Abstract
The Ly49 natural killer (NK)–cell receptor family comprises both activating and inhibitory members, which recognize major histocompatibility complex (MHC) class I or MHC class I–related molecules and are involved in target recognition. As previously shown, the Ly49E receptor fails to bind to a variety of soluble or cell-bound MHC class I molecules, indicating that its ligand is not an MHC class I molecule. Using BWZ.36 reporter cells, we demonstrate triggering of Ly49E by the completely distinct, non–MHC-related protein urokinase plasminogen activator (uPA). uPA is known to be secreted by a variety of cells, including epithelial and hematopoietic cells, and levels are up-regulated during tissue remodeling, infections, and tumorigenesis. Here we show that addition of uPA to Ly49E-positive adult and fetal NK cells inhibits interferon-γ secretion and reduces their cytotoxic potential, respectively. These uPA-mediated effects are Ly49E-dependent, as they are reversed by addition of anti-Ly49E monoclonal antibody and by down-regulation of Ly49E expression using RNA interference. Our results suggest that uPA, besides its established role in fibrinolysis, tissue remodeling, and tumor metastasis, could be involved in NK cell–mediated immune surveillance and tumor escape.
Introduction
Natural killer (NK) cells play a key role in host defense against pathogens and tumors.1 Recognition of targets is regulated by the balance between signals from activating and inhibitory receptors, recognizing non-self or stress-induced ligands and major histocompatibility complex (MHC) class I or MHC class I–like molecules, respectively. Whereas the killer cell immunoglobulin-like receptors serve as the major functional NK receptor family recognizing MHC class I molecules in humans, members of the Ly49 family carry out similar functions in mice.2 The Ly49 family comprises several members of which Ly49H and Ly49D are activating. These activating members possess a charged transmembrane residue and transmit an activating signal through the adaptor molecule DAP12, which contains an immunoreceptor tyrosine-based activation motif. In contrast, the inhibitory Ly49 members have an immunoreceptor tyrosine-based inhibitory motif in their cytoplasmic domain.2 One member, Ly49E, contains a charged transmembrane residue as well as an immunoreceptor tyrosine-based inhibitory motif3 and displays some other unique features, which further distinguish this receptor from the other family members. First, the Ly49e gene is highly conserved between different mouse strains (C57BL/6, 129S6, and BALB/c).4,5 Second, Ly49E is the first member to emerge during NK-cell development, being the only member expressed on fetal and neonatal cells, but is largely absent on resting, mature NK cells.6 However, its expression on mature NK cells can be induced progressively after culture with interleukin-2 (IL-2) or IL-15.7 Furthermore, fetal thymic and skin Vγ3 T cells do not express Ly49 receptors, with the exception of Ly49E.8 Finally, Ly49E fails to bind a variety of soluble or cell-bound MHC class I molecules, suggesting that its ligand is not MHC class I.9,10
In this report, we used BWZ.36 reporter cells to show engagement of Ly49E by uPA, a serine protease that converts plasminogen into plasmin.11 uPA is well known to play a role in the plasminogen activator system, which is responsible for degradation of intravascular blood clots and contributes to extracellular proteolysis in a wide variety of physiologic and pathologic processes, including tissue remodeling, tumor invasion, and tumor metastasis.12 Secreted as a single-chain zymogen, uPA becomes activated by a single cleavage into 2-chain uPA, which comprises 2 main regions.11 The low molecular weight region contains the catalytic triad, whereas the amino-terminal fragment binds the high affinity uPA receptor (uPAR). Binding to uPAR focuses proteolysis and initiates signaling cascades that are implicated in pathologic processes including tumor growth, metastasis, and inflammation.11,13 Here we describe uPA-mediated triggering of another receptor, Ly49E, resulting in NK-cell inhibition. Therefore, in addition to uPAR, Ly49E could be involved in uPA-mediated immunologic processes and in tumor biology.
Methods
Mice and ex vivo–derived cell populations
C57BL/6 and β2 microglobulin (β2m) knockout (KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). uPA and uPAR KO and wild-type (WT) mice were described previously.14,15 Mice were housed and bred in our breeding facility, and used conforming to the guidelines of the Ethical Committee for Experimental Animals at the Faculty of Medicine and Health Sciences of Ghent University. Mice were mated for 15 hours and fetuses were removed at fetal day (FD) 14 or FD17 (plug date = day 0). Adult thymus and whole fetus, thymus, intestine, liver, and spleen from FD14 or FD17 fetuses were disrupted using a Potter homogenizer. Erythrocytes from fetal and adult liver and adult spleen cell suspensions were lysed with 0.17 M NH4Cl. FD17 thymocytes used in the CD107 assay were cultured for 2 days with 1000 U/mL human recombinant IL-2 (Roche Diagnostics, Basel, Switzerland) and another 2 days with 150 U/mL.
