Abstract
Microvascular endothelial cell (MVEC) injury coupled to progression of platelet microthrombi facilitated by ADAMTS13 deficiency is characteristic of idiopathic and HIV-linked thrombotic thrombocytopenic purpura (TTP). Cytokines capable of inducing MVEC apoptosis in vitro are up-regulated in both TTP and HIV infection. However, the concentrations of these cytokines required to elicit EC apoptosis in vitro are 2- to 3-log–fold greater than present in patient plasmas. We report that clinically relevant levels of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) and interferon (IFN)–γ act in synergy to induce apoptosis in dermal MVECs, but have no effect on large-vessel ECs or pulmonary MVECs. This reflects the tissue distribution of TTP lesions in vivo. Sensitivity to TTP plasma or TRAIL plus IFN-γ is paralleled by enhanced ubiquitination of the caspase-8 regulator cellular FLICE-like inhibitory protein (c-FLIP), targeting it for proteasome degradation. c-FLIP silencing with anti-FLIP short interfering RNA (siRNA) in pulmonary MVECs rendered them susceptible to TTP plasma– and cytokine-mediated apoptosis, while up-regulation of c-FLIP by gene transfer partially protected dermal MVECs from such injury. TTP plasma–mediated apoptosis appears to involve cytokine-induced acceleration of c-FLIP degradation, sensitizing cells to TRAIL-mediated caspase-8 activation and cell death. Suppression of TRAIL or modulation of immunoproteasome activity may have therapeutic relevance in TTP.
Introduction
A variety of injuries to microvascular endothelial cells (MVECs) may precipitate episodes of thrombotic thrombocytopenic purpura (TTP) in the setting of deficiency of ADAMTS13 von Willebrand factor (VWF)–cleaving protease activity.1 However, the specific factors initiating MVEC injury and the mechanism of their lineage specificity are unknown. Autopsy and biopsy studies support the argument that EC lesions characteristic of TTP are primary events, not secondary to microthrombi formation.2 This injury appears to be apoptotic and restricted to certain lineages of ECs; large vessels are never involved, nor are pulmonary MVECs.3,4 These pathologic distinctions can be reproduced in vitro by exposure of primary human ECs to plasmas from patients with acute TTP.4
Cytokines linked to MVEC apoptosis and procoagulant phenotype in vitro are dysregulated during acute TTP in vivo. These include tumor necrosis factor (TNF)–α and interleukin (IL)–1.5 TNF-α and IL-1 also induce release of ultralarge VWF multimers from MVECs,6,7 another hallmark of TTP.1 Plasma levels of TNF-α and IL-1 are increased at the onset of disease, with normalization following TTP treatment by plasma exchange.6,8 In other types of thrombotic microangiopathy, including diarrhea/shigatoxin-associated (D+) hemolytic uremic syndrome (HUS), significant alterations in these cytokines have not been documented.9,10 However, levels of IL-1 or TNF-α required to induce MVEC apoptosis in vitro are 2- to 3-log–fold greater than those present in TTP plasma.
TNF-α, a related cytokine, TNF-related apoptosis-inducing ligand (TRAIL), and another soluble factor up-regulated during the acute phase of TTP, interferon (IFN)–γ,8 can interact to elicit apoptosis in several cell types.11,12 Although the levels required still far exceed those present in TTP, such synergy studies offer insight into modulation of cell-specific cytoprotective pathways that might underlie the differential sensitivity of divergent ECs to injury in TTP.
For example, EC viability depends upon several interdependent pathways involving proangiogenic, integrin, and VE-cadherin–associated molecules.13 These pathways are regulated by NF-κB, Akt, and other signaling mechanisms acting through cellular FLICE-like inhibitory protein (c-FLIP), inhibitors of apoptosis (IAP), and Bcl-2.13,14 Some of these systems may be involved in the EC injury of D+ HUS, for which the shiga-like toxins are etiologic agents. Shigatoxins down-regulate Bcl-215 and c-FLIP,16 sensitizing ECs to lipopolysaccharide-induced apoptosis.15 TNF-α and IFN-γ can facilitate the EC apoptosis-inducing activity of shigatoxin in vitro by up-regulation of shigatoxin receptor CD7717 and dephosphorylation and subsequent acceleration of ubiquitin-dependent degradation of Bcl-2 in the proteasome.18 Yet, levels of TNF-α and IFN-γ required to effect those changes—100 ng/mL TNF-α and 200 to 1000 U/mL (20-100 ng/mL) IFN-γ15,18 —still far exceed those found in D+ HUS or TTP.
We had explored the involvement of Bcl-2, Fas, and TNF-α in TTP plasma–associated EC apoptosis. We found that retroviral-mediated transfer of genes for bcl-XL or the bcl-2 homolog A1 into TTP plasma–susceptible MVECs blocked the ability of plasma from patients with idiopathic or HIV-associated TTP to induce apoptosis in these cells.19 Neither Fas nor TNF-α appeared to be involved.20 We now turn to other potential apoptosis-inducing factors in TTP plasmas, and to cytoprotective mechanisms upstream of Bcl. We hypothesized that interactions of IFN-γ with TRAIL, at clinically relevant concentrations, subserve the apoptosis-inducing activity of TTP plasma in susceptible lineages of ECs via modulation of c-FLIP, sensitizing these cells to TRAIL-mediated apoptosis. Pharmacologic suppression of just one of these interacting cytokines might influence the clinical course of TTP in at least some patients.
Methods
Patients
This study was approved by the Institutional Review Board of New York Presbyterian Hospital–Cornell. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Plasmas were obtained from citrated venous blood of 8 healthy controls and 7 patients with acute idiopathic TTP. All individuals were serologically negative for HIV-1 and -2, were not pregnant, and had no clinical evidence of systemic lupus erythematosus. All patients had schistocytes on peripheral blood smear. In addition, TTP was diagnosed according to one or more of the following criteria: fever: unexplained oral temperature higher than 38°C; neurologic dysfunction: any new abnormality on general medical neuropsychiatric examination; renal dysfunction: serum creatinine greater than 120 μM or greater than 150% of previous baseline; and platelet counts lower than 80 × 109/L. The renal and platelet abnormalities were present in all patients, while mental status changes occurred in 4 patients and fever in 5 patients. All patients had an initial complete remission following plasma exchange.
EC culture and confirmation of cell origin
Primary human MVECs of dermal and pulmonary origin were purchased from ScienCell Research Labs (San Diego, CA) and Clonetics (San Diego, CA). Human umbilical vein ECs (HUVECs) were isolated as described by our laboratory.19 The identity of all ECs had been confirmed by phenotypic and genotypic analyses, as described over the past decade by our laboratory. HUVECs and all MVECs expressed VWF and PECAM-1 by immunoflorescence.21 MVECs expressed CD34 up through passages 5 and 6.4 Dermal and pulmonary MVECs expressed transcripts for EC markers in the absence of mesosderm-specific markers such as desmin and vimentin.21
All ECs were maintained in T-25 flasks (Falcon; Becton Dickinson Labware, Lincoln Park, NJ), coated with either 0.1% gelatin in water or 50 μg/mL human plasma fibronectin (Chemicon International, Temecula, CA) in phosphate-buffered saline (PBS), in ECM 1001 medium (ScienCell Research Labs) containing a proprietary endothelial cell growth supplement, penicillin, streptomycin, and 15% fetal bovine serum (FBS). All ECs were used in passages 2 to 6. Subcultures involved a 5- to 10-minute exposure to 0.25% trypsin-EDTA, followed by washing with PBS (pH 7.2).
