Abstract
Phagocytosis and activation of the NADPH oxidase are important mechanisms by which neutrophils and macrophages engulf and kill microbial pathogens. We investigated the role of PI3K signaling pathways in the regulation of the oxidase during phagocytosis of Staphylococcus aureus and Escherichia coli by mouse and human neutrophils, a mouse macrophage-like cell line and a human myeloid-like cell line. Phagocytosis of these bacteria was promoted by serum, independent of serum-derived antibodies, and effectively abolished in mouse neutrophils lacking the β2-integrin common chain, CD18. A combination of PI3K isoform-selective inhibitors, mouse knock-outs, and RNA-interference indicated CD18-dependent activation of the oxidase was independent of class I and II PI3Ks, but substantially dependent on the single class III isoform (Vps34). Class III PI3K was responsible for the synthesis of PtdIns(3)P on phagosomes containing either bacteria. The use of mouse neutrophils carrying an appropriate knock-in mutation indicated that PtdIns(3)P binding to the PX domain of their p40phox oxidase subunit is important for oxidase activation in response to both S aureus and E coli. This interaction does not, however, account for all the PI3K sensitivity of these responses, particularly the oxidase response to E coli, suggesting that additional mechanisms for PtdIns(3)P-regulation of the oxidase must exist.
Introduction
Phagocytic leukocytes such as neutrophils and macrophages play several key roles in the innate immune system. One of their most important functions is to engulf small bacterial and fungal pathogens by the process of phagocytosis, subsequent formation of intracellular phagosomes and the destruction of the internalized pathogen.1 This process is coordinated into a whole-body response to infection through antigen presentation to the adaptive immune system and the tailored release of inflammatory mediators.
Phagocytosis and the maturation of the phagosome are regulated by an extensive signaling web downstream of several activated cell-surface receptors. The receptors used depend upon the nature of the prey, the extent to which the surface of the prey has been coated with host-derived opsonins (fragments of complement and/or antibodies), and soluble factors generated at the site of inflammation (cytokines, chemokines, and bacterially derived products such as formylated peptides and endotoxin).2 Under most physiologic circumstances several receptors will be cooperatively involved, including the major classes of ‘phagocytic’ receptors essential for regulating the process of internalization (such as β2-integrins, FcRs, and scavenger receptors), together with cytokine-, G-protein–coupled, and pattern recognition receptors, which modulate the host cells' response to phagocytosis (reviewed in Underhill and Ozinsky2 ). Particularly important in the absence of a prior or effective response from the adaptive immune system are antibody-independent phagocytic mechanisms, the clearest example of which is the deposition of complement fragments (C3b/C3bi) on the surface of bacteria by the alternative pathway of complement fixation and ligation of these fragments by the β2-integrin CD11b/CD18 (also known as CR3 or Mac-1). Thus, mice lacking CD18 (the common chain of β2-integrins) are more susceptible to bacterial infections,3 and patients deficient in CD11b/CD184 or possessing mutations in CD185 suffer recurrent and spontaneous microbial infections from prevalent but normally benign strains of bacteria, including strains of Staphylococcus aureus and Escherichia coli.
During engagement and internalization, phagocytes use several mechanisms to ensure effective killing of pathogens, including the use of antimicrobial peptides, broad spectrum proteases, and the production of reactive oxygen species (ROS). The generation of ROS by the NADPH oxidase complex is thought to play an important role in both direct and indirect killing of several species of bacteria and fungi.6 Sufferers of chronic granulomatous disease (CGD) carry genetic lesions in essential components of this NADPH oxidase complex, and present with recurring, life-threatening infections, commonly of Staphylococcus, Salmonella, or Aspergillus origin.7,8 The complex comprises 2 membrane-bound proteins gp91phox and p22phox, and 4 cytosolic components p40phox, p47phox, p67phox and the GTPase-Rac.6 In response to inflammatory or phagocytic stimuli, these components assemble on the plasma and/or phagocytic membrane to form the functional NADPH complex, which transfers electrons from NADPH on the cytosolic face of the membrane to molecular oxygen on the extracellular/phagosomal face, producing superoxide anions and other superoxide-derived ROS.
Most attempts to unravel the signaling mechanisms involved in the regulation of the NADPH oxidase have used simplified model systems designed to isolate the contribution of individual receptors; these have included the presentation of inert particles coated with single opsonins (eg, IgG or C3bi) or the use of soluble, inflammatory stimuli (eg, fMLP or C5a). These studies have indicated that there is a complex signaling web between receptors and the assembly of an active oxidase, involving several established signaling pathways. Key targets of these pathways are guanine nucleotide exchange on Rac2, leading to productive complex formation between GTP-Rac2, p67phox, and gp91phox and also phosphorylation of the C-terminus of p47phox, leading to productive complex formation between p47phox, p67phox, and p22phox.6 Moreover, while activation of certain receptor classes can, in isolation, initiate sufficient signaling to activate the oxidase, robust activation normally requires cooperative interaction between more than one receptor type. Thus, ligation of the major phagocytic complement receptor, CD11b/CD18 by C3bi-coated particles is sufficient to induce phagocytosis but does not induce substantial activation of a plasma membrane or phagosomal NADPH oxidase without costimulation with other receptor systems (eg, TLRs, cytokine receptors, or low level stimulation of FcγRs).9,10
There are now several lines of evidence that suggest that phosphoinositides synthesized by PI3Ks play important roles in regulating efficient assembly and activation of the oxidase in different contexts of cell stimulation.11,12 Class IA PI3Ks (PI3Kα, β, and δ) and class IB PI3K (PI3Kγ) play key roles in the signal transduction pathways downstream of a variety of protein tyrosine kinase and Gi-coupled cell-surface receptors, respectively.13,14 Upon receptor activation, class I PI3Ks synthesize the messenger lipids PtdIns(3,4,5)P3 and PtdIns(3,4)P2 in the plasma membrane and coordinate the recruitment and activation of several protein effectors, the best established of which possess PH-domains with high specificity for binding one or both head groups of these lipids (eg, PKB, BTK, GRP1, and ARAP3).13-15 Relevant here, both class IA and IB PI3Ks play important roles in the activation of the NADPH oxidase in cytokine-primed neutrophils stimulated by soluble chemoattractants.16-18 Furthermore, class IA PI3Ks are important in the phagocytosis and concomitant oxidase burst stimulated by engagement of macrophages with large IgG-opsonized particles,19 with PtdIns(3,4,5)P3 accumulating transiently on the nascent phagosomal cup, and disappearing rapidly upon phagosomal closure.20,21 The precise roles that PtdIns(3,4,5)P3 and/or PtdIns(3,4)P2 play in the signaling pathways to oxidase activation under these circumstances is still unclear but likely to include PtdIns(3,4,5)P3 activation of RacGEFs (P-Rex1, DOCK2, and Vav1-3) and PtdIns(3,4)P2 binding to p47phox.11 The involvement of class I PI3Ks in non–antibody-mediated phagocytosis and oxidase activation is undefined.
Class III PI3K (also known as Vps34) synthesizes the messenger lipid PtdIns(3)P in internal membrane compartments of the endosomal/lysosomal system.22 PtdIns(3)P is accepted to play a major regulatory role in these membranes by influencing the localization and activity of several protein effectors possessing PtdIns(3)P-specific, FYVE, and PX domains.23 PtdIns(3)P has also been shown to accumulate dramatically on phagosomal membranes subsequent to closure and separation from the plasma membrane.20,24 In one model system, in which nonphagocytic Chinese hamster ovary (CHO) cells were rendered competent to phagocytose IgG-opsonized particles via heterologous expression of FcγRIIA, phagosomal PtdIns(3)P accumulation was blocked by anti–class III PI3K antibodies.20 In all other examples of phagosomal PtdIns(3)P synthesis, however, the source of this lipid is unknown. In this regard, it has been suggested that PtdIns(3)P can also be produced in plasma membrane/endocytic vesicles by sequential dephosphorylation of class I–derived PtdIns(3,4,5)P3 via specific 5- and 4-phosphatases25 or, by one of the 3 still poorly characterized class II PI3Ks: PI3KinaseC2α, -β, and -γ.26
PtdIns(3)P plays an important role in phagosomal maturation and pathogen destruction20,27,28 and, indeed, some virulent strains of bacteria (eg, Mycobacterium tuberculosis) are thought to evade host defenses by interfering with its metabolism.29 Details of the molecular mechanisms by which PtdIns(3)P exerts these effects are still largely undefined, although the binding of PtdIns(3)P to the PX domain of p40phox has recently been shown to play an important role in phagosomal oxidase activation during engulfment of serum-opsonized S aureus and IgG-opsonized particles.27,30,31 The extent to which this interaction is important in other contexts of NADPH oxidase activation is unknown.
We set out to establish the relative involvement of class I, II, and III PI3Ks in non–antibody-dependent phagocytosis, phagosomal PtdIns(3)P accumulation, and NADPH oxidase activation in response to uptake of a gram-positive S aureus and a gram-negative E coli by both mouse and human neutrophils and a mouse macrophage cell line.
Methods
Materials
fMLP, luminol, murine granulocyte macrophage–colony-stimulating factor (GM-CSF), and human serum were from Sigma-Aldrich (Dorset, United Kingdom). Murine and human TNF-α were from R&D Systems (Abingdon, United Kingdom). Dulbecco phosphate-buffered saline (PBS) with Ca2+ and Mg2+ was from Sigma-Aldrich (D8662). Tissue culture reagents were from Invitrogen (Paisley, United Kingdom). Class I PI3K isoform–selective inhibitors were as previously described.16 All buffer components were from Sigma-Aldrich and were endotoxin-free or low-endotoxin, as available.