Retroviral vectors and BWZ.36 reporter assays
The vectors pMXs-puro and pMXs-IRES–green fluorescent protein (GFP) were provided by T. Kitamura (University of Tokyo, Tokyo, Japan),16 pMX-HD12 and pMX-HD12.Y2F17 by H. R. Smith (Howard Hughes Medical Institute, St Louis, MO). The construct of the hybrid Ly49H/E receptor, pMXs-H/E-IRES-GFP, included the Ly49H intracellular and transmembrane domain (amplified from pMX-HD12) and the Ly49E extracellular part. Replacement of GFP by DAP12 and DAP12.Y2F (amplified from pMX-HD12 and pMX-HD12.Y2F, respectively) eventually led to the pMXs-H/E-IRES-D12 and pMXs-H/E-IRES-D12.Y2F vectors, which were transduced into the BWZ.36 cell line18 to establish BWZ.36 H/ED12 and BWZ.36 H/ED12.Y2F reporter cells. In a similar way, BWZ.36 H/KLRG1D12 cells were generated with the KLRG1 construct provided by L. Brossay (Brown University, Providence, RI). Reporter cells were seeded at 25 000 cells per well into 96-well tissue-culture plates for 16 hours and were cocultured with 75 000 to 150 000 other cells or incubated in wells precoated for 6 hours with uPA, low molecular weight uPA, plasmin (Molecular Innovations, Southfield, MI), or control bovine serum albumin (BSA; Roche Diagnostics). 4D12 F(ab′)2 fragments (10 μg/mL) were added 30 minutes before and during incubation. β-Galactosidase (β-gal) activity was determined by 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal) staining or using the substrate chlorophenol red β-d-galactoside (CPRG; Roche Diagnostics) as described.18 Maximal β-gal activity was induced by adding 5 ng/mL phorbol myristate acetate and 0.5 μg/mL ionomycin. For microscopy, an Axiovert 25 inverted microscope (Carl Zeiss, Jena, Germany) with a CP Achromat lens at 10×/0.25 PH1 (Carl Zeiss) was used, and images were captured by a Moticam 480 digital camera (DC Imaging, West Chester, OH). Images were processed with Motic Images Plus 2.0 software (DC Imaging) and Microsoft Office Picture Manager (Microsoft Corporation, Redmond, WA).
Expression cloning
A retroviral cDNA library was constructed from J774.1 cells. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA), and mRNA was purified using the poly(A) Quik mRNA Isolation Kit (Stratagene, La Jolla, CA). mRNA was converted to cDNA using the ZAP-cDNA Synthesis Kit (Stratagene), which allowed unidirectional cloning in the retroviral pMXs-puro vector. After electroporation, 4.5 × 106 independent colonies were obtained. The cDNA library was transfected in a packaging cell line, and viral supernatant was used to transduce BaF/3 cells. For the initial screening, 1.2 × 106 transduced and puromycin-selected BaF/3 cells were seeded at 500 cells per well into 96-well plates. After 3 to 4 days, each plate was duplicated and H/ED12 reporter cells were added to the original plate. After 16 hours, cells were stained with X-gal. Four wells contained more than 20 positive cells (background = 5). The cells from the duplicate plates corresponding to the positive wells were split out over 10 96-well plates at 10 cells per well. After 6 to 7 days, plates were duplicated, H/ED12 reporter cells were added to the original plate for 16 hours, and CPRG analysis was performed. The cells from the duplicate plates corresponding to the positive wells were cloned at 1 cell per well and were, after expansion and duplication, subjected to the CPRG assay. Eventually, polymerase chain reaction on retroviral inserts from the clonal, strongly stimulating BaF/3 populations was performed with primers on the vector backbone.