Reagents and gene constructs
Human recombinant IFN-γ was purchased from PeproTech (Rocky Hill, NJ). An amount of 0.1 ng/mL was equivalent to 1 activity unit (AU). MG132 was obtained from Calbiochem (La Jolla, CA) and prepared as a 20 mM stock solution in DMSO. Rabbit anti–caspase-8 antibody (Ab) was purchased from BD Pharmingen (San Diego, CA). Affinity-purified rabbit anti–human/mouse c-FLIP Ab was purchased from R&D Systems (Minneapolis, MN). It recognizes the predominant, long (L) isoform (molecular weight [MW] 55-58 kDa) of c-FLIP, which is a much more potent inhibitor of apoptosis than the short (S) isoform (MW 28 kDa) at comparable levels of expression.22 Polyclonal Abs against IFN-γ, TNF-α, and IL-6 were obtained from R&D Systems. Rabbit polyclonal Ab against TRAIL was obtained from Millennium Biotechnology (Ramona, CA).
c-FLIP (L and S forms) was delivered into cells via adenovirus (Ad) type 5 vectors with E1 and E3 deletions, driven by a CMV promoter (Ad-FLIP; Vector Biolabs, Philadelphia, PA). A control vector, lacking a transgene, was also used (Ad-CMV-Null; Vector Biolabs). Stocks were prepared at 1010 pfu/mL.
Short interfering RNAs (siRNAs) were used to suppress FLIP translation. A siRNA targeted to c-FLIP (L and S forms), with the sense sequence 5′-GGGACCUUCUGGAUAUUUUdTT-3′ and antisense sequence 5′-AAAAUAUCCAGAAGGUCCCdTG-3′, and a control siRNA containing a scrambled sequence screened for lack of effect on human transcripts were prepared by Ambion (Austin, TX). GeneSilencer, a proprietary siRNA transfection reagent, was obtained from Genlantis (San Diego, CA).
Apoptosis assays
We have used multiple assays to confirm that TTP plasma–mediated injury of restricted lineages of MVECs in vitro is apoptotic, including: genomic DNA fragmentation documented by ethidium bromide staining20 ; propidium iodide (PI) staining in the presence of RNaseA and quantitation of hypodiploid DNA content (A0) by fluorescence-activated cell sorter (FACS)–derived DNA histogram analysis4,20 ; morphologic changes highlighted by DAPI/isorhodamine staining4,20 ; immunoblotting for caspase-3 activation19 ; and inhibition of TTP plasma activity by specific inhibitors of caspase-1 and -3.19 In the present experiments, the degree of apoptosis is recorded as an apoptotic index, assessed by 2 methods: FACS-based DNA histogram analysis, with percentage of A0 peaks defined by computer software (MCycle Av; Phoenix Flow Systems, San Diego, CA),20 and enzyme-linked immunosorbent assay (ELISA)–based quantitation of cytoplasmic histone-associated DNA fragments, performed as per the manufacturer's directions (Roche Diagnostics, Indianapolis, IN).
ECs were washed with PBS and plated in chambers of 12-well plates, coated with 0.1% gelatin in water, at 0.15 × 106 viable cells/mL. Dermal MVECs, susceptible to TTP plasma–mediated apoptosis, as well as TTP plasma–resistant pulmonary MVECs and HUVECs, were exposed to 1% to 2% (vol/vol) TTP or normal donor plasma for 18 to 24 hours, then harvested by trypsinization.
Cytokine assays
Plasma levels of TNF-α and IFN-γ were assessed by ELISA (R&D Systems). Plasma levels of TRAIL were assessed by Immundiagnostik AG ELISA (Wiesenstraße, Germany).
Caspase-8 assays
Activation of caspase-8 was evaluated by a functional assay based on hydrolysis of acetyl-Ile-Glu-Thr-Asp-p-nitraniline, performed according to the manufacturer's instructions (Caspase-8 Assay Kit, colorimetric; Sigma-Aldrich, St Louis, MO).
Immunoblotting for c-FLIP
A total of 0.5 × 106 cells per condition were treated with lysis buffer (20 mM dTT, 6% SDS, 0.25 M Tris, and 10% glycerol [pH 6.8]). A total of 50 μg protein lysate, quantitated by bicinchoninic acid (BCA) assay (Pierce, Rockford, IL), were separated by SDS-PAGE. Proteins were transferred to PVDF membranes using an LKB transfer apparatus (Pharmacia, Uppsala, Sweden) and probed with 1:100 dilutions of anti–c-FLIP polyclonal Ab. The secondary antibody was a horseradish peroxidase (HRP)–rabbit anti–human IgG. Bands were detected by a chemiluminescence kit (GE Healthcare, Piscataway, NJ). Controls for c-FLIP quantitation involved immunoblotting for GAPDH.
Manipulation of c-FLIP levels
Gene silencing.
ECs were washed twice with OptiMEM, a serum-free medium (Invitrogen, Carlsbad, CA), followed by suspension at 5 × 105 cells per condition in OptiMEM. They were exposed to 50 or 100 nM c-FLIP or 100 nM control GeneSilencer oligonucleotides for 15 minutes at 37°C in a total volume of 200 μL. Cells plus transfection mix were then combined with culture medium to a total of 1 mL, transferred to 12-well culture plates coated with gelatin, and incubated for 18 hours. TTP or control plasmas were then added to a final volume of 2%, and cells were incubated for an additional 18 to 24 hours. Efficacy of transcriptional suppression was determined in cell aliquots by Western blotting for FLIP.
Gene transfer.
A total of 3 to 4 × 105 ECs in 1 mL culture medium were combined with 1.5 μL stock adenovirus containing either a c-FLIP–encoding or control insert at a multiplicity of infection of 30. Cultures were incubated for 18 hours at 37°C, followed by addition of 2% TTP or control plasma. Cells were harvested after an additional 12- to 18-hour incubation.
Analysis of oligonucleotide array data
Our group had previously published an analysis of transcripts in primary human dermal versus pulmonary MVECs following an 18-hour exposure to normal or acute TTP plasmas.21 These experiments were conducted using Hu6800 Genechip arrays (Affymetrix, Santa Clara, CA), and data analysis was performed using Genechip 3.1 software. A greater than 2-fold change in cRNA expression was indicative of a significant difference.21 These data were reanalyzed here, focusing on potential cytoprotective pathways.