Mouse strains
PI3KδD910A/D910A,32 PI3KC2β−/−,33 FcRγ−/−,34 mice (each on a C57BL/6J background) and CD18−/−,3 PI3Kγ−/−,35 p40phox−/−,31 and gp91phox−/−36 mice (each on a mixed 129/Sv, C57BL/6J background) have been described previously. p40phoxR58A/R58A mice were created through interbreeding of p40phoxR58A/+ mice,27 as described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). In all experiments, mice were compared with appropriate age- and strain-matched wild-type controls. Animals were housed in the small animal barrier unit (SABU) at the Babraham Institute. This work was approved by Home Office Project License PPL 80/1875.
Preparation of cells
RAW264.7 cells stably expressing a green fluorescence protein– (GFP-) tagged probe for PtdIns(3)P (GFP-PX-RAW)22 were maintained at 37°C in a 5% CO2 humidified incubator and harvested by centrifugation (1000g for 5 minutes at room temperature) and resuspension in Dulbecco PBS with Ca2+ and Mg2+, 1 g/L glucose, 4 mM sodium bicarbonate (DPBS+).
Human neutrophils were isolated from the peripheral blood of healthy volunteers (REC approval number 06/Q0108/165), and mature mouse neutrophils were isolated from bone marrow, as described previously.16 Purity was determined by cytospin and REASTAIN Quick-Diff (Reagena, Toivala, Finland) staining, and were at least 95% (human) or 70% to 85% (mouse) pure. After washing, neutrophils were resuspended in DPBS+. Use of human blood for this study was approved by the National Health Service (NHS) Research Ethics Committee.
Preparation of mouse serum
Mouse serum was isolated from either C57BL/6J (normal) or antibody-deficient RAG2/γc37 mice and prepared as previously described.31 Where indicated, serum was heat-inactivated at 56°C for 60 minutes, before opsonization of bacteria. Antibody depletion of serum was performed by incubating 250 μL normal mouse serum with 125 μL protein G sepharose (45 minutes, 4°C, mixing end-on-end).
Preparation of bacterial strains
Bacteria (S aureus Wood 46 and E coli E2348169) were subcultured at 37°C to logarithmic growth from overnight cultures. Bacteria were washed in DPBS+ and opsonized by incubation in DPBS+ with 10% serum (mouse or human as appropriate) at 37°C with end-over-end mixing for 15 minutes, followed by washing in DPBS+. Opsonized bacteria were resuspended in DPBS+ with 10% serum at 5 × 108/mL. Nonopsonized bacteria were prepared in the absence of serum. For some assays, bacteria were labeled with 20 μg/mL RITC (Sigma) before opsonization.27
Measurement of ROS production
Neutrophils (6.25 × 106/mL) were primed with TNFα (200 U/mL human, 1000 U/mL mouse) and GM-CSF (100 ng/mL) for 1 hour at 37°C with occasional gentle mixing. Where indicated, primed neutrophils or GFP-PX-RAW cells were preincubated with PI3K inhibitors or vehicle control (DMSO, 0.1%) for 10 minutes before stimulation. Rate kinetics of intracellular ROS production were measured using a luminol-based assay in polystyrene 96-well plates (no. 23 300; Berthold Technologies, Harpenden, United Kingdom) as described previously.16 Briefly, 5 × 105 cells in DPBS+ were incubated with luminol (150 μM) for 3 minutes, 37°C. Cells were then added manually to prewarmed bacteria (final ratio 1:20), and measurement started immediately. Assays were conducted without horseradish peroxidase (HRP) and thus represent intracellular ROS production (addition of 18.75 U/mL HRP revealed negligible extracellular ROS production). Light emission was recorded by a Berthold Mircolumat Plus luminomter (Berthold Technologies). Data output is relative light units per second (RLU/s) or total RLUs integrated over the indicated periods of time.
RNAi knockdown of class III PI3K
RNAi was performed using Expression Arrest pSM2 retroviral shRNAmir (Open Biosystems, Huntsville, AL), using nonsilencing shRNAmir retroviral control (RHS1707) and 2 independent Vps34RNAi constructs (RMM1766-96740308, RHS1766-96881245) per manufacturer's instructions. Optimal viral titer of 1:1 dilution in media supplemented with 5 μg/mL polybrene was added to GFP-PX-RAW cells seeded into 6-well dishes at 20% confluence. Viral supernatant was removed after 24 hours, and targeted cells selected with 3 μg/mL puromycin. Cells were expanded for at least 2 weeks, replacing media every second day. For maximal superoxide responses, puromycin selection was relieved for 3 days before analysis.
In several experiments GFP-PX-RAW cells, adhered to glass coverslips, were incubated with RITC-labeled, serum-opsonized bacteria for 30 minutes. Cells were washed, fixed in 4% paraformaldehyde, and mounted as previously described.27 GFP-positive endosomes and/or phagosomes and phagocytosed bacteria were visualized using a Zeiss LSM 510 META point-scanning confocal microscope (Carl Zeiss, Obekochen, Germany) using a plan apochromat 63×/1.40 lens. GFP accumulation of specific regions of interest (endosomes, phagosomal membrane) or average intensity of three 3-μm regions of the cell (cytosol) was quantitated using LSM 510 Image browser software.
Western blotting
Neutrophils or GFP-PX-RAW cells (5 × 105) were sonicated into 1× SDS loading buffer, subjected to SDS-PAGE, transferred, and blotted for PI3KC2β (611342; BD Biosciences Transduction Laboratories, Lexington, KY), Vps34 (38-2100; Zymed, South San Francisco, CA), p67phox (07-502; Upstate Biotechnology, Charlottesville, VA), p47phox (07-500; Upstate Biotechnology), p40phox (07-501; Upstate Biotechnology) and/or β-actin (clone AC-15 A5441; Sigma). Signal was detected by ECL and quantified using a Quality One Gel Doc (Bio-Rad Laboratories, Hercules, CA) and Aida Image Analyzer 2.2 software (GmbH).
Results
Neutrophil ROS production in response to phagocytosis of serum-opsonized S aureus and E coli requires complement receptors but is antibody-independent
Rate measurements of intracellular ROS production were performed in human peripheral neutrophils and mouse bone marrow–derived neutrophils, using a luminol-based chemiluminescence assay. Under the conditions described here, detection of ROS by this assay is abolished by the general flavocytochrome inhibitor diphenyleneiodonuim (DPI) and absent in mouse neutrophils derived from gp91phox−/− mice, indicating the ROSs detected are derived from the neutrophil NADPH oxidase complex (Figure S1). This assay was chosen because of its sensitivity and accuracy but is compromised by its dependency on peroxidase activity in the vicinity of the superoxide anions being measured. (During phagocytosis, this is provided by enzymes like myeloperoxidase.) For this reason, key observations were also confirmed, albeit with lower fidelity, using an assay based on direct, O2-reduction of soluble tetrazolium salts to form insoluble formazan deposits (nitroblue tetrazolium [NBT] assay; see Document S1).38
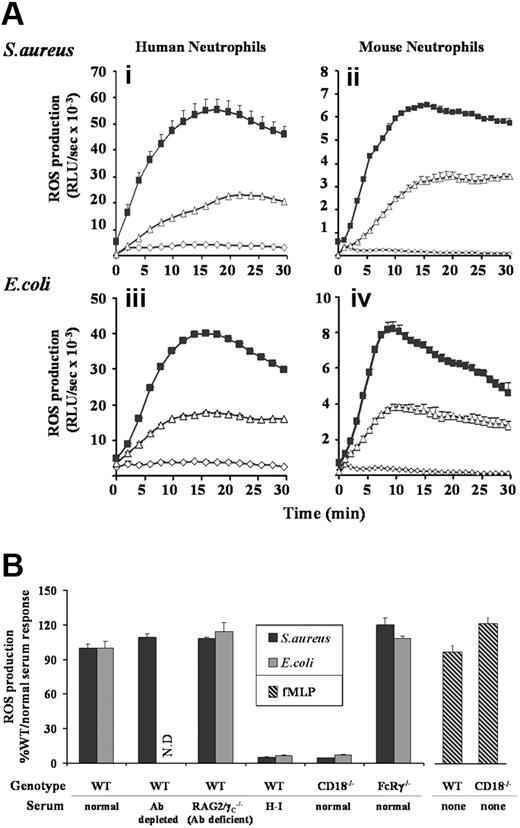
Incubation of TNFα/GM-CSF–primed human neutrophils with S aureus or E coli resulted in substantial ROS generation, peaking between 15 and 20 minutes (Figure 1Ai,iii). Similar kinetics of ROS generation were observed in mouse neutrophils, although overall production was nearly an order of magnitude lower (Figure 1Aii,iv). In the absence of neutrophil priming, rates of ROS generation induced by either S aureus or E coli were slower (peaking at around 30 minutes), although reaching equivalent maximal levels (data not shown). Most of our analyses were subsequently performed with primed neutrophils to mimic more closely the physiologic context, but key observations were repeated with unprimed cells where specifically noted.