Flow cytometric analysis, intracellular staining, and CD107 assays
Cells were analyzed with a FACSCalibur (BD Biosciences, San Jose, CA). In both the intracellular staining and the CD107 assay, cells were seeded at 30 000 to 60 000 cells per well into 96-well tissue-culture plates precoated for 18 hours with anti-NK1.1 (BD Biosciences), of which the last 6 hours together with uPA or BSA. 4D12 F(ab′)2 fragments (10 μg/mL) were added 30 minutes before and during incubation of the cells. Cells were FcR-blocked with unlabeled 2.4G2 monoclonal antibody (mAb; provided by J. Unkeless, Mount Sinai School of Medicine, New York, NY) and stained with anti–Ly49E/C-biotin, clone 4D126 or CM47 , or with the IgG2aκ-biotin isotype control (BD Biosciences), followed by streptavidin-allophycocyanin staining (BD Biosciences). For intracellular staining, the KY19 and NK0320 cell lines, the latter provided by N. Matsumoto (University of Tokyo, Tokyo, Japan), were seeded for 15 hours. Brefeldin A (BD Biosciences) was added during the last 12 hours. Cells were fixed and permeabilized with the Cytofix/Cytoperm Kit (BD Biosciences) and stained with anti–interferon-γ (IFN-γ)–phycoerythrin (PE) (BD Biosciences). For the CD107 assay, FD17 thymocytes were incubated for 4 hours in the presence of anti–CD107a–fluorescein isothiocyanate and monensin (BD Biosciences) and subsequently stained with anti–NK1.1-PE and anti–CD3-Cy5.5-PE (BD Biosciences).
RNA interference
Lentiviral constructs expressing a short hairpin (sh) RNA cassette from the pol III promoter H1 and enhanced GFP (EGFP from a CMV promoter were designed as described previously.21 The expression vectors were provided by B. Verhasselt (Ghent University, Ghent, Belgium). The 19-nucleotide targeted sequences inserted into the shRNA cassette were 5′-AGATGAAGATGAACTGAAA-3′ (recognizing Ly49e) and the control 5′-AGGAAGAAATGTTGACAA A-3′.
Results
Expression cloning of a putative Ly49E ligand
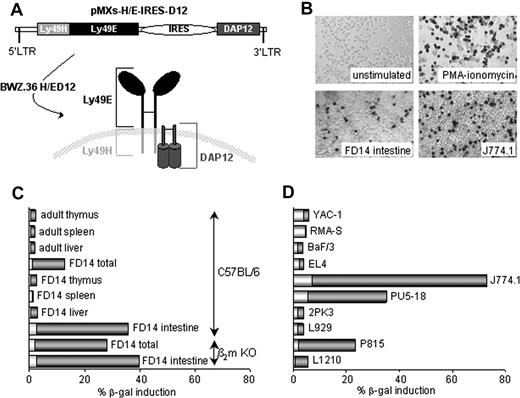
To identify a ligand for Ly49E, we used BWZ.36 reporter cells18 , in which a hybrid Ly49H/E receptor together with DAP12 (referred to as H/ED12) was expressed. The hybrid receptor consisted of the extracellular part of Ly49E and the transmembrane and intracellular regions of Ly49H (Figure 1A). The Ly49H regions as well as DAP12 were used to ensure activation of nuclear factor of activated T cells and subsequent induction of β-gal after Ly49E-triggering because it was unknown whether Ly49E is an activating or inhibitory receptor. A wide range of murine tissues and cell lines were screened. No β-gal induction was observed with thymus, spleen, or liver cell suspensions derived from adult or FD14 C57BL/6 mice (Figure 1C). In contrast, total FD14 cell suspension significantly stimulated H/ED12 cells. This appeared to be mainly because of the intestinal cells (Figure 1B,C). Importantly, a comparable stimulation was seen with intestinal cells from β2m KO mice (Figure 1C), confirming the assumption that the Ly49E ligand is not an MHC class I molecule or a β2m-dependent MHC class I–like molecule.9,10 Adult intestinal cells could not be analyzed because of endogenous β-gal activity (data not shown). Only 3 of 50 examined cell lines significantly triggered H/ED12 cells: the PU5-18 and J774.1 macrophage cell lines and the P815 mastocytoma cell line (Figure 1B,D; and data not shown). Because J774.1 cells induced the strongest stimulation, a retroviral cDNA library from these cells was constructed. BaF/3 cells, which did not stimulate H/ED12 cells (Figure 1D), were screened after transduction with the cDNA library. Eventually, 4 strongly stimulating BaF/3 clones were obtained. Surprisingly, their common retroviral insert encoded uPA (data not shown), a soluble protein completely different from all NK receptor ligands identified so far.