Qualitative analysis of TRAIL-related transcripts
mRNA expression was assessed by qualitative reverse transcriptase–polymerase chain reaction (RT-PCR). Total RNA was extracted by the TRIZOL method (Gibco Life Technologies [Invitrogen]). First-strand cDNAs were reverse-transcribed from total RNAs using random hexamers and murine leukemia virus (MuLV) RT, then amplified by PCR using Taq polymerase (GeneAmp kit; PerkinElmer, Waltham, MA). Oligomers for TRAIL receptors DR4 and DR5 and TRAIL decoy receptors DcR1 and DcR2 were based on published sequences.23,24 Commercial β-actin primers were used as controls for DNA integrity. The primers are listed in Table 1, together with their expected amplicon size.
c-FLIP ubiquitination
A total of 6 × 105 cells per condition were incubated with buffer, normal plasma (2% vol/vol), TTP plasma (2% vol/vol), or TRAIL (2 ng/mL) plus IFN-γ (0.1 ng/mL) for 30 minutes. Cell lysate (100 μg) from each condition was precleared with protein G Plus-Agarose (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at 4°C and then incubated with 2 μg rabbit polyclonal anti-FLIP Ab overnight at 4°C. Protein G Plus-Agarose was added for 1 hour, immunoprecipitates were washed with lysis buffer and denatured at 90°C for 10 minutes in sample buffer, and the samples were resolved on a 4% to 15% polyacrylamide gel. Proteins were transferred onto nitrocellulose, probed with antiubiquitin mouse IgG mAb (Santa Cruz Biotechnology), and detected using HRP-conjugated bovine anti–mouse IgG (Santa Cruz Biotechnology) and chemiluminescence (GE Healthcare).
Statistics
Groups were compared using paired and nonpaired 2-tailed t tests.
Results
Patient characteristics and cytokine levels
The female predominance (male-female ratio, 2:5) among our patients with TTP is characteristic of the thrombotic microangiopathies. Their mean level of circulating TRAIL (1330 ± 195 pg/mL) was markedly higher than our normal controls (378 ± 49 pg/mL; P = .03). We found no difference in circulating TNF-α levels between patients with TTP (1.32 ± 0.29 pg/mL) and controls (1.19 ± 0.35 pg/mL; P = .14), nor was there a difference in IFN-γ plasma levels (mean, 1.08 ± 0.18 pg/mL for patients vs 1.09 ± 0.11 pg/mL for controls; P = .87). The levels of IFN-γ and TNF-α for our patients are somewhat lower than those seen in other TTP cohorts (mean circulating IFN-γ, 6.5 pg/mL8 and 14 pg/mL [range, 0-28 pg/mL])6 ; mean TNF-α of 11.4 pg/mL8 and 5.2 pg/mL (range, 0-36.8 pg/mL)25 ). TRAIL has not previously been investigated in TTP. While cytokine levels were assessed in all patients and controls, subsequent experiments used plasma from the 5 available patients with acute idiopathic TTP (4 female, 1 male) and 3 controls. Of these 5, 4 patients with TTP had IgG autoantibodies reactive with ADAMTS13, which was determined by the Imubind ELISA kit (American Diagnostica, Stamford, CT).
Differential sensitivity of ECs to TTP plasma, TRAIL, and IFN-γ
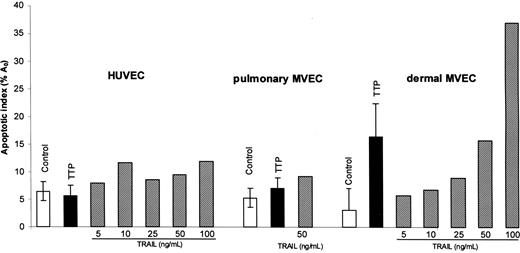
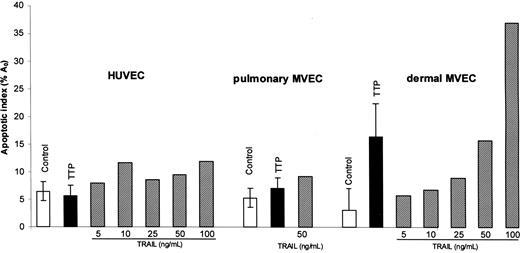
HUVECs and pulmonary and dermal MVECs were exposed to control or TTP plasmas (1% vol/vol) for 18 hours. Apoptosis was assessed by PI staining and quantitation of the A0 hyperdiploid peak. As shown in Figure 1, representing 3 (HUVECs and pulmonary MVECs) or 6 (dermal MVECs) separate experiments using 3 to 4 different TTP plasmas per cell lineage, there was no effect of TTP plasma on the viability of HUVECs (control A0 peak, 6.5% ± 1.7%; TTP plasma, 5.8% ± 1.7%; P = .32) or pulmonary MVECs (control, 4.8 ± 3.3%; TTP plasma, 6.8% ± 1.6%). Significant apoptosis was noted for dermal MVECs (control, 3.1% ± 4.0%; TTP plasma, 16.5% ± 5.9%; P < .001). These data reproduce findings reported from our laboratory.4,20
Effect of TTP plasma and TRAIL on survival of HUVECs and pulmonary and dermal MVECs. ECs were exposed to control plasma (1% vol/vol), acute TTP plasma (1% vol/vol), or varying concentrations of recombinant TRAIL for 18 hours. The extent of apoptosis was quantitated by propidium iodide–based DNA histogram analysis and measurement of hypodiploid A0 peaks. There was no difference in apoptosis of HUVECs or pulmonary MVECs in the presence of control versus TTP plasma, while TTP plasma induced apoptosis in the dermal MVECs (P < .007). Standard deviations are noted for mean values for control and TTP plasma.
Effect of TTP plasma and TRAIL on survival of HUVECs and pulmonary and dermal MVECs. ECs were exposed to control plasma (1% vol/vol), acute TTP plasma (1% vol/vol), or varying concentrations of recombinant TRAIL for 18 hours. The extent of apoptosis was quantitated by propidium iodide–based DNA histogram analysis and measurement of hypodiploid A0 peaks. There was no difference in apoptosis of HUVECs or pulmonary MVECs in the presence of control versus TTP plasma, while TTP plasma induced apoptosis in the dermal MVECs (P < .007). Standard deviations are noted for mean values for control and TTP plasma.
HUVECs and pulmonary and dermal MVECs were then exposed to TNF-α (1-10 ng/mL) or TRAIL (5-100 ng/mL), and apoptosis was assessed by A0 measurements 18 hours later. No significant effect was seen in HUVECs or pulmonary MVECs with doses of TNF-α to 10 ng/mL (data not shown) or TRAIL to doses of 100 ng/mL (Figure 1). Dermal and pulmonary MVECs were also not susceptibility to TNF-α at doses to 10 ng/mL (not shown). Dermal MVECs were more sensitive to TRAIL-mediated injury, with an A0 peak of 15.8% at 50 ng/mL and 36.9% at 100 ng/mL (Figure 1). However, these concentrations are still some 50-fold greater than we found in plasmas of patients with acute TTP.