ROS generation in human and mouse neutrophils in response to S aureus and E coli. (A) Human peripheral (i,iii), and mouse bone marrow–derived (ii,iv) neutrophils were prepared, primed with TNFα and GM-CSF, and preincubated with luminol as described in “Preparation of cells”, and “Measurement of ROS production.” Cells (5 × 105/well) were added to 107 unopsonized (▵) or serum-opsonized (■) S aureus (i,ii) or E coli (iii,iv), or incubated in the absence of bacteria (◇) and chemiluminesence recorded using a Berthold Microlumat Plus luminometer. Incubations were performed in duplicate, and data (mean ± range) from one experiment representative of 3 are shown and are expressed as relative light units/sec (RLU/s). (B) Primed bone marrow neutrophils derived from C57BL/6J mice (WT) or mice lacking either the β2-integrin common chain CD18 (CD18−/−), or the Fc receptor γ chain (FcRγ−/−) were incubated with S aureus or E coli opsonized with 10% serum generated from C57BL/6J (normal) or RAG2/γC−/− (Ab-deficient) mice. Where indicated, normal serum was depleted of antibody before opsonization, by incubation with protein G sepharose, or heat-inactivated (H-I) at 56°C for 1 hour. Cells (5 × 105/well) were added to 107 serum-opsonized S aureus (■), E coli ( ), or 10 μM fMLP (
), or 10 μM fMLP ( ), and light emission measured over 40 minutes as described in Figure 1A. ROS production in response to E coli opsonized with antibody-depleted serum was not determined (ND). All experiments were performed in duplicate and data (mean ± SEM) are accumulated light emission for a combination of at least 2 experiments, expressed as a percentage of the response in WT mouse neutrophils to 10% normal serum-opsonized bacteria.
), and light emission measured over 40 minutes as described in Figure 1A. ROS production in response to E coli opsonized with antibody-depleted serum was not determined (ND). All experiments were performed in duplicate and data (mean ± SEM) are accumulated light emission for a combination of at least 2 experiments, expressed as a percentage of the response in WT mouse neutrophils to 10% normal serum-opsonized bacteria.
ROS generation in human and mouse neutrophils in response to S aureus and E coli. (A) Human peripheral (i,iii), and mouse bone marrow–derived (ii,iv) neutrophils were prepared, primed with TNFα and GM-CSF, and preincubated with luminol as described in “Preparation of cells”, and “Measurement of ROS production.” Cells (5 × 105/well) were added to 107 unopsonized (▵) or serum-opsonized (■) S aureus (i,ii) or E coli (iii,iv), or incubated in the absence of bacteria (◇) and chemiluminesence recorded using a Berthold Microlumat Plus luminometer. Incubations were performed in duplicate, and data (mean ± range) from one experiment representative of 3 are shown and are expressed as relative light units/sec (RLU/s). (B) Primed bone marrow neutrophils derived from C57BL/6J mice (WT) or mice lacking either the β2-integrin common chain CD18 (CD18−/−), or the Fc receptor γ chain (FcRγ−/−) were incubated with S aureus or E coli opsonized with 10% serum generated from C57BL/6J (normal) or RAG2/γC−/− (Ab-deficient) mice. Where indicated, normal serum was depleted of antibody before opsonization, by incubation with protein G sepharose, or heat-inactivated (H-I) at 56°C for 1 hour. Cells (5 × 105/well) were added to 107 serum-opsonized S aureus (■), E coli ( ), or 10 μM fMLP (
), or 10 μM fMLP ( ), and light emission measured over 40 minutes as described in Figure 1A. ROS production in response to E coli opsonized with antibody-depleted serum was not determined (ND). All experiments were performed in duplicate and data (mean ± SEM) are accumulated light emission for a combination of at least 2 experiments, expressed as a percentage of the response in WT mouse neutrophils to 10% normal serum-opsonized bacteria.
), and light emission measured over 40 minutes as described in Figure 1A. ROS production in response to E coli opsonized with antibody-depleted serum was not determined (ND). All experiments were performed in duplicate and data (mean ± SEM) are accumulated light emission for a combination of at least 2 experiments, expressed as a percentage of the response in WT mouse neutrophils to 10% normal serum-opsonized bacteria.
Opsonization of S aureus or E coli with 10% serum (human or mouse as appropriate) substantially increased both the rate and total production of ROS in both human and mouse neutrophils (Figure 1A). The main serum-derived opsonin receptors of neutrophils are FcγRs, which engage IgGs, and a subgroup of β2-integrins that bind complement fragments.2 Depletion of antibody from serum prior to bacterial opsonization in either human or mouse neutrophil ROS assays or the use of antibody-deficient mouse serum in mouse neutrophil ROS assays had no significant effect (Figure 1B and data not shown). Furthermore, ROS responses to serum-opsonized S aureus and E coli were equivalent in neutrophils derived from wild-type (WT) mice, or mice lacking the γ-chain of their activating FcγRs (FcRγ−/−, Figure 1B). These results suggest that the S aureus– and E coli–induced ROS responses are independent of IgG-FcγR signaling.
Heat inactivation of serum effectively abolished (> 95% inhibition) serum-enhanced ROS production (serum H-I bars in Figure 1B), suggesting a role for heat-labile complement factors in these bacterial responses. Both phagocytosis (data not shown) and ROS production (Figure 1B) were reduced by more than 95% in neutrophils derived from mice lacking the β2-integrin subunit of their major complement receptor (CD18−/−), whereas responses to the soluble ligand fMLP remained unaffected. These results support an important role for complement opsonization in the serum-enhanced responses to S aureus and E coli. Additionally, since the loss of CD18 reduced ROS responses to below the level induced by unopsonized bacteria, β2-integrins appear to act as phagocytic receptors for these bacteria in the absence of serum-derived opsonins.
PI3Ks are involved in S aureus– and E coli–induced neutrophil ROS responses
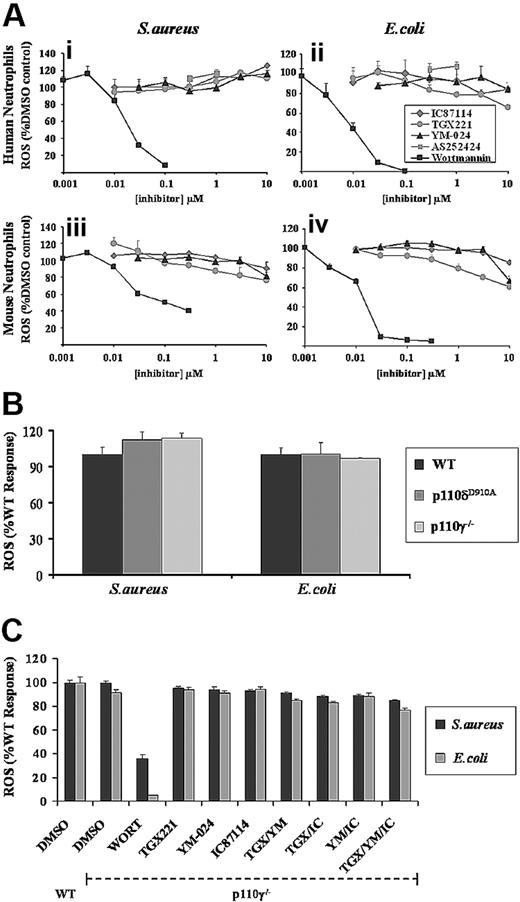
Human or mouse neutrophils were preincubated with increasing concentrations of the general PI3K inhibitor wortmannin before measurement of ROS responses to serum-opsonized E coli or S aureus. Low concentrations of wortmannin inhibited ROS generation in both human and mouse neutrophils in response to either bacterium (Figure 2A), demonstrating strong PI3K involvement. There were, however, some significant differences in the sensitivities of the individual responses to wortmannin; ROS production to E coli in both human and mouse neutrophils was more sensitive to wortmannin than equivalent responses to S aureus and, as noted previously,27 there was a significant wortmannin-insensitive component of the ROS response to S aureus in mouse neutrophils (Figure 2Aiii). These differences in wortmannin sensitivity were preserved in the absence of serum opsonization, suggesting their origin is not directly connected to complement-dependent signaling (data not shown).