Expression of a putative Ly49E ligand by murine tissues and cell lines. (A) Schematic illustration of the pMXs-H/E-IRES-D12 retroviral construct, used to generate BWZ.36 H/ED12 reporter cells expressing the hybrid Ly49H/E together with DAP12. (B) X-gal staining of H/ED12 cells either unstimulated or stimulated as indicated (original magnification ×100). (C,D) CPRG analysis of H/ED12 cells, cocultured with the indicated cell suspensions from murine tissues (C) and with the indicated cell lines (D). Representative data from 1 of 2 to 4 experiments are shown. Bars show CPRG data expressed as a percentage of maximal β-gal induction by phorbol myristate acetate + ionomycin. Background CPRG percentages of the indicated cells in the absence of H/ED12 cells are indicated by the white fraction of each bar.
Expression of a putative Ly49E ligand by murine tissues and cell lines. (A) Schematic illustration of the pMXs-H/E-IRES-D12 retroviral construct, used to generate BWZ.36 H/ED12 reporter cells expressing the hybrid Ly49H/E together with DAP12. (B) X-gal staining of H/ED12 cells either unstimulated or stimulated as indicated (original magnification ×100). (C,D) CPRG analysis of H/ED12 cells, cocultured with the indicated cell suspensions from murine tissues (C) and with the indicated cell lines (D). Representative data from 1 of 2 to 4 experiments are shown. Bars show CPRG data expressed as a percentage of maximal β-gal induction by phorbol myristate acetate + ionomycin. Background CPRG percentages of the indicated cells in the absence of H/ED12 cells are indicated by the white fraction of each bar.
Specific triggering of Ly49E by uPA
uPA is secreted as a soluble, catalytically inactive enzyme. Activated by cleavage, uPA reshapes into a 2-chained form. Recombinant, 2-chain active uPA was used in all further experiments. Added soluble, uPA only weakly induced β-gal activity in H/ED12 cells, suggesting the need for Ly49E cross-linking. Indeed, coated uPA significantly stimulated H/ED12 cells in a concentration-dependent manner (Figure 2A). Preincubation with anti-Ly49E (4D12) F(ab′)2 fragments almost completely abrogated uPA-mediated β-gal induction, indicating Ly49E specificity (Figure 2A). Furthermore, uPA-mediated β-gal induction was not seen in untransduced BWZ.36 cells or in BWZ.36 cells transduced with Ly49H/E together with a signaling-deficient DAP12 molecule in which a tyrosine residue was substituted by phenylalanine (referred to as H/ED12.Y2F). In addition, no uPA-mediated stimulation was observed in control H/KLRG1D12 cells, in which the extracellular part of Ly49E was substituted by that of the KLRG1 NK receptor (Figure 2B). Importantly, intestinal cells from FD14 uPA KO fetuses did not induce β-gal activity in H/ED12 cells (Figure 2C), which further confirmed that the observed β-gal induction by WT intestinal cells was uPA-mediated. uPA activates plasminogen by its conversion into the broad-spectrum protease plasmin, which in turn processes several other proteins.12 To examine whether the true Ly49E ligand was a factor downstream in the uPA proteolytic cascade, several experiments were performed. Soluble or coated plasmin did not stimulate H/ED12 cells. The coated low molecular weight region of uPA, which contains the catalytic site and maintains the ability to activate plasminogen,11 only weakly induced β-gal activity. Furthermore, culture medium containing 10% fetal calf serum and incubated for 16 hours in uPA-coated wells was transferred to H/ED12 cells and did not induce β-gal activity (Figure 2D). Finally, as already mentioned, soluble uPA, although catalytically active, hardly induced β-gal activity.
Specific and Ly49E-dependent activation of H/ED12 cells by coated uPA. (A) CPRG analysis of H/ED12 cells incubated in the presence of indicated amounts of uPA. uPA was coated onto the wells (■) or added soluble during incubation ( ). No (
). No ( , ■) or anti-Ly49E (4D12) F(ab′)2 fragments (□) were added. (B) CPRG analysis of the indicated reporter cells incubated in uPA-coated wells (200 ng/well). (C) X-gal staining of H/ED12 cells incubated with uPA KO or WT FD14 intestinal cells. Representative data from 1 of 3 experiments are shown (original magnification ×100). (D) CPRG analysis of H/ED12 cells incubated with the indicated proteins (200 ng/well) or with medium preincubated in uPA-coated wells (200 ng/well) (referred to as uPA-conditioned medium). CPRG data are expressed as mean plus or minus SD of 3 experiments.