The potential for cytokines elevated in TTP plasma to act in synergy at clinically relevant concentrations to induce EC apoptosis was next investigated. We used 2 ng/mL of TRAIL, reflecting concentrations seen in our patient population and in HIV+ patients,26 because untreated HIV infection is the single greatest risk factor for TTP. We also used 1 U/mL (0.1 ng/mL) IFN-γ. This concentration is 5-fold greater than that reported in acute TTP plasmas,8 but is typical of tissue (lymph node extract) levels found in HIV infection and some healthy individuals.27
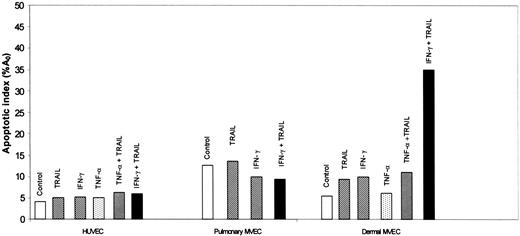
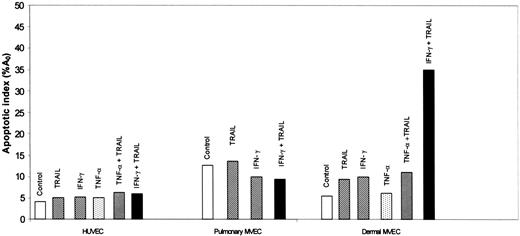
HUVECs and pulmonary MVECs were unaffected by TNF-α (1 ng/mL), TRAIL (2 ng/mL), or IFN-γ (0.1 ng/mL) when used alone (Figure 2; data not shown). Combinations of TNF-α with TRAIL (Figure 2), IFN-γ (not shown), or TRAIL plus IFN-γ (Figure 2) also had no effect on survival of these cells. In contrast, while these cytokines alone, or the combination of TNF-α plus TRAIL, did not alter dermal MVEC survival, IFN-γ plus TRAIL led to marked apoptosis in the dermal MVECs (Figure 2).
Effect of TTP-associated cytokines on survival of HUVECs and pulmonary and dermal MVECs. Apoptosis was assessed, as described in the legend to Figure 1, 18 hours after exposure of cells to buffer or various cytokines. IFN-γ, 0.1 ng/mL; TNF-α, 1 ng/mL; and TRAIL, 2 ng/mL.
Effect of TTP-associated cytokines on survival of HUVECs and pulmonary and dermal MVECs. Apoptosis was assessed, as described in the legend to Figure 1, 18 hours after exposure of cells to buffer or various cytokines. IFN-γ, 0.1 ng/mL; TNF-α, 1 ng/mL; and TRAIL, 2 ng/mL.
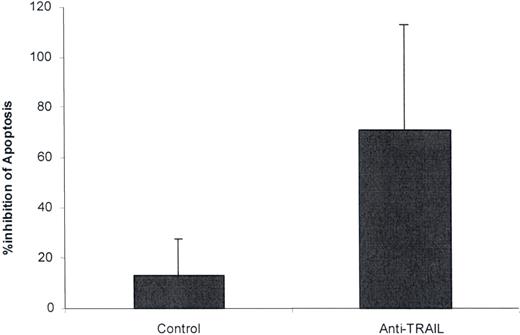
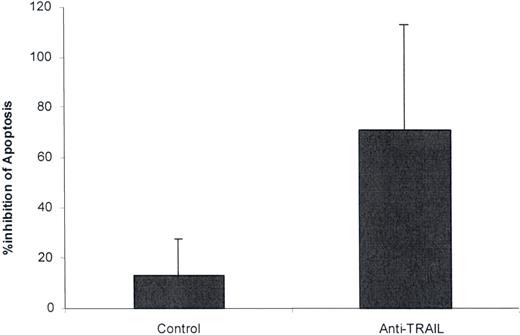
The ability of anti-TRAIL polyclonal Ab to affect TTP plasma–mediated apoptosis was next investigated. A 1:500 dilution of antibody suppressed the apoptotic activity of 4 of 5 TTP plasmas tested (mean suppression, 87.5% vs 13.2% for control serum). This was a significant difference when comparing results for all 5 TTP plasmas tested versus controls (Figure 3; P = .02). It is consistent with a pilot study from our group showing that another type of TRAIL antagonist, the decoy receptor osteoprotegerin (OPG), depleted apoptosis-inducing activity from TTP plasma.28
Effect of anti-TRAIL antibody on TTP plasma–mediated apoptosis. Aliquots of 5 different acute TTP plasmas were incubated with control rabbit serum (1:500 dilution) or polyclonal rabbit anti–human TRAIL IgG (1:500 dilution) for 2 hours at 4°C. Final concentrations (1% vol/vol) of the TTP plasmas were then incubated with dermal MVECs for 18 hours at 37°C. Apoptosis was assessed as in the legend to Figure 1. Anti-TRAIL Ab decreased TTP plasma–mediated apoptosis (P = .02). Standard deviations are noted for the mean values.
Effect of anti-TRAIL antibody on TTP plasma–mediated apoptosis. Aliquots of 5 different acute TTP plasmas were incubated with control rabbit serum (1:500 dilution) or polyclonal rabbit anti–human TRAIL IgG (1:500 dilution) for 2 hours at 4°C. Final concentrations (1% vol/vol) of the TTP plasmas were then incubated with dermal MVECs for 18 hours at 37°C. Apoptosis was assessed as in the legend to Figure 1. Anti-TRAIL Ab decreased TTP plasma–mediated apoptosis (P = .02). Standard deviations are noted for the mean values.
TRAIL receptors
Upon binding of TRAIL to cells, the membrane receptors DR4 and DR5 are capable of recruiting Fas-associated death domain (FADD) and, in turn, caspase-8 to form a death-inducing signal complex.29 There are also 3 soluble decoy receptors for TRAIL: DcR1, DcR2, and OPG. Lacking death domains, they can protect cells from TRAIL-mediated apoptosis.29 HUVECs and dermal MVECs are known to express similar levels of mRNA and protein for DR4 and DR5.29 HUVECs also express both DcR1 and DcR2,23,30 while dermal MVECs express DcR1 but lack both message and expressed protein for DcR2.28,29 We sought to examine the effect of TTP plasma on these receptors in TTP plasma–sensitive and –resistant MVECs. mRNAs for DR4, DR5, and DcR1 were unchanged in dermal and pulmonary MVECs following an 18-hour exposure to 1% TTP plasma (data not shown). We confirmed that dermal MVECs lack mRNA for decoy DcR2 (data not shown). We also found that pulmonary MVECs express DcR2, as has been reported for HUVECs,29,30 and this expression was unaffected by TTP plasma (data not shown).
Caspase-8 activation by TTP plasma
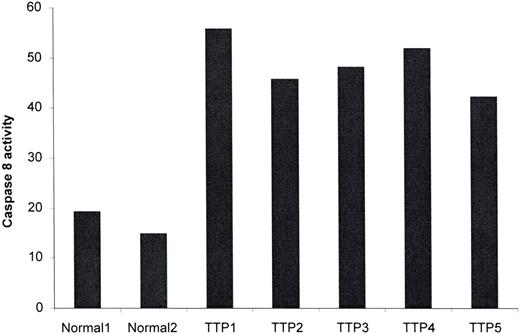
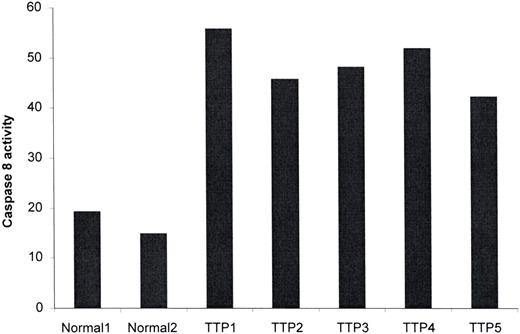
Caspase-8 exists in the cell as an inactive proenzyme of 55 kDa. It is converted into an active form, consisting of 40-kDa and 23-kDa subunits, upon recruitment to the cytoplasmic domain of activated death receptors.29 As noted here, TRAIL recruits caspase-8, and IFN-γ can induce procaspase-8 in certain cells.31 We thus anticipated that TTP plasma would also activate this enzyme. As shown in Figure 4, all of 5 acute TTP plasmas tested induced caspase-8 activity in dermal MVEC cultures exposed to TTP versus 2 normal plasmas (P < .009).