Effect of general and class I PI3K isoform–selective inhibitors on S aureus– and E coli–induced ROS formation in neutrophils. (A) Primed human (i,ii) and mouse (iii,iv) neutrophils were preincubated for 10 minutes with wortmannin (■), TGX-221 ( ), IC87114 (gray diamonds), YM-024 (▴), or AS252424 (
), IC87114 (gray diamonds), YM-024 (▴), or AS252424 ( ), as indicated, in the presence of luminol as described in “Measurement of ROS production.” Cells were added to serum-opsonized S aureus (i,iii) or E coli (ii,iv), and ROS responses measured over 40 minutes, as described in Figure 1. Data (mean ± SEM, n ≥ 3) are accumulated light emission, expressed as a percentage of response in the absence of inhibitor (DMSO control). (B) Primed bone marrow neutrophils, derived from wild-type mice (WT, ■) or mice lacking p110γ (p110γ−/−,
), as indicated, in the presence of luminol as described in “Measurement of ROS production.” Cells were added to serum-opsonized S aureus (i,iii) or E coli (ii,iv), and ROS responses measured over 40 minutes, as described in Figure 1. Data (mean ± SEM, n ≥ 3) are accumulated light emission, expressed as a percentage of response in the absence of inhibitor (DMSO control). (B) Primed bone marrow neutrophils, derived from wild-type mice (WT, ■) or mice lacking p110γ (p110γ−/−,  ), or expressing a kinase-dead version of p110δ (p110δD910A,
), or expressing a kinase-dead version of p110δ (p110δD910A,  ) were prepared and ROS generation in response to S aureus or E coli measured as described in Figure 1. Data are mean plus or minus SEM from at least 2 independent experiments performed in duplicate, and expressed as a percentage of response from neutrophils derived from WT mice. (C) Primed bone marrow neutrophils derived from WT or PI3Kγ−/− mice were preincubated with either 100 nM wortmannin, 0.1 μM TGX221 (TGX), 3 μM YM-024 (YM), or 3 μM IC87114 (IC) as indicated, alone or in combination, for 10 minutes before addition of serum-opsonized S aureus (■) or E coli
) were prepared and ROS generation in response to S aureus or E coli measured as described in Figure 1. Data are mean plus or minus SEM from at least 2 independent experiments performed in duplicate, and expressed as a percentage of response from neutrophils derived from WT mice. (C) Primed bone marrow neutrophils derived from WT or PI3Kγ−/− mice were preincubated with either 100 nM wortmannin, 0.1 μM TGX221 (TGX), 3 μM YM-024 (YM), or 3 μM IC87114 (IC) as indicated, alone or in combination, for 10 minutes before addition of serum-opsonized S aureus (■) or E coli ). ROS generation was measured as described in panel A. Data are mean plus or minus SEM (n ≥ 6) and are expressed as a percentage of WT untreated responses.
). ROS generation was measured as described in panel A. Data are mean plus or minus SEM (n ≥ 6) and are expressed as a percentage of WT untreated responses.
Effect of general and class I PI3K isoform–selective inhibitors on S aureus– and E coli–induced ROS formation in neutrophils. (A) Primed human (i,ii) and mouse (iii,iv) neutrophils were preincubated for 10 minutes with wortmannin (■), TGX-221 ( ), IC87114 (gray diamonds), YM-024 (▴), or AS252424 (
), IC87114 (gray diamonds), YM-024 (▴), or AS252424 ( ), as indicated, in the presence of luminol as described in “Measurement of ROS production.” Cells were added to serum-opsonized S aureus (i,iii) or E coli (ii,iv), and ROS responses measured over 40 minutes, as described in Figure 1. Data (mean ± SEM, n ≥ 3) are accumulated light emission, expressed as a percentage of response in the absence of inhibitor (DMSO control). (B) Primed bone marrow neutrophils, derived from wild-type mice (WT, ■) or mice lacking p110γ (p110γ−/−,
), as indicated, in the presence of luminol as described in “Measurement of ROS production.” Cells were added to serum-opsonized S aureus (i,iii) or E coli (ii,iv), and ROS responses measured over 40 minutes, as described in Figure 1. Data (mean ± SEM, n ≥ 3) are accumulated light emission, expressed as a percentage of response in the absence of inhibitor (DMSO control). (B) Primed bone marrow neutrophils, derived from wild-type mice (WT, ■) or mice lacking p110γ (p110γ−/−,  ), or expressing a kinase-dead version of p110δ (p110δD910A,
), or expressing a kinase-dead version of p110δ (p110δD910A,  ) were prepared and ROS generation in response to S aureus or E coli measured as described in Figure 1. Data are mean plus or minus SEM from at least 2 independent experiments performed in duplicate, and expressed as a percentage of response from neutrophils derived from WT mice. (C) Primed bone marrow neutrophils derived from WT or PI3Kγ−/− mice were preincubated with either 100 nM wortmannin, 0.1 μM TGX221 (TGX), 3 μM YM-024 (YM), or 3 μM IC87114 (IC) as indicated, alone or in combination, for 10 minutes before addition of serum-opsonized S aureus (■) or E coli
) were prepared and ROS generation in response to S aureus or E coli measured as described in Figure 1. Data are mean plus or minus SEM from at least 2 independent experiments performed in duplicate, and expressed as a percentage of response from neutrophils derived from WT mice. (C) Primed bone marrow neutrophils derived from WT or PI3Kγ−/− mice were preincubated with either 100 nM wortmannin, 0.1 μM TGX221 (TGX), 3 μM YM-024 (YM), or 3 μM IC87114 (IC) as indicated, alone or in combination, for 10 minutes before addition of serum-opsonized S aureus (■) or E coli ). ROS generation was measured as described in panel A. Data are mean plus or minus SEM (n ≥ 6) and are expressed as a percentage of WT untreated responses.
). ROS generation was measured as described in panel A. Data are mean plus or minus SEM (n ≥ 6) and are expressed as a percentage of WT untreated responses.
Importantly, wortmannin did not inhibit phagocytosis of opsonized S aureus or E coli in either human or mouse neutrophils (Figure S2i), consistent with previous reports that phagocytic uptake of small particles is relatively insensitive to PI3K inhibition.39 Thus, the wortmannin-sensitive, PI3K-dependent components of these bacterial responses are downstream of phagocytosis and most likely reflect direct involvement in the signaling pathways to NADPH oxidase activation.
S aureus– and E coli–induced neutrophil ROS responses are substantially independent of class I PI3K activity
Wortmannin displays similar potency toward all reported classes of PI3K (with the exception of class II PI3KC2α)40 and thus is a poor tool for distinguishing among them. Hence, we assessed the role of class I PI3Ks in S aureus– and E coli–induced ROS production using recently characterized, isoform-selective class I PI3K inhibitors and mouse class I PI3K models.
Pretreatment of human or mouse neutrophils with isoform-selective inhibitors for p110α (YM-024; IC50 18 nM),16 p110β (TGX221; IC50 7 nM),41 or p110δ (IC87114; IC50 500 nM)42 at concentrations up to and beyond their reported IC50s, and which we have previously demonstrated to effectively inhibit fMLP-induced ROS production in human neutrophils,16 failed to significantly inhibit ROS generation induced by serum-opsonized S aureus or E coli (Figure 2A). The p110γ inhibitor (AS252424; IC50 1 μM)16 demonstrated no inhibition at concentrations up to 1 μM (coloration at concentrations above 1 μM affected the luminol-based assay). ROS responses to S aureus and E coli in neutrophils derived from mice lacking the p110γ catalytic subunit (p110γ−/−),35 or possessing a kinase-dead version of p110δ, p110δD910A/D910A (p110δD910A)32 (Figure 2B) were normal.
To investigate whether there is any functional redundancy among class I PI3K isoforms in these responses, we also examined the effects of combining inhibitors at concentrations approximately 10 to 20 times greater than their IC50s against their individual targets, using mouse neutrophils from a WT or p110γ−/− background. Paired combinations of 0.1 μM TGX221, 3 μM IC87114, and 3 μM YM-024, in either the presence or absence of p110γ, had no significant effect on ROS responses in either primed (Figure 2C) or unprimed (Figure S3) neutrophils to either S aureus or E coli. Pretreating p110γ−/− neutrophils with all 3 inhibitors before addition of bacteria resulted in a minor reduction in ROS generation compared with untreated WT neutrophils (Figure 2C), but this was less than 15% of the inhibition induced by low concentrations of wortmannin.
Taken together, these results suggest that S aureus– and E coli–induced ROS responses in both mouse and human neutrophils, under primed or nonprimed conditions, are largely independent of class I PI3Ks.
S aureus– and E coli–induced neutrophil ROS responses are substantially independent of class IIβ PI3K activity
Class IIα and β PI3K isoforms are widely expressed; class IIγ is limited to liver, breast, prostate, and salivary glands.43 The α and β isoforms differ greatly in their sensitivity to wortmannin, with class IIβ displaying inhibition at 2 nM to 30 nM (similar to class I and III PI3Ks), whereas the α isoform requires concentrations greater than 400 nM for significant inhibition.43 Given the observed wortmannin sensitivity of the E coli– and S aureus–induced ROS production described above (Figure 2A, IC50 10-30 nM), it would appear that they do not involve class IIα.
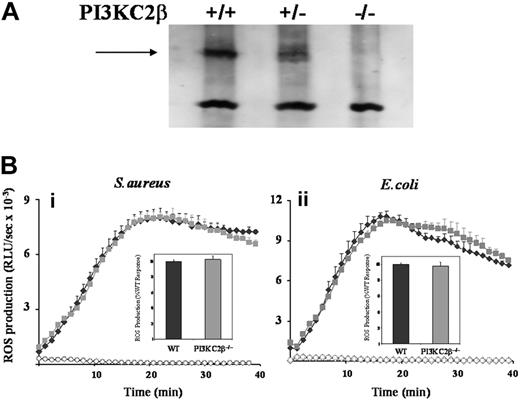
The potential role of class IIβ PI3K was investigated using both primed and unprimed neutrophils derived from mice lacking this isoform (Figure 3A and data not shown).33 S aureus– and E coli–induced ROS responses were the same in class IIβ−/− and WT neutrophils (Figure 3B). Moreover, the wortmannin sensitivities of these responses were similar for both genotypes (data not shown) confirming the lack of involvement of the class IIβ PI3K isoform in the wortmannin-sensitive component of the ROS responses to S aureus and E coli.