, ■) or anti-Ly49E (4D12) F(ab′)2 fragments (□) were added. (B) CPRG analysis of the indicated reporter cells incubated in uPA-coated wells (200 ng/well). (C) X-gal staining of H/ED12 cells incubated with uPA KO or WT FD14 intestinal cells. Representative data from 1 of 3 experiments are shown (original magnification ×100). (D) CPRG analysis of H/ED12 cells incubated with the indicated proteins (200 ng/well) or with medium preincubated in uPA-coated wells (200 ng/well) (referred to as uPA-conditioned medium). CPRG data are expressed as mean plus or minus SD of 3 experiments.
Specific and Ly49E-dependent activation of H/ED12 cells by coated uPA. (A) CPRG analysis of H/ED12 cells incubated in the presence of indicated amounts of uPA. uPA was coated onto the wells (■) or added soluble during incubation ( ). No (
). No ( , ■) or anti-Ly49E (4D12) F(ab′)2 fragments (□) were added. (B) CPRG analysis of the indicated reporter cells incubated in uPA-coated wells (200 ng/well). (C) X-gal staining of H/ED12 cells incubated with uPA KO or WT FD14 intestinal cells. Representative data from 1 of 3 experiments are shown (original magnification ×100). (D) CPRG analysis of H/ED12 cells incubated with the indicated proteins (200 ng/well) or with medium preincubated in uPA-coated wells (200 ng/well) (referred to as uPA-conditioned medium). CPRG data are expressed as mean plus or minus SD of 3 experiments.
, ■) or anti-Ly49E (4D12) F(ab′)2 fragments (□) were added. (B) CPRG analysis of the indicated reporter cells incubated in uPA-coated wells (200 ng/well). (C) X-gal staining of H/ED12 cells incubated with uPA KO or WT FD14 intestinal cells. Representative data from 1 of 3 experiments are shown (original magnification ×100). (D) CPRG analysis of H/ED12 cells incubated with the indicated proteins (200 ng/well) or with medium preincubated in uPA-coated wells (200 ng/well) (referred to as uPA-conditioned medium). CPRG data are expressed as mean plus or minus SD of 3 experiments.
uPA inhibits IFN-γ production by NK cell lines
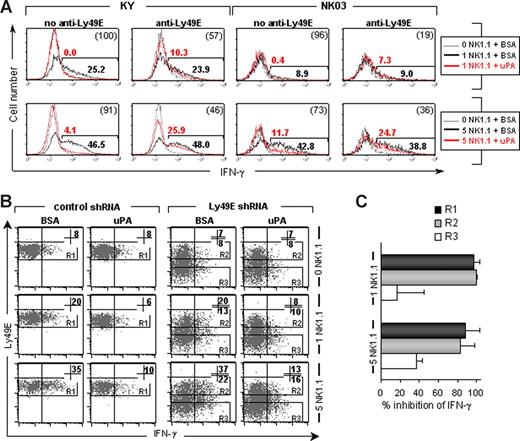
Next, we investigated whether uPA induced or inhibited Ly49E-expressing NK cells. Although a low number of adult NK cells expresses the Ly49E receptor,6 the amount of positive cells increases dramatically on IL-2 or IL-15 culture.7 Indeed, both the KY and NK03 cell lines, which were obtained after long-term IL-2 culture,19,20 strongly express Ly49E (Figure 3B; and data not shown). NK03 cells also express Ly49A and Ly49G2; KY cells do not express other Ly49 members (data not shown). Using NK1.1-mediated induction of IFN-γ as a marker of activation, we exposed the NK-cell lines either to coated uPA or to control BSA. IFN-γ production by both cell lines was almost completely inhibited by coated uPA (Figure 3A). Addition of anti-Ly49E (4D12) F(ab′)2 fragments restored the IFN-γ production by 50% to 80% (Figure 3A), indicating that the inhibition of IFN-γ production by coated uPA was Ly49E-dependent. In addition, Ly49E expression on KY cells was down-regulated using Ly49E shRNA. Only part of the Ly49E shRNA-transduced cells showed a strong decrease in Ly49E expression, giving rise to a population with low Ly49E expression levels and an internal control population with normal Ly49E expression. KY cells transduced with control shRNA were used as an extra control population (Figure 3B). As expected, uPA-mediated inhibition of NK1.1-induced IFN-γ production was almost complete in control shRNA-transduced cells as well as in the internal control population, whereas it was partially restored in the population with decreased Ly49E expression (Figure 3B,C). In conclusion, these data demonstrate that uPA-mediated inhibition of IFN-γ secretion by NK cells depends on Ly49E triggering and strongly argue against the possibility of anti-NK1.1 mAb degradation by uPA.