TTP plasma activates procaspase-8 in dermal MVECs. Dermal MVECs were exposed to 2% plasma from 2 healthy donors and 5 patients with acute TTP for 2 hours, followed by cell lysis and assessment of caspase-8 activity by a colorimetric assay. Caspase-8 activity, expressed as percent over baseline optical density values, was increased significantly by TTP versus normal plasma (P < .009).
TTP plasma activates procaspase-8 in dermal MVECs. Dermal MVECs were exposed to 2% plasma from 2 healthy donors and 5 patients with acute TTP for 2 hours, followed by cell lysis and assessment of caspase-8 activity by a colorimetric assay. Caspase-8 activity, expressed as percent over baseline optical density values, was increased significantly by TTP versus normal plasma (P < .009).
Role of c-FLIP in TTP plasma–mediated apoptosis
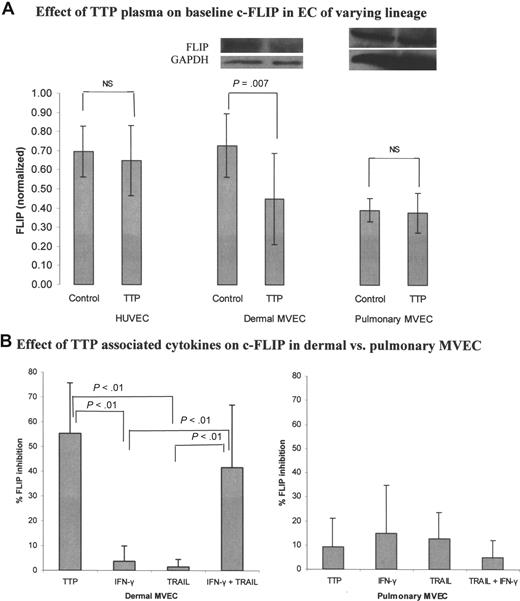
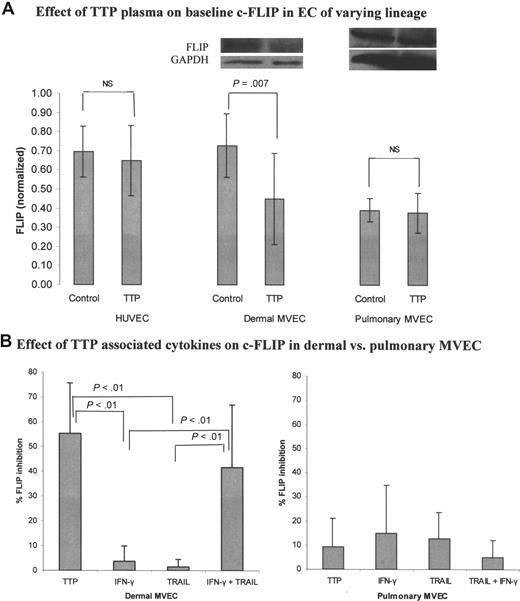
We examined basal levels of the caspase-8 inhibitor c-FLIP in HUVECs and pulmonary and dermal MVECs, and the impact of TTP plasma (2%) on this expression. All data represent densitometry tracings of immunoblots from 3 to 5 separate experiments, with calculation of FLIP/GAPDH ratios. Basal FLIP levels were virtually identical in HUVECs and dermal MVECs (Figure 5A; P = .72). Basal FLIP was significantly lower in pulmonary MVECs compared with either HUVECs or dermal MVECs (Figure 5A; P = .007). However, only dermal MVECs showed a change in FLIP expression following exposure to TTP plasma. All of the 5 acute TTP patient plasmas tested suppressed FLIP expression in dermal MVECs (mean, 28.3%), while FLIP in pulmonary MVECs was unaffected by this treatment (mean, 3.9% suppression; P = .007).
Effect of TTP plasma and TTP-associated cytokines on c-FLIP expression in pulmonary versus dermal MVECs. Levels of c-FLIP, normalized to GAPDH, were assessed by Western blotting 18 hours after exposure of ECs to normal plasma (2% vol/vol), TTP plasma (2% vol/vol), or cytokines. (A) Basal levels of c-FLIP in HUVECs and dermal MVECs were equivalent, and significantly greater than basal FLIP levels in pulmonary MVECs (summary of 4 experiments; P = .007). TTP plasma suppressed basal FLIP expression only in dermal MVECs (5 different TTP plasmas tested; P = .007). (B) TTP plasma (P < .01) and the combination of TRAIL (2 ng/mL) plus IFN-γ (0.1 ng/mL; P < .01) suppressed FLIP expression in dermal MVECs, while either cytokine alone had no effect. Neither TTP nor cytokine combinations significantly affected basal FLIP levels in pulmonary MVECs. Standard deviations for all mean values are provided.
Effect of TTP plasma and TTP-associated cytokines on c-FLIP expression in pulmonary versus dermal MVECs. Levels of c-FLIP, normalized to GAPDH, were assessed by Western blotting 18 hours after exposure of ECs to normal plasma (2% vol/vol), TTP plasma (2% vol/vol), or cytokines. (A) Basal levels of c-FLIP in HUVECs and dermal MVECs were equivalent, and significantly greater than basal FLIP levels in pulmonary MVECs (summary of 4 experiments; P = .007). TTP plasma suppressed basal FLIP expression only in dermal MVECs (5 different TTP plasmas tested; P = .007). (B) TTP plasma (P < .01) and the combination of TRAIL (2 ng/mL) plus IFN-γ (0.1 ng/mL; P < .01) suppressed FLIP expression in dermal MVECs, while either cytokine alone had no effect. Neither TTP nor cytokine combinations significantly affected basal FLIP levels in pulmonary MVECs. Standard deviations for all mean values are provided.
In contrast to these protein data, examination of c-FLIP mRNA expression from our prior DNA microarray experiments21 showed little difference between dermal and pulmonary MVECs, and TTP plasma had no impact on this expression.
We then explored whether we could recapitulate the effects of TTP plasma on c-FLIP protein expression in TTP plasma–susceptible MVECs using levels of TRAIL and IFN-γ consistent with those found in vivo. In this series of experiments, using 3 TTP plasmas, dermal MVEC FLIP levels declined by 55.3% from control levels in the presence of TTP plasma, while neither IFN-γ (0.1 ng/mL) nor TRAIL (2 ng/mL) alone altered c-FLIP expression (Figure 5B; summary of 3 experiments; P < .01 for all conditions). The combination of TRAIL plus IFN-γ decreased FLIP levels by 41.6% (Figure 5B; P < .01), paralleling the TTP plasma effect. In contrast, neither cytokine, alone or in combination, altered FLIP levels in pulmonary MVEC (Figure 5B).