Effect of absence of PI3KC2β on neutrophil ROS responses to S aureus and E coli. (A) Bone marrow–derived neutrophils from PI3KC2β+/+, PI3KC2β+/−, and PI3KC2β−/− animals were sonicated into SDS sample buffer, subjected to SDS-PAGE, and immunoblotted for PI3KC2β as described in “Western blotting.” PI3KC2β is indicated by →. (B) Primed bone marrow neutrophils derived from C57BL/6J WT (♦) and PI3KC2β−/− ( ) mice were prepared as described in “Preparation of cells” and ROS production in response to serum-opsonized S aureus (i) and E coli (ii) measured, in the presence of luminol, as described for Figure 1. Open symbols (♦, WT;
) mice were prepared as described in “Preparation of cells” and ROS production in response to serum-opsonized S aureus (i) and E coli (ii) measured, in the presence of luminol, as described for Figure 1. Open symbols (♦, WT;  , PI3KC2β−/−) represent neutrophil ROS generation in the absence of bacterial addition. Shown are mean plus or minus range from duplicate measurements of one representative experiment of 3. Insets show mean plus or minus SEM accumulated light emission over a 40-minute measurement period for combined data, expressed as a percentage of the wild-type neutrophil responses.
, PI3KC2β−/−) represent neutrophil ROS generation in the absence of bacterial addition. Shown are mean plus or minus range from duplicate measurements of one representative experiment of 3. Insets show mean plus or minus SEM accumulated light emission over a 40-minute measurement period for combined data, expressed as a percentage of the wild-type neutrophil responses.
Effect of absence of PI3KC2β on neutrophil ROS responses to S aureus and E coli. (A) Bone marrow–derived neutrophils from PI3KC2β+/+, PI3KC2β+/−, and PI3KC2β−/− animals were sonicated into SDS sample buffer, subjected to SDS-PAGE, and immunoblotted for PI3KC2β as described in “Western blotting.” PI3KC2β is indicated by →. (B) Primed bone marrow neutrophils derived from C57BL/6J WT (♦) and PI3KC2β−/− ( ) mice were prepared as described in “Preparation of cells” and ROS production in response to serum-opsonized S aureus (i) and E coli (ii) measured, in the presence of luminol, as described for Figure 1. Open symbols (♦, WT;
) mice were prepared as described in “Preparation of cells” and ROS production in response to serum-opsonized S aureus (i) and E coli (ii) measured, in the presence of luminol, as described for Figure 1. Open symbols (♦, WT;  , PI3KC2β−/−) represent neutrophil ROS generation in the absence of bacterial addition. Shown are mean plus or minus range from duplicate measurements of one representative experiment of 3. Insets show mean plus or minus SEM accumulated light emission over a 40-minute measurement period for combined data, expressed as a percentage of the wild-type neutrophil responses.
, PI3KC2β−/−) represent neutrophil ROS generation in the absence of bacterial addition. Shown are mean plus or minus range from duplicate measurements of one representative experiment of 3. Insets show mean plus or minus SEM accumulated light emission over a 40-minute measurement period for combined data, expressed as a percentage of the wild-type neutrophil responses.
Class III PI3K is required for maximal ROS responses to S aureus and E coli in model cell lines
A single class III PI3K (Vps34) exists, which directly phosphorylates PtdIns to generate PtdIns(3)P.22 Deletion of this enzyme in mice has not been described, and there are no available class III–selective PI3K inhibitors. Fully differentiated neutrophils are nondividing and difficult to manipulate genetically. We therefore decided to assess the role of class III PI3K in both phagosomal PtdIns(3)P and ROS production in response to S aureus or E coli by an RNAi-mediated knockdown strategy in a model cell line. For most of our analyses, we chose a previously described mouse macrophage-like cell line, RAW264.7, stably expressing a GFP-tagged reporter for PtdIns(3)P (the isolated PX domain of p40phox; GFP-PX-RAW).24
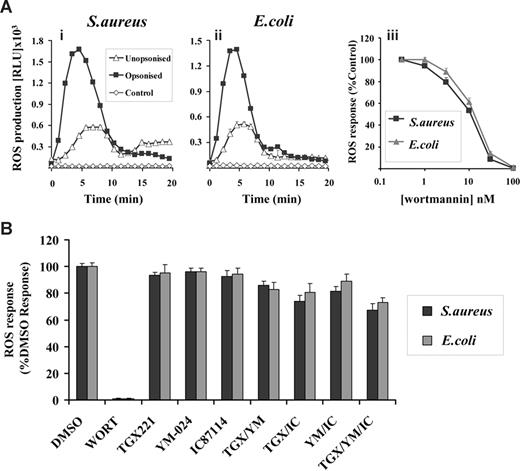
GFP-PX-RAW cells demonstrated clear DPI-sensitive ROS responses to both S aureus and E coli (Figure S1 and Figure 4A) although, as detected by the luminol-dependent chemiluminescence assay, these responses were considerably smaller and more transient than those seen in either human or mouse neutrophils (compare with Figure 1A). This is probably due in part to the relative lack of phagosomal peroxidases in RAW cells, since more equivalent ROS responses were seen between mouse neutrophils and RAW cells using the NBT reduction assay (data not shown). As with the neutrophil responses, both the rate and magnitude of this ROS production increased with serum-opsonization (Figure 4A) and this effect was independent of antibodies but sensitive to serum heat inactivation (data not shown). Further, ROS responses to both S aureus and E coli, but not phagocytosis (Figure S2ii), were inhibited by wortmannin (IC50 15 nM, Figure 4Aiii) and insensitive to class I PI3K-selective inhibitors (Figure 4B). Thus, the GFP-PX-RAW cells used in this study exhibit similar characteristics with respect to S aureus– and E coli–induced ROS responses to human and mouse neutrophils.
Characterization of ROS responses in GFP-PX-RAW cells in response to S aureus and E coli. RAW264.7 cells expressing a GFP-PX probe for PtdIns(3)P (GFP-PX-RAW cells) were prepared as described in “Preparation of cells.” (A) Cells (5 × 105/well) were added to 1 × 107 unopsonized (▵) or serum-opsonized (■) S aureus (i) or E coli (ii), and chemiluminesence recorded as described in Figure 1. ◇ represent ROS generation in the absence of bacterial addition. Incubations were performed in duplicate, and data (mean ± range) from one representative experiment of 3 are shown, and are expressed as relative light units/sec (RLU/s). (iii) GFP-PX-RAW cells were preincubated with 0-100 nM wortmannin in the presence of luminol as described in “Measurement of ROS production.” Cells (5 × 106/well) were added to 107 serum-opsonized S aureus (■) or E coli ( ), and light emission measured over 40 minutes as described in Figure 1. All incubations were performed in duplicate and data from at least 3 experiments (mean ± SEM, n ≥ 6), are accumulated light emission, expressed as a percentage of response in the absence of wortmannin. (B) GFP-PX-RAW cells were preincubated for 10 minutes at 37°C with either DMSO vehicle control or 100 nM wortmannin, 0.1 μM TGX221, 3 μM YM-024, 3 μM IC87114 as indicated, alone or in combination, or in the presence of 1 μM AS252424, before addition of serum-opsonized S aureus (■) or E coli (
), and light emission measured over 40 minutes as described in Figure 1. All incubations were performed in duplicate and data from at least 3 experiments (mean ± SEM, n ≥ 6), are accumulated light emission, expressed as a percentage of response in the absence of wortmannin. (B) GFP-PX-RAW cells were preincubated for 10 minutes at 37°C with either DMSO vehicle control or 100 nM wortmannin, 0.1 μM TGX221, 3 μM YM-024, 3 μM IC87114 as indicated, alone or in combination, or in the presence of 1 μM AS252424, before addition of serum-opsonized S aureus (■) or E coli ( ). ROS generation was measured as described in Figure 1. Data are mean plus or minus SEM (n ≥ 6) and are expressed as a percentage of WT untreated responses.
). ROS generation was measured as described in Figure 1. Data are mean plus or minus SEM (n ≥ 6) and are expressed as a percentage of WT untreated responses.
Characterization of ROS responses in GFP-PX-RAW cells in response to S aureus and E coli. RAW264.7 cells expressing a GFP-PX probe for PtdIns(3)P (GFP-PX-RAW cells) were prepared as described in “Preparation of cells.” (A) Cells (5 × 105/well) were added to 1 × 107 unopsonized (▵) or serum-opsonized (■) S aureus (i) or E coli (ii), and chemiluminesence recorded as described in Figure 1. ◇ represent ROS generation in the absence of bacterial addition. Incubations were performed in duplicate, and data (mean ± range) from one representative experiment of 3 are shown, and are expressed as relative light units/sec (RLU/s). (iii) GFP-PX-RAW cells were preincubated with 0-100 nM wortmannin in the presence of luminol as described in “Measurement of ROS production.” Cells (5 × 106/well) were added to 107 serum-opsonized S aureus (■) or E coli ( ), and light emission measured over 40 minutes as described in Figure 1. All incubations were performed in duplicate and data from at least 3 experiments (mean ± SEM, n ≥ 6), are accumulated light emission, expressed as a percentage of response in the absence of wortmannin. (B) GFP-PX-RAW cells were preincubated for 10 minutes at 37°C with either DMSO vehicle control or 100 nM wortmannin, 0.1 μM TGX221, 3 μM YM-024, 3 μM IC87114 as indicated, alone or in combination, or in the presence of 1 μM AS252424, before addition of serum-opsonized S aureus (■) or E coli (
), and light emission measured over 40 minutes as described in Figure 1. All incubations were performed in duplicate and data from at least 3 experiments (mean ± SEM, n ≥ 6), are accumulated light emission, expressed as a percentage of response in the absence of wortmannin. (B) GFP-PX-RAW cells were preincubated for 10 minutes at 37°C with either DMSO vehicle control or 100 nM wortmannin, 0.1 μM TGX221, 3 μM YM-024, 3 μM IC87114 as indicated, alone or in combination, or in the presence of 1 μM AS252424, before addition of serum-opsonized S aureus (■) or E coli ( ). ROS generation was measured as described in Figure 1. Data are mean plus or minus SEM (n ≥ 6) and are expressed as a percentage of WT untreated responses.
