection afforded by CD44 deficiency, robust and consistent therapeutic effects were achieved in several inflammatory disease models (including collagen-induced arthritis and PGIA) with CD44-specific antibodies,8,11-14,31,32 suggesting major differences in the mechanisms by which CD44 deficiency and antibody treatment affect the recruitment of leukocytes.
Here we report that in vivo cross-linking of CD44 in WT mice with anti-CD44 mAbs elicits an instant reaction from leukocytes (primarily neutrophils) in the vascular bed of the inflamed synovium. Rolling is immediately disabled, platelets are deposited, and the antibody- and platelet-covered neutrophils disappear from the circulation. These effects are not reminiscent of the consequences of CD44 deficiency, nor do they appear to be resulting from an antibody-mediated inhibition of the interaction of CD44 with HA. Moreover, these effects can be reproduced in mice treated with a mAb against Gr-1, a major cell surface molecule on neutrophils, which has no known relationship with CD44 or an established role in cell adhesion.
In an attempt to interfere with IM7-induced platelet deposition on the leukocytes, thought to be mediated through an interaction between platelet P-selectin and neutrophil PSGL-1,27 we injected mice with rmP-selectin and were surprised to find that this soluble receptor elicited the same reaction as the anti-CD44 mAb. This observation, together with the finding that administration of a mAb against Gr-1 produces in vivo effects indistinguishable from those occurring in anti-CD44 and P-selectin–treated mice, suggests that leukocyte/platelet responses to all these treatments may be driven by common mechanisms.
We show that cross-linking of CD44 with bivalent mAb is important for triggering of the leukocyte/platelet responses. Ligation of CD44 with mAbs has been reported to lead to rapid activation of tyrosine kinases, mainly those belonging to the Src family,36,37 which are involved in integrin signaling. Similarly, in vitro treatment of neutrophils with P-selectin has been shown to activate the αMβ2 (CD11b/CD18, Mac-1) integrin by signaling through Src family kinases, followed by the integrin-mediated adhesion of the neutrophils to platelets.38 This suggests that outside-in signaling, initiated by CD44 or PSGL-1 cross-linking in neutrophils, may lead to rapid integrin activation and subsequent platelet adhesion.
The strong antagonistic effect of heparin on all IM7-triggered reactions indicates interplay between the coagulation system and cell adhesion pathways. Excessive fibrin deposition has been observed in mice treated with soluble P-selectin (and in those that shed P-selectin because of genetic manipulation28 ) as well as in mice undergoing anti–Gr-1 treatment after TNF-α stimulation.29 Because Mac-1 has been shown to bind fibrinogen/fibrin via a specific binding motif,39 it is conceivable that fibrin(ogen) binding to neutrophils, possibly mediated by activated Mac-1, can become a precipitating event in the sequelae of IM7 treatment. Reduction of both IM7-induced platelet deposition and neutrophil loss by heparin and (to a lesser degree) by platelet depletion suggests that CD44 cross-linking induces procoagulant activity on the neutrophil surface, which, through progressive accumulation of platelets, should lead to a profound alteration of the cell membrane. Although both cell surface–bound component(s) of the coagulation system and platelets seem to contribute to the rapid deterioration of neutrophils and their removal from the circulation, 40% to 70% of the cells disappear in the presence of anticoagulant and in the absence of platelets in IM7-treated mice. This may provide a “safe therapeutic window” for combined anti-CD44 and anticoagulant treatment, where the antibody-initiated leukocyte disposal still proceeds at a lower rate, whereas the procoagulant effect is eliminated. Consistent with this, we show that joint inflammation is still suppressed in arthritic mice undergoing combined heparin and IM7 treatment.
Our study implicates granulocyte depletion as a major mechanism underlying the therapeutic effect of anti-CD44 antibodies in inflammatory arthritis, a disease characterized by predominant recruitment of granulocytes in the joints.24,35,40 We also show that cross-linking of other abundantly expressed molecules, such as Gr-1 and PSGL-1, on the granulocyte surface with antibody and soluble receptor, respectively, leads to a similar sequence of events, culminating in the removal of these cells from the circulation. This warrants caution in the interpretation of in vivo intervention studies that use antibodies or multivalent proteins targeted to prominent leukocyte cell surface molecules, where favorable treatment outcomes might result from leukocyte depletion rather than from interference with the function of the target molecule.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Colt Egelston and Bálint Farkas for technical assistance.
This work was supported by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants P01 AR45652, K.M., A.F., and T.T.G.; and R01 AR051163, K.M.).
National Institutes of Health
Authorship
Contribution: G.H. and E.B. designed and performed the experiments, analyzed the data, made the figures, and wrote the manuscript; I.G. performed preliminary experiments and provided essential reagents; A.F. provided reagents and revised the manuscript; T.T.G. participated in the design of the experiments, provided important materials, and revised the manuscript; and K.M. initiated and coordinated the project, revised the figures, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katalin Mikecz, Department of Orthopedic Surgery, Rush University Medical Center, 1735 W Harrison Street, Cohn 712, Chicago, IL 60612; e-mail: Katalin_Mikecz@rsh.net.
References
Author notes
*G.H. and E.B. contributed equally to this work.