As pretreatment of most TTP plasmas with anti-TRAIL Ab or TRAIL decoy OPG suppressed its ability to induce dermal MVEC apoptosis, we examined whether pretreatment of TTP plasma with anti-TRAIL or anti–IFN-γ polyclonal Ab could also abrogate its ability to down-regulate FLIP expression. Pre-exposure of TTP plasma to anti-TRAIL Ab resulted in a 64.2% reduction in its ability to suppress FLIP expression (Table 2; mean of 2 experiments). A similar effect was noted with anti–IFN-γ pretreatment (74.6% blockade of the effect of TTP plasma on FLIP expression; Table 2). Antibodies to TNF-α and IL-6 had little to no impact (Table 2). This supports the concept that synergy between IFN-γ and TRAIL is required for the ability of TTP plasma to decrease FLIP protein levels and to induce apoptosis in susceptible ECs.
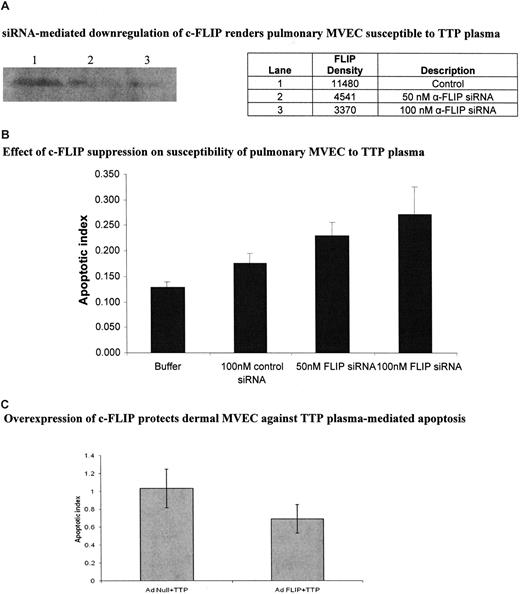
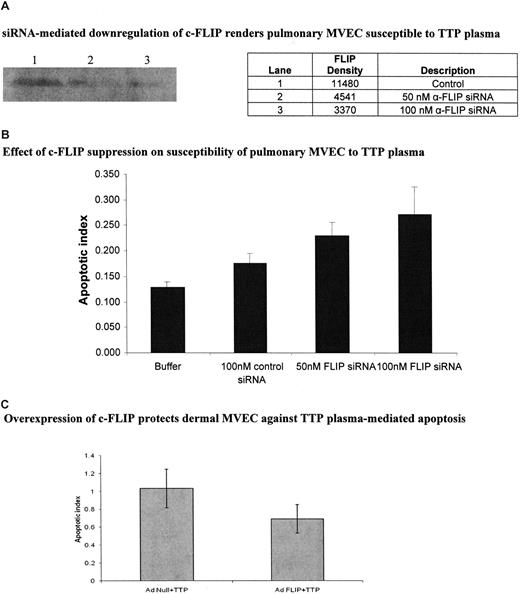
We directly addressed the role of c-FLIP in TTP plasma–mediated EC apoptosis via siRNA silencing and adenovirus-mediated gene transfer experiments. Compared with a control siRNA, anti–c-FLIP siRNA suppressed FLIP expression in pulmonary MVECs by 60.4% at 50 nM and 70.6% at 100 nM, as assessed by immunoblotting (Figure 6A), while having no effect on cell viability (data not shown). (The control siRNA led to less than 10% suppression of FLIP over cells exposed to buffer only [data not shown]). Pulmonary MVECs were rendered sensitive to TTP plasma–mediated apoptosis by both 50 nM (P < .001) and 100 nM (P < .001) concentrations of c-FLIP siRNA (Figure 6B). A total of 100 nM control siRNA had little impact on the apoptotic index of these cells (Figure 6B). Alternately, up-regulation of c-FLIP in dermal MVECs via AdFLIP vector partially protected these cells against TTP plasma–mediated injury, an effect not seen when an Adnull vector was used (Figure 6C; P = .01). The magnitude of apoptosis suppression, determined by histone-associated DNA fragment measurements, was similar to that seen by quantiation of viable cells by trypan blue dye exclusion (data not shown).
Effect of modulation of c-FLIP expression in pulmonary and dermal MVECs on their sensitivity to TTP plasma–mediated apoptosis. (A) Representative immunoblot for c-FLIP in pulmonary MVECs 18 hours after culture with 100 nM of control or 50 nM or 100 nM of anti-FLIP siRNA. (B) Effect of anti-FLIP siRNA on susceptibility of pulmonary MVECs to TTP plasma. Cells were exposed to siRNAs for 18 hours followed by addition of TTP plasma (2% vol/vol) and an additional 18-hour culture. Apoptosis was assessed by ELISA-based quantitation of DNA-histone complexes in cell lysates. The apoptotic index was significantly increased over baseline (MVECs treated with control siRNA) when pulmonary MVEC were treated with either 50 nM (P < .001) or 100 nM (P < .001) of anti-FLIP siRNA, then exposed to 2 different TTP plasmas. (C) Dermal MVECs were infected with adenovirus-bearing null or c-FLIP-coding sequences for 18 hours, followed by addition of control or TTP plasmas (2% vol/vol) and an additional 18 hours of incubation. Apoptosis, assessed as in panel B, was significantly reduced by AdFLIP treatment (P = .01). Buffer alone gave an index of 0.4. Standard deviations for mean values are provided.
Effect of modulation of c-FLIP expression in pulmonary and dermal MVECs on their sensitivity to TTP plasma–mediated apoptosis. (A) Representative immunoblot for c-FLIP in pulmonary MVECs 18 hours after culture with 100 nM of control or 50 nM or 100 nM of anti-FLIP siRNA. (B) Effect of anti-FLIP siRNA on susceptibility of pulmonary MVECs to TTP plasma. Cells were exposed to siRNAs for 18 hours followed by addition of TTP plasma (2% vol/vol) and an additional 18-hour culture. Apoptosis was assessed by ELISA-based quantitation of DNA-histone complexes in cell lysates. The apoptotic index was significantly increased over baseline (MVECs treated with control siRNA) when pulmonary MVEC were treated with either 50 nM (P < .001) or 100 nM (P < .001) of anti-FLIP siRNA, then exposed to 2 different TTP plasmas. (C) Dermal MVECs were infected with adenovirus-bearing null or c-FLIP-coding sequences for 18 hours, followed by addition of control or TTP plasmas (2% vol/vol) and an additional 18 hours of incubation. Apoptosis, assessed as in panel B, was significantly reduced by AdFLIP treatment (P = .01). Buffer alone gave an index of 0.4. Standard deviations for mean values are provided.