). ROS generation was measured as described in Figure 1. Data are mean plus or minus SEM (n ≥ 6) and are expressed as a percentage of WT untreated responses.
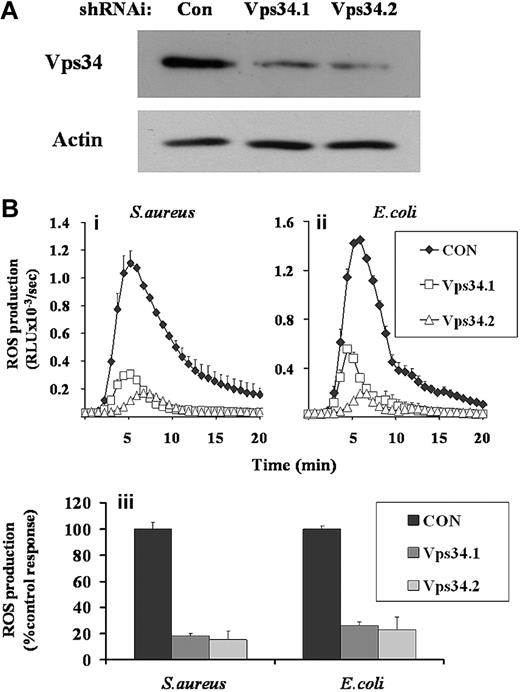
GFP-PX-RAW cells were infected with retroviruses expressing shRNAi directed against 2 separate regions of class III PI3K (Vps34.1 and Vps34.2) and a nonsilencing control (CON). Western blot analysis demonstrated significant knockdown of class III PI3K expression in both Vps34shRNAi cell populations (Figure 5A, Vps34.1, 32%, and Vps34.2, 20% of control expression). Phagocytosis of S aureus and E coli by Vps34shRNAi and control GFP-PX-RAW cells were equivalent (Figure S2iii). However, ROS generation in Vps34shRNAi cell populations in response to either S aureus or E coli were significantly impaired compared with CONshRNAi populations (Figure 5B), indicating a significant role for class III PI3K in these responses. These reductions in ROS responses measured by luminol-dependent chemiluminescence were mirrored by parallel reductions in phagosomal ROS measured by the NBT assay (data not shown), suggesting they are driven by lowered NADPH oxidase activity and not peroxidase availability.
Effect of RNAi-mediated knock-down of class III PI3K expression on ROS responses to S aureus and E coli in GFP-PX-RAW cells. GFP-PX-RAW cells were infected with retroviral constructs directing expression of nonsilencing control shRNAi (CON), or 2 independent class III PI3K shRNAi constructs (Vps34.1, Vps34.2), as described in “RNAi knockdown of class III PI3K.” (A) shRNAi-treated GFP-PX-RAW cells were harvested, sonicated into SDS sample buffer, subjected to SDS-PAGE, and immunoblotted for class III PI3K and, as a loading control, actin. (B) Control (♦) and 2 Vps34 (□ and ▵) shRNAi GFP-PX-RAW cell populations were harvested, incubated with luminol, and ROS generation measured over time in response to serum-opsonized S aureus (i) or E coli (ii) as described in Figure 1. Data shown are mean plus or minus range for duplicate measurements in one representative experiment of 3. (iii) Total accumulated superoxide production for control and Vps34shRNAi GFP-PX-RAW cells in response to S aureus and E coli from 4 independently derived cell populations, was calculated over a 20-minute measurement period. Data are mean plus or minus SEM.
Effect of RNAi-mediated knock-down of class III PI3K expression on ROS responses to S aureus and E coli in GFP-PX-RAW cells. GFP-PX-RAW cells were infected with retroviral constructs directing expression of nonsilencing control shRNAi (CON), or 2 independent class III PI3K shRNAi constructs (Vps34.1, Vps34.2), as described in “RNAi knockdown of class III PI3K.” (A) shRNAi-treated GFP-PX-RAW cells were harvested, sonicated into SDS sample buffer, subjected to SDS-PAGE, and immunoblotted for class III PI3K and, as a loading control, actin. (B) Control (♦) and 2 Vps34 (□ and ▵) shRNAi GFP-PX-RAW cell populations were harvested, incubated with luminol, and ROS generation measured over time in response to serum-opsonized S aureus (i) or E coli (ii) as described in Figure 1. Data shown are mean plus or minus range for duplicate measurements in one representative experiment of 3. (iii) Total accumulated superoxide production for control and Vps34shRNAi GFP-PX-RAW cells in response to S aureus and E coli from 4 independently derived cell populations, was calculated over a 20-minute measurement period. Data are mean plus or minus SEM.
We also attempted to confirm some of the key observations made in the GFP-PX-RAW cells using the human myeloid-derived cell line PLB-985. Both phagocytosis and ROS production in differentiated PLB-985 cells (0.5% N,N-dimethylformamide in cell media for 6 days; see Document S1) challenged with S aureus or E coli showed very similar sensitivities to wortmannin and class I PI3K inhibitors as the equivalent responses in RAW cells and neutrophils described above (data not shown). Furthermore, we were able to generate populations of differentiated PLB-985 cells with modest reductions in the expression of class III PI3K using lentiviral-mediated delivery of control- and Vps34-directed shRNAi (Figure S4A) and these populations exhibited parallel reductions in ROS responses to S aureus and E coli (Figure S4B), supporting a conserved role for class III PI3K in their regulation.
Class III PI3K is required for PtdIns(3)P synthesis on S aureus and E coli phagosomes
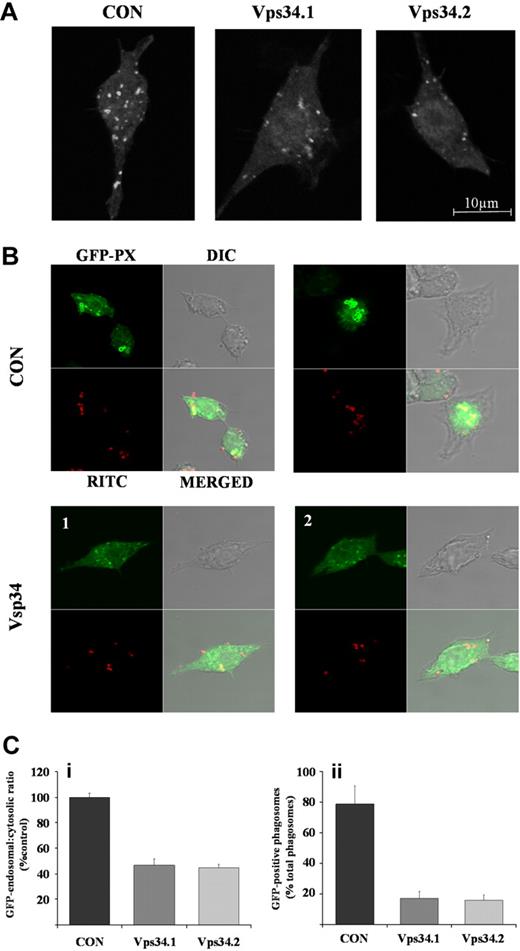
As previously described,24 basal distribution of the GFP-PX, PtdIns(3)P-probe in GFP-PX-RAW cells was partially cytosolic and partially distributed in vesicular, endosomal structures. Uptake of S aureus or E coli resulted in strong phagosomal accumulation of the GFP-PX probe, which could be completely inhibited by wortmannin pretreatment (data not shown). Quantitation of more than 100 phagocytic events demonstrated that approximately 75% of S aureus taken up by the cells during the first 7 minutes was surrounded by GFP-positive phagosomes.
The distributions of the GFP-PX probe in CON- or Vps34shRNAi cells, in the absence (Figure 6A) or presence (Figure 6B) of RITC-labeled S aureus, were carefully quantified by confocal microscopy (Figure 6C). In the absence of bacteria, CONshRNAi cells displayed similar cytosolic and vesicular distributions of the GFP-PX probe compared with uninfected GFP-PX-RAW cells (data not shown). In contrast, Vps34shRNAi cells exhibited an approximately 50% reduction in the ratio of vesicular to cytosolic GFP-fluorescence (Figure 6Ci), indicating knockdown of class III PI3K expression results in effective reduction of vesicular PtdIns(3)P levels.
Effect of RNAi-mediated knock-down of class III PI3K expression on endosomal and phagosomal PtdIns(3)P accumulation. GFP-PX-RAW cells expressing nonsilencing control (CON) or independent class III PI3K (Vps34.1, Vps34.2) shRNAi were adhered to glass coverslips and incubated in the absence (A) or presence (B) of RITC-labeled, serum-opsonized S aureus as described in “RNAi knockdown of class III PI3K.” Samples were fixed, mounted, and GFP-positive endosomes (A) and phagosomes (B) visualized on a Zeiss LSM 510 META point-scanning microscope. Shown are images from a single 1 μm confocal plane. (C) GFP-endosomal and cytosolic accumulation (i) and GFP-positive phagosomes (ii) from cells described in panels A and B, respectively, were quantified from 6 × 1 μm z-section confocal images using LSM 510 software. Data are mean plus or minus SEM for at least 20 cells, or 100 phagocyte events in control (■) and Vps34.1 ( ), Vps34.2 (
), Vps34.2 ( ) shRNAi-treated cells, and are expressed as endosomal GFP accumulation as a ratio of cytosolic GFP accumulation in subpanel i or GFP-positive phagosomes expressed as a percentage of total phagosomes in subpanel ii.