Coated uPA inhibits IFN-γ production by NK-cell lines in an Ly49E-dependent manner. (A) IFN-γ staining of KY and NK03 cells incubated in coated wells, in the presence or absence of anti-Ly49E (4D12) F(ab′)2 fragments as indicated. Wells were coated with no (dotted lines) or anti-NK1.1 mAb (solid lines) at 1 and 5 μg/mL as indicated, together with uPA (red lines) or BSA (black lines) at 200 ng/well. In each histogram, the percentage IFN-γ expression, corrected for its background at 0 μg/mL anti-NK1.1, is indicated above (uPA) or below (BSA) the marker line. The percentage inhibition of IFN-γ by uPA compared with BSA is given in parentheses in the top right corner. Representative data from 1 of 3 experiments are shown. (B) KY cells were transduced with a control or Ly49E shRNA construct, both containing the EGFP marker gene. Cells were seeded into wells coated with no or anti-NK1.1 mAb at 1 and 5 μg/mL, together with uPA or BSA at 200 ng/well, as indicated. Thereafter, cells were costained for IFN-γ and Ly49E. The gated EGFP+Ly49E+ populations are indicated by R1 or R2, the gated EGFP+Ly49E− cells by R3. In each dot plot, the percentage IFN-γ expression in the gated regions is given in the top right corner. Representative data from 1 of 4 experiments are shown. (C) The percentage uPA-mediated inhibition of IFN-γ production by the populations and in the conditions as indicated in panel B. The mean plus or minus SD of 4 experiments is shown.
Coated uPA inhibits IFN-γ production by NK-cell lines in an Ly49E-dependent manner. (A) IFN-γ staining of KY and NK03 cells incubated in coated wells, in the presence or absence of anti-Ly49E (4D12) F(ab′)2 fragments as indicated. Wells were coated with no (dotted lines) or anti-NK1.1 mAb (solid lines) at 1 and 5 μg/mL as indicated, together with uPA (red lines) or BSA (black lines) at 200 ng/well. In each histogram, the percentage IFN-γ expression, corrected for its background at 0 μg/mL anti-NK1.1, is indicated above (uPA) or below (BSA) the marker line. The percentage inhibition of IFN-γ by uPA compared with BSA is given in parentheses in the top right corner. Representative data from 1 of 3 experiments are shown. (B) KY cells were transduced with a control or Ly49E shRNA construct, both containing the EGFP marker gene. Cells were seeded into wells coated with no or anti-NK1.1 mAb at 1 and 5 μg/mL, together with uPA or BSA at 200 ng/well, as indicated. Thereafter, cells were costained for IFN-γ and Ly49E. The gated EGFP+Ly49E+ populations are indicated by R1 or R2, the gated EGFP+Ly49E− cells by R3. In each dot plot, the percentage IFN-γ expression in the gated regions is given in the top right corner. Representative data from 1 of 4 experiments are shown. (C) The percentage uPA-mediated inhibition of IFN-γ production by the populations and in the conditions as indicated in panel B. The mean plus or minus SD of 4 experiments is shown.