Mechanism of TTP plasma–mediated inhibition of c-FLIP protein
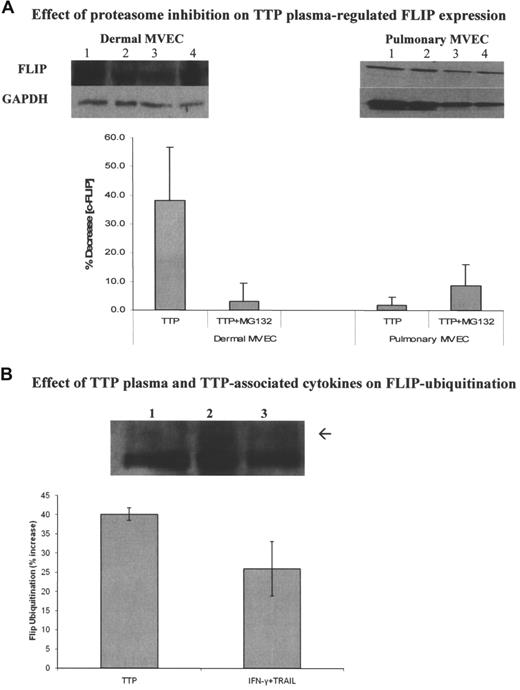
IFN-γ accelerates degradation of many regulatory proteins,32 including c-FLIP,33 via induction of the proteasome activator P28 and other immunoproteasome components, resulting in enhanced ubiquitination of those proteins. TRAIL can also suppress FLIP via protein kinase activation.34 However, the concentrations or TRAIL or IFN-γ required for inhibition of c-FLIP by isolated cytokines far exceed levels found in TTP plasmas. We reasoned that the synergistic interaction between IFN-γ and TRAIL in suppression of FLIP expression, documented here, was a result of their acting via these 2 divergent pathways, and postulated that interference with proteasome function would disrupt this cooperative activity. It is known that c-FLIP inhibition by certain pathologic stimuli can be blocked by proteasome inhibitors such as MG132.16,35,36
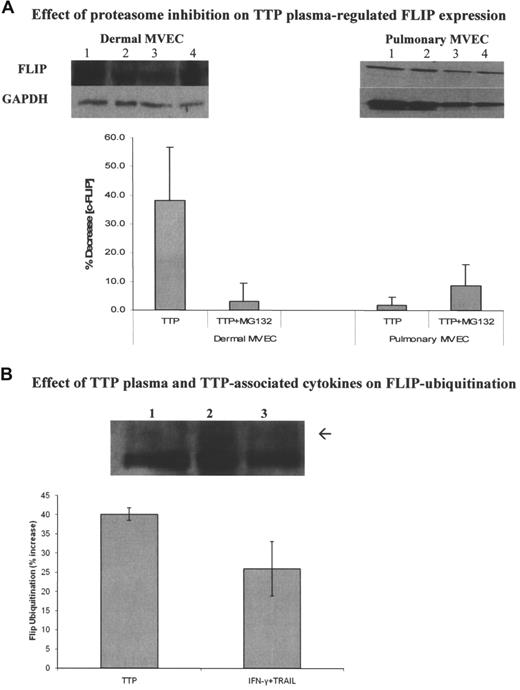
We first showed that MG132 alone, at levels (5 μM) that did not affect the viability of ECs, showed no enhancement of basal FLIP levels in either pulmonary or dermal MVECs. Indeed, there was a small decrease in basal FLIP levels (Figure 7A), which was consistent with its activity in other cell systems.35 However, while MG132 had no effect on FLIP expression in pulmonary MVECs exposed to TTP plasma (Figure 7A; summary of 3 experiments; P = .21), it restored c-FLIP levels in dermal MVECs exposed to TTP plasma (Figure 7A; summary of 3 experiments; P = .048) or TRAIL plus IFN-γ (data not shown).
Modulation of the effect of TTP plasma on c-FLIP through proteasome inhibition and alteration of c-FLIP ubiquitination. (A) Effect of MG132 on total FLIP expression in MVECs. Top panels show representative immunoblots. Pulmonary and dermal MVECs were incubated with normal (lane 1) or TTP plasma (lane 2; 2% vol/vol) for 30 minutes. Cultures treated with MG132 (5 μM) were exposed to this proteasome inhibitor for 1 hour prior to addition of normal (lane 3) or TTP (lane 4) plasmas. Bottom panel shows summary of 3 experiments using 2 normal and 2 TTP plasmas. MG132 had no effect on FLIP expression in pulmonary MVECs (P = .21), but it blocked TTP plasma–mediated FLIP suppression by TTP plasma in dermal MVECs (P = .048). (B) Effect of TTP plasma and cytokines on FLIP ubiquitination. Dermal MVECs were immunoprecipitated with an anti-FLIP polyclonal Ab. Equal amounts of protein (100 μg) were then loaded on gels and immunoblotted with an antiubiquitin mAb. A representative immunobot and a summary of 3 experiments are presented. Levels of monoubiquitinated FLIP (arrow; just above the dense heavy chain IgG band) were increased by TTP plasma (P = .008) and TRAIL plus IFN-γ (P = .03) compared with cells incubated with normal plasma. Lane 1, control plasma; lane 2, TTP plasma; lane 3, TRAIL plus IFN-γ. Standard deviations for all mean values are provided.
Modulation of the effect of TTP plasma on c-FLIP through proteasome inhibition and alteration of c-FLIP ubiquitination. (A) Effect of MG132 on total FLIP expression in MVECs. Top panels show representative immunoblots. Pulmonary and dermal MVECs were incubated with normal (lane 1) or TTP plasma (lane 2; 2% vol/vol) for 30 minutes. Cultures treated with MG132 (5 μM) were exposed to this proteasome inhibitor for 1 hour prior to addition of normal (lane 3) or TTP (lane 4) plasmas. Bottom panel shows summary of 3 experiments using 2 normal and 2 TTP plasmas. MG132 had no effect on FLIP expression in pulmonary MVECs (P = .21), but it blocked TTP plasma–mediated FLIP suppression by TTP plasma in dermal MVECs (P = .048). (B) Effect of TTP plasma and cytokines on FLIP ubiquitination. Dermal MVECs were immunoprecipitated with an anti-FLIP polyclonal Ab. Equal amounts of protein (100 μg) were then loaded on gels and immunoblotted with an antiubiquitin mAb. A representative immunobot and a summary of 3 experiments are presented. Levels of monoubiquitinated FLIP (arrow; just above the dense heavy chain IgG band) were increased by TTP plasma (P = .008) and TRAIL plus IFN-γ (P = .03) compared with cells incubated with normal plasma. Lane 1, control plasma; lane 2, TTP plasma; lane 3, TRAIL plus IFN-γ. Standard deviations for all mean values are provided.
Next, we examined the effect of TTP plasma and TRAIL plus IFN-γ on ubiquitination of c-FLIP in dermal MVECs. Levels of monoubiquitinated FLIP, determined by loading equal amounts of immunoprecipitated FLIP protein from each condition then immunoblotting with an antiubiquitin mAb, were increased over levels seen in the presence of normal plasma by both TTP plasma (P = .008) and TRAIL plus IFN-γ (P = .03; Figure 7B; summary of 3 experiments).
Discussion
MVEC injury is central to the pathophysiology of TTP, but its etiology remains poorly understood.37 Mortality remains unacceptably high, and relapses cannot be predicted. More specific strategies aimed at manipulating key autoimmune phenomena, such as ADAMTS13 autoantibody, and the initiating events for MVEC injury are required. In this study we document the ability of IFN-γ and TRAIL to act in synergy, at concentrations typical of those found in TTP and HIV patient plasmas, to induce apoptosis in restricted lineages of MVECs in vitro, mimicking the vascular distribution and pathology of TTP in vivo. This process appears to involve cooperation between IFN-γ and TRAIL in suppressing, via posttranscriptional mechanisms, a c-FLIP cytoprotective pathway, sensitizing cells to TRAIL-mediated apoptosis.