) shRNAi-treated cells, and are expressed as endosomal GFP accumulation as a ratio of cytosolic GFP accumulation in subpanel i or GFP-positive phagosomes expressed as a percentage of total phagosomes in subpanel ii.
Effect of RNAi-mediated knock-down of class III PI3K expression on endosomal and phagosomal PtdIns(3)P accumulation. GFP-PX-RAW cells expressing nonsilencing control (CON) or independent class III PI3K (Vps34.1, Vps34.2) shRNAi were adhered to glass coverslips and incubated in the absence (A) or presence (B) of RITC-labeled, serum-opsonized S aureus as described in “RNAi knockdown of class III PI3K.” Samples were fixed, mounted, and GFP-positive endosomes (A) and phagosomes (B) visualized on a Zeiss LSM 510 META point-scanning microscope. Shown are images from a single 1 μm confocal plane. (C) GFP-endosomal and cytosolic accumulation (i) and GFP-positive phagosomes (ii) from cells described in panels A and B, respectively, were quantified from 6 × 1 μm z-section confocal images using LSM 510 software. Data are mean plus or minus SEM for at least 20 cells, or 100 phagocyte events in control (■) and Vps34.1 ( ), Vps34.2 (
), Vps34.2 ( ) shRNAi-treated cells, and are expressed as endosomal GFP accumulation as a ratio of cytosolic GFP accumulation in subpanel i or GFP-positive phagosomes expressed as a percentage of total phagosomes in subpanel ii.
) shRNAi-treated cells, and are expressed as endosomal GFP accumulation as a ratio of cytosolic GFP accumulation in subpanel i or GFP-positive phagosomes expressed as a percentage of total phagosomes in subpanel ii.
In the presence of S aureus, CONshRNAi cells showed the expected, dramatic accumulation of GFP-PX probe around S aureus–containing phagosomes and associated drop in cytosolic GFP-fluorescence (Figure 6B and data not shown). In marked contrast, Vps34shRNAi cells showed much reduced accumulation of the GFP-PX probe around the phagosome with less than 20% of engulfed, RITC-labeled S aureus surrounded by a GFP-positive phagosome (Figure 6B,Cii). Similar results were observed in Vps34shRNAi cells after phagocytosis of serum-opsonized, RITC-labeled E coli (data not shown).
These results strongly suggest that class III PI3K plays a key role in regulating the accumulation of PtdIns(3)P in phagosomes created by uptake of serum-opsonized S aureus or E coli.
PtdIns(3)P binding to the PX domain of p40phox contributes to the regulation of neutrophil ROS production in response to S aureus and E coli
The results described here suggest that class III PI3K–generated PtdIns(3)P is responsible for most, perhaps all, of the wortmannin-sensitive component of phagosomal ROS produced in response to uptake of serum-opsonized S aureus and E coli. Previous studies have demonstrated a role for direct interaction of PtdIns(3)P with the core NADPH oxidase component p40phox in several contexts of ROS production, including uptake of S aureus by mouse neutrophils.27,30,44-46 We therefore sought to establish the extent to which the effects of class III–generated PtdIns(3)P on oxidase regulation may be explained by this interaction.
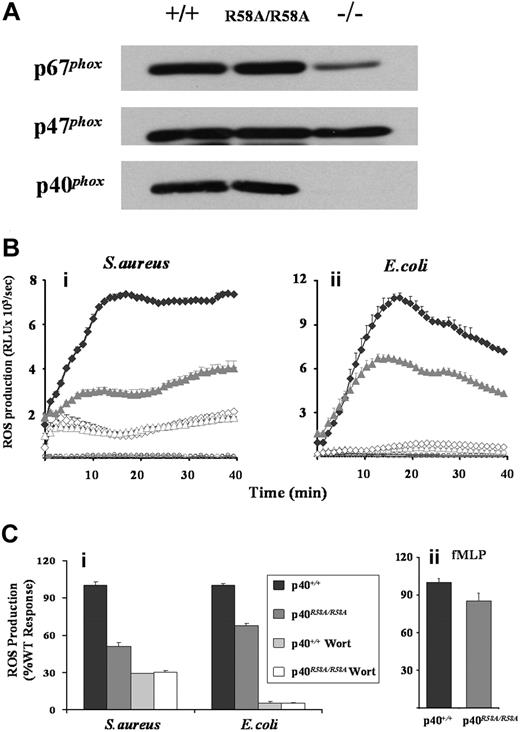
ROS production was measured in neutrophils derived from mice carrying a homozygous mutation in the PX domain of p40phox (p40phoxR58A/R58A), which prevents high-affinity binding to PtdIns(3)P. This mutation did not affect NADPH oxidase subunit expression, in contrast to significantly reduced p67phox expression when p40phox is absent (Figure 7A).31 While ROS responses to fMLP were only mildly inhibited by the R58A mutation (< 15%, Figure 7Cii), ROS generated in response to either S aureus or E coli was substantially reduced (50% and 30% overall inhibitions, respectively, Figure 7B,Ci). As previously noted, in a comparison between S aureus responses in p40phoxR58A/− versus p40phox+/− neutrophils,27 this R58A sensitivity represents a large proportion (approximately 70%) of the wortmannin-sensitive component of the S aureus response. However, the R58A-sensitive component of the ROS response to E coli represented a much smaller proportion (approximately 30%) of the wortmannin-sensitive response. This clearly suggests there are likely to be additional p40phox PX domain–independent roles for PtdIns(3)P in the regulation of phagosomal ROS production. These additional roles are unlikely to include the relatively trivial explanation that PtdIns(3)P regulates delivery of peroxidase-containing granules to the phagosome, affecting the peroxidase-dependent luminol assay, because analogous reductions in ROS production were seen using the NBT assay (Figure S5).
Effects of R58A mutation in p40phox on mouse neutrophil ROS responses. (A) Bone marrow neutrophils derived from wild-type (p40phox+/+), p40phoxR58A/R58A, or p40phox−/− animals were sonicated into SDS sample buffer, subjected to SDS-PAGE, and immunoblotted for p40phox, p47phox, and p67phox as described in “Western blotting.” (Bi,ii and Ci). Primed bone marrow neutrophils from p40phox+/+ (♦) and p40phoxR58A/R58A ( ) mice were prepared and preincubated with luminol in the absence (solid symbols) or presence (open symbols) of 100 nM wortmannin, as described in “Preparation of cells” and “Measurement of ROS production.” Cells (5 × 105/well) were added to 107 serum-opsonized S aureus (Bi) or E coli (Bii), and light emission measured over 40 minutes as described in Figure 1. (Bi,ii) Open circles represent ROS production in the absence of bacteria for p40phox+/+ (black) and p40phoxR58A/R58A (gray) neutrophils. Rate measurements of ROS production (mean ± range) from a single experiment representative of 3. (Ci) Accumulated light emissions over the 40-minute measurement period combined from 3 individual experiments and expressed as a percentage of WT responses (mean ± SEM). (Cii) Primed bone marrow neutrophils from p40phox+/+ and p40phoxR58A/R58A mice were incubated, in the presence of luminol and HRP (18.75 U/mL), with fMLP (10 μM final) and ROS production measured as described in Figure 1, except light emission was recorded for 3 minutes. Data presented are accumulated light emissions from 3 independent experiments (mean ± SEM).
) mice were prepared and preincubated with luminol in the absence (solid symbols) or presence (open symbols) of 100 nM wortmannin, as described in “Preparation of cells” and “Measurement of ROS production.” Cells (5 × 105/well) were added to 107 serum-opsonized S aureus (Bi) or E coli (Bii), and light emission measured over 40 minutes as described in Figure 1. (Bi,ii) Open circles represent ROS production in the absence of bacteria for p40phox+/+ (black) and p40phoxR58A/R58A (gray) neutrophils. Rate measurements of ROS production (mean ± range) from a single experiment representative of 3. (Ci) Accumulated light emissions over the 40-minute measurement period combined from 3 individual experiments and expressed as a percentage of WT responses (mean ± SEM). (Cii) Primed bone marrow neutrophils from p40phox+/+ and p40phoxR58A/R58A mice were incubated, in the presence of luminol and HRP (18.75 U/mL), with fMLP (10 μM final) and ROS production measured as described in Figure 1, except light emission was recorded for 3 minutes. Data presented are accumulated light emissions from 3 independent experiments (mean ± SEM).