uPA reduces the cytotoxic potential of fetal NK cells
Unlike other Ly49 members, Ly49E is expressed on fetal thymic NK cells.6 Because these cells hardly produce IFN-γ (data not shown), we assessed their cytotoxic potential in the presence or absence of uPA by measuring the NK1.1-mediated induction of CD107 cell membrane expression. CD107, expressed on the membrane of cytotoxic granules, temporarily appears on the cell surface of activated cytotoxic cells as a result of degranulation.22 It has been demonstrated that CD107 surface expression correlates with the cytotoxic potential of activated NK cells.23,24 IL-2–cultured FD17 thymocytes were incubated for 4 hours in anti-NK1.1 plus uPA- or BSA-coated wells. To discriminate between Ly49E-expressing and nonexpressing cells, the thymocytes were stained with anti-Ly49E mAb. In the gated CD3−NK1.1+ NK population, 3 subpopulations were observed according to their Ly49E expression, referred to as Ly49Eneg, Ly49Edim, and Ly49Ebright cells (Figure 4A). A clear correlation was observed between the Ly49E expression level and reduction of CD107 surface expression by coated uPA (Figure 4B). Although much lower, also the Ly49E− population showed reduction of CD107 expression. A possible explanation is the low level of Ly49E expression on Ly49E− cells, as indicated by the higher Ly49E staining compared with isotype control staining (Figure 4A). Alternatively, there might be a small Ly49E-independent, uPA-mediated reduction in CD107 expression. To further determine Ly49E specificity of the observed uPA effect, anti-Ly49E (4D12) F(ab′)2 fragments were added. To still distinguish the different Ly49E-expressing populations during analysis, cells were stained with another anti-Ly49E mAb, CM4, which shows minimal competition with 4D12 for Ly49E binding.7 Anti-Ly49E F(ab′)2 fragments restored CD107 expression on Ly49Ebright and Ly49Edim cells almost to the level of the Ly49E− population (Figure 4B). This indicates that the uPA-induced reduction of CD107 expression is mainly Ly49E-specific. Finally, we wanted to exclude a possible role for uPAR, weakly expressed on NK cells (data not shown), in the observed effects of uPA. Lacking a cytosolic domain, the glycosylphosphatidylinositol-anchored uPAR is known to signal through other receptors, including integrins and G protein–coupled receptors.13 Similarly, uPA-binding to uPAR might result in Ly49E-mediated signaling. However, coated uPA induced an equal reduction in CD107 expression on FD17 NK cells from uPAR KO compared with WT mice, excluding uPAR involvement (Figure 4B).
Coated uPA reduces the cytotoxic potential of Ly49E-positive fetal NK cells independent from uPAR expression. (A) Anti-Ly49E mAb (CM4) staining of IL-2–cultured FD17 cells (solid line) discriminated Ly49Eneg, Ly49Edim, and Ly49Ebright cells (M1, M2, and M3, respectively) after NK1.1+CD3− gating. Isotype control staining is shown as a dotted line. (B) The percentage uPA-mediated reduction of CD107 expression in the 3 gated NK populations. C57BL/6 or uPAR KO FD17 cells were incubated in anti-NK1.1 (10 μg/mL) plus uPA or BSA (200 ng/well) coated wells. No (filled symbols; C57BL/6: n = 7; uPAR KO: n = 5) or anti-Ly49E (4D12) F(ab′)2 fragments (open symbols; n = 4) were added. The mean is represented by a horizontal bar. *P < .05; **P < .01 (Student t test).
Coated uPA reduces the cytotoxic potential of Ly49E-positive fetal NK cells independent from uPAR expression. (A) Anti-Ly49E mAb (CM4) staining of IL-2–cultured FD17 cells (solid line) discriminated Ly49Eneg, Ly49Edim, and Ly49Ebright cells (M1, M2, and M3, respectively) after NK1.1+CD3− gating. Isotype control staining is shown as a dotted line. (B) The percentage uPA-mediated reduction of CD107 expression in the 3 gated NK populations. C57BL/6 or uPAR KO FD17 cells were incubated in anti-NK1.1 (10 μg/mL) plus uPA or BSA (200 ng/well) coated wells. No (filled symbols; C57BL/6: n = 7; uPAR KO: n = 5) or anti-Ly49E (4D12) F(ab′)2 fragments (open symbols; n = 4) were added. The mean is represented by a horizontal bar. *P < .05; **P < .01 (Student t test).
Discussion
Our present data surprisingly demonstrate a direct role for uPA in Ly49E-triggering. Immobilized recombinant uPA specifically stimulated H/ED12 reporter cells expressing Ly49E. Furthermore, stimulation was observed after coculture with intestinal cells from WT but not from uPA KO mice. On the other hand, downstream proteolytic products of uPA were not involved in Ly49E-mediated stimulation. IFN-γ experiments on Ly49E-expressing adult NK cells as well as CD107 degranulation assays on fetal NK cells demonstrated an Ly49E-dependent inhibition of NK-cell activity by immobilized uPA. Blocking experiments using anti-Ly49E F(ab′)2 fragments supported the Ly49E dependence, in addition to knockdown experiments with shRNA.