Overproduction of TRAIL is characteristic of idiopathic TTP as well as infection with several viruses capable of directly injuring MVECs, including cytomegalovirus, parvovirus, and HIV. All have been linked epidemiologically to TTP.8,20,38,39 HIV is of particular interest in this regard, in terms of the magnitude of its association—1 in 200 patients with untreated HIV disease develop TTP40 —and the similarities between idiopathic and HIV-linked TTP, including the presence of ADAMTS13 autoantibody and response to plasma exchange.20,41 Individual cytokine levels in TTP plasmas may be indistinguishable from those of many other disorders, including hepatitis B infection and malignancy,42 in the absence of a thrombotic microangiopathy. However, the combination of specific cytokines elevated in TTP, and the chronicity of their elevation, may characterize only a few conditions. HIV disease fits this description.26,27 Development of a clinical thrombotic microangiopathy may also require a “second hit,” such as deficiency of ADAMTS1341 or a complement regulatory protein.28 The fact that we could influence c-FLIP expression and induce apoptosis in vitro with 1% to 2% (vol/vol) TTP plasma, but optimal cytokine synergy required levels of TRAIL and IFN-γ equivalent to 50% to 100% TTP plasma, may relate to 2 factors. First, other investigators employing a human dermal MVEC cell line found that concentrations of TTP plasma in the 50% (vol/vol) range were required to induce cell death.43 Differences in basal EC activation state, influenced by plasma factors, may play a role in determining the level of insult required to induce apoptosis. Second, additional factors capable of modulating c-FLIP may be present in TTP plasma, including other cytokines and components of the alternate pathway of complement.28,43
Sensitivity or resistance of ECs to TTP plasma may be mediated through at least 3 pathways: cell signaling, TRAIL receptor expression, and c-FLIP processing. We had previously reported that MVECs susceptible to TTP plasma–mediated apoptosis exhibit marked and prolonged up-regulation of several mitogen-activated protein (MAP) kinases, including p38, JNK, and extracellular signal–regulated kinase (ERK)–1, upon exposure to such plasma.44 In contrast, HUVECs and pulmonary MVECs had only a transient rise in ERK-1 activation when cultured with TTP plasmas,44 a pattern linked to protection of these cells from TRAIL-mediated injury.45 Other groups have shown that manipulation of these kinase pathways can alter c-FLIP levels in HUVECs, sensitizing them to apoptosis.34
We found that TTP plasma–sensitive and –resistant ECs expressed similar levels of transcripts for TRAIL receptors DR4 and DR5 and TRAIL decoy receptor DcR1. However, unlike HUVECs and pulmonary MVECs, TTP plasma–sensitive dermal MVECs lacked mRNA for a second TRAIL decoy, DcR2. Whether this difference accounts for the heightened sensitivity of dermal MVECs versus pulmonary MVECs and HUVECs to TRAIL plus IFN-γ or TTP plasma is uncertain. However, in preliminary experiments we up-regulated DcR2 expression in dermal MVECs by gene transfer with no impact on their sensitivity to TTP plasma.
The most striking difference between TTP plasma–sensitive and –resistant ECs was the susceptibility of their c-FLIP protein to suppression by TTP plasma and disease-relevant concentrations of TRAIL plus IFN-γ. We explored the mechanism of this effect and its relevance to TTP-linked apoptosis.
First, there was no difference in basal FLIP levels between TTP-resistant HUVECs and TTP-sensitive dermal MVECs. TTP-resistant pulmonary MVECs had even lower basal expression of FLIP than the dermal MVECs. In some clonal tumor cell lines an inverse relationship between c-FLIP levels and sensitivity to TRAIL-induced apoptosis has been described, but no such general correlation between basal FLIP levels and apoptosis has been reported.46
Second, we documented the ability of TTP plasma and TRAIL plus IFN-γ to suppress c-FLIP levels in conjunction with increased c-FLIP ubiquitination. The importance of this cytokine combination was established by the ability of 2 inhibitors, anti-TRAIL Ab and the TRAIL decoy OPG, to block the apoptotic effect of TTP plasmas in most cases, and the ability of antibodies to either TRAIL or IFN-γ to inhibit TTP plasma–mediated suppression of FLIP expression. The role of c-FLIP as a critical intermediary to the function of these cytokines was further illustrated by the caspase-8 activation and c-FLIP siRNA silencing and adenovirus-mediated gene transfer experiments. TTP plasmas consistently activated caspase-8 in MVECs. TRAIL is known to initiate apoptosis via caspase-3 and -8 activation,29 and c-FLIP blocks caspase-8 activation.35 Reduction of FLIP levels by IFN-γ plus TRAIL might thus be expected to sensitize cells to apoptosis induction by low levels of TRAIL.
We also explored the mechanism by which IFN-γ plus TRAIL interacts in the posttranslational suppression of c-FLIP. The fact that a general proteasome inhibitor, MG132, blocked this phenomenon suggests involvement of the proteasome, and IFN-γ regulates immunoproteasome formation.32,33 In addition, certain protein kinases modulate FLIP expression in ECs,34 and these enzymes are altered by both TRAIL and TTP plasma.44 The molecular basis for cooperation between IFN-γ and TRAIL in regulating c-FLIP likely involves these 2 distinct pathways. IFN-γ/TRAIL synergy is a potentially very complex interaction, however. For example, IFN-γ can also enhance NFκB activation by TRAIL, which can then alter FLIP-dependent caspase-8 processing,33 and MG132 affects NF-κB induction via IκB-dependent proteasomal processing.
Finally, as noted here, IFN-γ and TRAIL need not be the only soluble factors involved in MVEC injury linked to TTP and related disorders that could act via c-FLIP. The C5b-9 complement membrane attack complex (MAC) has been implicated in MVEC apoptosis in TTP/HUS in vivo and in vitro.28,43 Subcytotoxic levels of MAC up-regulate c-FLIP in some cells, protecting them from apoptotic death.47 TTP plasma–susceptible MVECs might fail to up-regulate FLIP in response to MAC exposure, a concept being pursued in our laboratory. The possibility that specific modulation of TRAIL or, more generally, manipulation of c-FLIP–related pathways could block progression of EC injury characteristic of at least subsets of patients with TTP is worthy of further study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Hong Wu, PhD; Jiyun Kim, MD, PhD; and Emily Olfson for performing some of the FLIP Western blot and RT-PCR assays, and Brian Di Carlo, MD, for assistance with the apoptosis assays.
This work was supported by grants from the National Institutes of Health (HL55646, DK65511, AI65200), the Angelo Donghia and Hagedorn Funds, and amfAR, The Foundation for AIDS Research.
National Institutes of Health
Authorship
Contribution: R.S., D.B., M.F., and F.S. performed the apoptosis assays and immunoblots; D.B., R.M., and F.S. performed the ubiquitin experiments; and J.L. directed the project in collaboration with R.S. and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeffrey Laurence, Laboratory for AIDS Virus Research, Division of Hematology-Oncology, Weill Medical College of Cornell University, 411 East 69th Street, New York, NY 10021; e-mail: jlaurenc@med.cornell.edu.