Effects of R58A mutation in p40phox on mouse neutrophil ROS responses. (A) Bone marrow neutrophils derived from wild-type (p40phox+/+), p40phoxR58A/R58A, or p40phox−/− animals were sonicated into SDS sample buffer, subjected to SDS-PAGE, and immunoblotted for p40phox, p47phox, and p67phox as described in “Western blotting.” (Bi,ii and Ci). Primed bone marrow neutrophils from p40phox+/+ (♦) and p40phoxR58A/R58A ( ) mice were prepared and preincubated with luminol in the absence (solid symbols) or presence (open symbols) of 100 nM wortmannin, as described in “Preparation of cells” and “Measurement of ROS production.” Cells (5 × 105/well) were added to 107 serum-opsonized S aureus (Bi) or E coli (Bii), and light emission measured over 40 minutes as described in Figure 1. (Bi,ii) Open circles represent ROS production in the absence of bacteria for p40phox+/+ (black) and p40phoxR58A/R58A (gray) neutrophils. Rate measurements of ROS production (mean ± range) from a single experiment representative of 3. (Ci) Accumulated light emissions over the 40-minute measurement period combined from 3 individual experiments and expressed as a percentage of WT responses (mean ± SEM). (Cii) Primed bone marrow neutrophils from p40phox+/+ and p40phoxR58A/R58A mice were incubated, in the presence of luminol and HRP (18.75 U/mL), with fMLP (10 μM final) and ROS production measured as described in Figure 1, except light emission was recorded for 3 minutes. Data presented are accumulated light emissions from 3 independent experiments (mean ± SEM).
) mice were prepared and preincubated with luminol in the absence (solid symbols) or presence (open symbols) of 100 nM wortmannin, as described in “Preparation of cells” and “Measurement of ROS production.” Cells (5 × 105/well) were added to 107 serum-opsonized S aureus (Bi) or E coli (Bii), and light emission measured over 40 minutes as described in Figure 1. (Bi,ii) Open circles represent ROS production in the absence of bacteria for p40phox+/+ (black) and p40phoxR58A/R58A (gray) neutrophils. Rate measurements of ROS production (mean ± range) from a single experiment representative of 3. (Ci) Accumulated light emissions over the 40-minute measurement period combined from 3 individual experiments and expressed as a percentage of WT responses (mean ± SEM). (Cii) Primed bone marrow neutrophils from p40phox+/+ and p40phoxR58A/R58A mice were incubated, in the presence of luminol and HRP (18.75 U/mL), with fMLP (10 μM final) and ROS production measured as described in Figure 1, except light emission was recorded for 3 minutes. Data presented are accumulated light emissions from 3 independent experiments (mean ± SEM).
Discussion
Phagocytosis of S aureus or E coli by both human and mouse neutrophils in vitro induced a robust, intracellular ROS response. Serum opsonization increased the initial rate of these responses approximately 2-fold. This effect was independent of serum-derived antibodies and probably dependent on the deposition of heat-labile complement factors. In mouse neutrophils, both phagocytosis and ROS production, in the presence or absence of serum, were dependent on CD18, the β2-integrin common chain and independent of the FcR γ-chain. There is now strong in vivo evidence that CD18-dependent mechanisms are critical for the efficient removal and destruction of bacteria by our innate immune system, including strains of S aureus and E coli.3-5
CD18-dependent phagocytosis of S aureus and E coli was insensitive to inhibition by wortmannin at concentrations expected to inhibit class I, class IIβ, and class III PI3Ks. This differs with a significant body of work indicating class I PI3Ks play an important role in the phagocytosis of IgG-opsonized particles, particularly when the particle is large.19-21,39,47 Several lines of evidence now suggest that this difference may relate to the different extents to which class I PI3K–dependent actin rearrangement and membrane extension is used during these distinct modes of phagocytosis.48 However, the CD18-dependent ROS production in response to both S aureus and E coli, in both human and mouse neutrophils, was potently inhibited by wortmannin, indicating that, while wortmannin-sensitive PI3Ks do not regulate phagocytosis under these conditions, they are involved in the signaling pathways regulating activation of the NADPH oxidase.
The insensitivity of the S aureus and E coli ROS responses, in both human and mouse neutrophils, to a variety of class I PI3K isoform-selective inhibitors, used either alone, in combination, or together with mouse models lacking PI3Kγ or δ activity, indicate they are very substantially independent of all class I PI3K activity. This is a surprise, given the clear importance of this signaling pathway in both GPCR- and FcR-driven activation of the oxidase in neutrophils and macrophages.16-18,49,50 It is also a surprise given that integrin-mediated adhesion and spreading on a matrix stimulates the neutrophil oxidase via a class I PI3K-dependent mechanism51 (data not shown). Recently it has been shown that CD18-dependent signaling to the oxidase during matrix adhesion requires the FcR γ-chain.52 Thus, there appears to be a significant and interesting difference in the signaling pathways used by CD18 integrins with respect to the use of both the FcR γ-chain and class I PI3Ks, depending on the context in which they are operating (ie, adhesion versus phagocytosis). It is also important to note that there is currently a great deal of interest within the pharmaceutical sector in developing novel therapies based on isoform-selective, class I PI3K inhibitors, particularly anti-PI3Kα agents in oncology and anti-PI3Kγ anti-PI3Kδ agents in inflammation.40,53-55 The results presented here suggest there is no a priori reason to predict these agents will inhibit nonantibody-mediated phagocytosis and killing of bacterial pathogens.
ROS production in response to S aureus or E coli was normal in class IIβ−/− neutrophils, effectively ruling out this enzyme in these responses, at least in the mouse. This leaves the single class III PI3K isoform as the most likely candidate for a PI3K mediating the wortmannin sensitivity of ROS responses to these bacteria. shRNAi-mediated reduction in the expression of class III PI3K in RAW 264.7 cells substantially reduced both phagosomal accumulation of a GFP-PtdIns(3)P reporter and ROS production in response to both serum-opsonized S aureus or E coli. In addition, shRNAi-mediated reduction in the expression of class III PI3K in differentiated PLB-985 cells also caused parallel reductions in ROS responses to these bacteria. Furthermore, the similar sensitivities of ROS responses to serum opsonization, wortmannin, and class I-selective PI3K inhibitors between RAW cells, PLB-985 cells, mouse and human neutrophils, together with the data described above, provides strong evidence that class III PI3K indeed plays a major role in CD18-dependent ROS responses to S aureus and E coli in neutrophils.
Previous work has implicated class III PI3K in phagosomal PtdIns(3)P production during FcR-mediated uptake of IgG-latex beads in CHO cells heterologously expressing FcγRIIa,20 suggesting that class III PI3K may be a common route for PtdIns(3)P synthesis in phagosomes generated by distinct uptake pathways. The mechanisms coupling phagocytic receptors to activation of class III PI3K are unknown but could in principle involve direct recruitment of class III PI3K from the cytosol to the formed phagosome or indirect recruitment via the docking of class III PI3K–enriched endosomal compartments. Good circumstantial evidence suggests that the small GTPase Rab5 may be involved in the recruitment mechanism since it is known to be both a key player in early phagosomal maturation56 and to bind directly to class III PI3K.25
Given the number of effectors in intracellular trafficking that specifically bind PtdIns(3)P,23,57 blocking its synthesis by reducing the expression of class III PI3K could, in principle, reduce phagosomal ROS responses by several direct or indirect routes. Previous work has suggested that the binding of PtdIns(3)P to the PX domain of p40phox is important for regulation of the oxidase in several different contexts of cell stimulation.27,30,44-46 We had previously created a mouse strain carrying an R58A knock-in mutation in the PX domain of p40phox to directly assess the significance of this interaction but, unfortunately, high rates of lethality among p40phoxR58A/R58A embryos forced a comparison between p40phoxR58A/− and p40phox+/− neutrophils.27 However, further breeding against a C57BL/6J background has now enabled us to create matched cohorts of p40phoxR58A/R58A and p40phox+/+ mice and allowed a direct comparison of neutrophil ROS responses between them. Results using these neutrophils strengthen our previous conclusion that PtdIns(3)P binding to the PX domain of p40phox can largely explain the wortmannin-sensitivity of the ROS response to S aureus but, surprisingly, also indicate that this interaction cannot effect more than approximately 30% of the wortmannin-sensitive ROS response to E coli. This then provides a natural explanation for why the ROS responses to these 2 bacteria differ in their wortmannin sensitivity and brings sharply into focus the notion that PtdIns(3)P must also regulate ROS formation via p40phox PX domain-independent routes that are yet to be elucidated, these might include, for example, PtdIns(3)P-dependent membrane fusion events required for optimal NADPH oxidase assembly and activation.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sandra Dye, Fiona Kemp, and Caro Wilson from the Small Animal Barrier Unit at the Babraham Institute for assistance with importing and care of animals. We also thank Geoff Morgan and Arther Davis for assistance in FACS analysis and sorting, Chris Wilton for valuable assistance, and Michael Fry for helpful discussions. PI3KC2β−/− mice and gp91phox−/− mice were kindly provided by Amy Truong and Paul Khavair, Stanford University (Stanford, CA), and Dr Ajay Shah, King's College London, respectively.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (BBS/B/01979) UK, Medical Research Council (Grant ID 79003, reference G0600840) UK, and British Lung Foundation (POS/1). T.C. is a UCB BBSRC-CASE award student.
Authorship
Contribution: K.E.A. designed and performed research, analyzed and interpreted data, wrote the manuscript; K.B., K.D., T.C., and S.K. performed research; G.J., A.S., K.S.-K., and O.R. contributed vital reagents; and L.R.S. and P.T.H. designed experiments, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Phillip Hawkins, The Inositide Laboratory Babraham Institute, Babraham Research Campus, Cambridge CB22 3AT, United Kingdom; e-mail: phillip.hawkins@bbsrc.ac.uk.
References
Author notes
*L.R.S. and P.T.H. contributed equally to this article.