Although our data provide clear evidence that uPA is specifically involved in Ly49E triggering, no Ly49E-specific binding was observed on BWZ.36 reporter cells, NK cells, or Ly49E-transduced Jurkat cells after incubation with fluorescein- or 125I-labeled uPA or with uPA-coated polystyrene beads. Binding of soluble Ly49E/IgFc fusion protein to uPA-expressing cells or to coated recombinant uPA was not observed either (data not shown). This suggests the requirement for a cofactor to bind Ly49E. The multitude of possible cofactors11,12 requires further intensive research, which is beyond the scope of this article.
In contrast to the effects observed with immobilized uPA, soluble uPA did not stimulate H/ED12 reporter cells nor inhibited NK-cell activity (the latter, data not shown). Therefore, to trigger NK cells physiologically, we assume that uPA has to be presented in vivo. Although uPA is secreted as a soluble protein, the majority of uPA is found at the cell membrane, bound on its high-affinity receptor uPAR.13 Furthermore, based on data revealed from uPAR KO mice, it is assumed that extracellular matrix components can substitute for uPAR as a binding site for uPA.13 Coated uPA in vitro might mimic uPA bound onto uPAR or extracellular matrix components in vivo.
Until recently, it was thought that NK-cell inhibitory receptors primarily recognize MHC class I molecules, supporting a role of these receptors in classic “missing-self” recognition. However, the recent identification of non MHC class I ligands for several inhibitory immune receptors, including KLRG1, LAIR-1, and NKR-P1D, indicates that also MHC class I–independent inhibitory receptors play a crucial role in inducing peripheral tolerance.25,26 Similar to the other inhibitory molecules,25,26 uPA is broadly expressed, and expression levels are up-regulated in transformed cells. In several types of cancer, elevated uPA expression levels are correlated with bad prognosis,12 which is described to be the result of promotion of tumor invasion and metastasis through uPAR-mediated signaling. Our data suggest that NK-cell immune suppression could play an additional role.
Similar to some of the other non-MHC class I inhibitory molecules,25,26 uPA production is up-regulated during severe infections in several cell types, including epithelial and endothelial cells, activated T cells, monocytes, and neutrophils. In that way, uPA potentiates neutrophil activation and migration and possibly participates in T-cell priming. Moreover, uPA-dependent cleavage of cell-bound plasminogen to plasmin enhances the release of pro-inflammatory mediators, amplifying the acute inflammatory reaction.11 After activation of the immune system and pathogen clearance, mechanisms to limit and ultimately terminate the response are crucial. Gays et al7 showed that de novo acquisition of Ly49E on adult NK cells by the inflammatory cytokine IL-2 was clearly linked to cell proliferation. Therefore, one could speculate that uPA-mediated Ly49E triggering could serve as a negative feedback mechanism for NK cells, after their initial expansion and activation by IL-2. Further in vivo experiments using Ly49E or uPA KO animals in infection models will provide insights into uPA-related physiologic functions of Ly49E.
Finally, we have previously shown that Ly49E is constitutively expressed on a subset of skin Vγ3 T cells.8 uPA expression, on the other hand, is known to be up-regulated during wound healing27 and in several types of skin tumors.28,29 Hence, besides a possible role for uPA in NK-mediated immunity, uPA could also play a regulatory role in skin immune surveillance through Ly49E-expressing Vγ3 T cells.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank C. Collier, J. Robin, and E. Vandenberghe for animal care and F. Timmermans for supportive discussions.
This work was supported by grants from the Fund for Scientific Research-Flanders (FWO) and the Foundation against Cancer, a foundation of public interest. T.V.D.B. and T.T. were supported by the FWO. S.T. was supported by the Institute for the Promotion of Innovation by Science and Technology in Flanders.
Authorship
Contribution: T.V.D.B., F.S., and G.L. designed the research; T.V.D.B., F.S., S.T., V.D., C.V., W.H., and G.L. performed experiments; B.V., T.T., P.M., J.P., W.H., M.D., and W.M.Y. contributed mice and reagents; T.V.D.B., F.S., and G.L. analyzed results; and T.V.D.B., and G.L. made the figures and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Georges Leclercq, Department of Clinical Chemistry, Microbiology and Immunology, Ghent University, Building A, 4th Floor, De Pintelaan 185, 9000 Ghent, Belgium; e-mail: georges.leclercq@ugent.be.
References
Author notes
*T.V.D.B. and F.S. contributed equally to this study.