Abstract
Immunodeficient mice are increasingly used to assay human hematopoietic repopulating cells as well as leukemia-initiating cells. One method commonly used to isolate these rare cells is to sort cells stained with fluorochrome-conjugated antibodies into fractions, then transplant the different fractions into immunodeficient mice to test their repopulating ability. The antibodies are generally treated as being neutral in terms of their effects on the experiment. Human repopulating cells are thought to express CD34 and lack CD38. Here we present evidence that anti-CD38 antibodies have a profound inhibitory effect on engraftment of cord blood and leukemia cells. We show that this effect is Fc-mediated and can be overcome by treating mice with immunosuppressive antibodies. When this inhibitory effect is prevented, we demonstrate that the CD34+CD38+ fraction of certain acute myeloid leukemia samples contains all, or at least most, leukemia-initiating cell capacity. This study highlights the potential pitfall of antibody-mediated clearance of repopulating cells and is important for any groups working with this model. More importantly, the work suggests that there is greater variation in the phenotypes of leukemia-initiating cells than previously suggested.
Introduction
Immunodeficient animals, such as the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse, are used to assay human hematopoietic stem and progenitor cells. Long-term repopulating cells, thought to be hematopoietic stem cells (HSCs), are able to initiate a graft in these mice over prolonged periods as well as in secondary recipients. One common strategy to isolate repopulating cells has been to sort fractions of cells based on expression of antigens and transplant these into NOD/SCID mice. These experiments indicate that normal human repopulating cells are enriched in the CD34+CD38− fraction.1,2 Some studies indicate that the CD34+CD38+ fraction of normal hematopoietic tissue cannot repopulate NOD/SCID mice.1 Other studies in which enhanced immunosuppression was used suggest this fraction has repopulating ability.2-4
Immunodeficient mice have also been used to assay sorted fractions of acute myeloid leukemia (AML). SCID-leukemia–initiating cells (SL-ICs) are the cells capable of inducing a leukemic graft when transplanted into immunodeficient mice. SL-ICs are detected only within the CD34+CD38− fraction of most AML samples.5-7
Most authors have treated the antibodies used for these experiments as being neutral. The NOD/SCID mice (that have a defect in T cells, B cells, natural killer cells, and complement8 ) were thought to be sufficiently immunodeficient so as not to clear antibody-coated cells.
We have previously observed loss of NOD/SCID repopulating potential after sorting of hematopoietic cells. While exploring this phenomenon, we noted that anti-CD33 antibody reduced the engraftment of repopulating cells in NOD/SCID mice.9 Here, we sought to assess the effects of a range of antibodies to human antigens thought to be present (or not) on repopulating cells to identify any potential bias in xenotransplantation experiments. We report that anti-CD38 antibodies have a profound inhibitory effect on the engraftment of both normal and leukemic repopulating cells. We show that the effects are mediated via the Fc receptor and are reversible by treating mice with immunosuppressive antibodies. Furthermore, we provide direct evidence that the SL-ICs from some AML samples are CD34+CD38+, indicating a greater heterogeneity in the leukemic stem cell compartment.
Methods
Primary cells
Cord blood and AML cells were obtained after informed consent at St Bartholomew's and the Royal London Hospitals. The protocol was approved by the East London Ethical Committee. Informed consent was obtained in accordance with the Declaration of Helsinki. Mononuclear cells were obtained by density centrifugation. AML samples were selected on the basis of cytogenetic risk group for further study in immunodeficient mice.10 Samples that had a karyotype that was not in the good risk category11 were screened for their ability to engraft immunodeficient mice before use in this study (Table 1).
Antibodies
All antibodies were obtained from BD Biosciences (Oxford, United Kingdom) except the AT13/5 clone, which was a kind gift of Prof Martin Glennie12 (Southampton, United Kingdom). The clones used are detailed as follows. Cluster differentiation marker clone isotype: CD3 HIT3a IgG2a κ, CD13 WM15 IgG1 κ, CD20 2H7 IgG2b κ, CD34 581 IgG1 κ, CD38 HIT2 IgG1 κ, CD38 HB7 IgG1 κ, CD38 AT13/5 IgG1 κ, CD45 H130 IgG1 κ, CD123 9F5 IgG1 κ.
F(ab′)2 fragments
F(ab′)2 fragments of anti-CD38 antibody (AT13/5) were produced by enzymatic digestion using ficin (Pierce Chemical, Rockford, IL) and then run through a column containing protein A to remove residual Fc-bearing molecules. The quality of the F(ab′)2 fragments was checked using sodium dodecyl sulfate gel. The binding of the F(ab′)2 fragments was also assessed based on the ability of the fragment to block binding of whole antibody.
Mice
NOD/SCID, NOD/SCID/β2-microglobulin null (NOD/SCID/β2m−/−) and NOD/SCID/interleukin 2 receptor γ chain null (NOD/SCID/IL2rγ−/−) mice were a kind gift of Dr Leonard Shultz and were used as detailed previously.9,10 All animal experiments were performed in accordance to Home Office and Cancer Research United Kingdom guidelines. Immunosuppression was increased for specific experiments using either human immunoglobulin (IVIG; Bio Products Laboratory, Elstree, United Kingdom) or anti-CD122 antibody that was produced from the TM beta 1 hybridoma kindly given by Dr Tanaka (Osaka University Medical Center, Osaka, Japan).13 A total of 0.5 mg/g of IVIG was administered by intraperitoneal injection on the morning of the day before transplantation and on the day of transplantation (total dose of 1 mg/g).14 A total of 200 μg of anti-CD122 antibody was administered by intraperitoneal injection on the day before transplantation.15 Cells were injected by intravenous route unless stated otherwise. Direct intra–bone marrow injection was performed under general anesthesia as described before.16
Assessment of engraftment
For experiments in which the effect of antibody on engraftment was determined, mice were killed at 6 weeks. For experiments in which sorted cells were transplanted, mice were killed at 8 to 12 weeks. Engraftment was assessed by immunophenotyping as described before.9,10 Briefly, normal multilineage engraftment was defined by the presence of separate CD45+CD33+ and CD45+CD19+ populations with the appropriate scatter characteristics. AML engraftment was defined by the presence of a single CD45+CD33+ population. Where there was doubt as to the origin of engrafted cells, we assayed the cells for leukemia-specific mutations, where possible.
For analysis of engraftment at 16 hours after transplantation, mononuclear cells were stained with PKH26 dye (Sigma-Aldrich, Poole, United Kingdom) according to manufacturer's instructions. Stained cells were injected intravenously and mice were killed 16 hours after transplantation. The bone marrow was analyzed by flow cytometry. Repopulating cells reside in the undivided PKH26 bright population.17 The percentage of PKH26 bright cells was determined (Figure S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). More than 500 000 events were recorded for each mouse analyzed.
Effect of antibodies on engraftment
Mononuclear cells from cord blood or AML patients were incubated with test antibody or isotype control for 30 minutes at 4°C. For initial experiments, 5 μL of the relevant conjugated antibody was used to stain 1.5 × 106 cells in 50 μL as if for cell sorting (Figure 1). For later experiments, 0.5 μg of antibody was incubated with 1.5 × 106 cells in a volume of 50 μL. The cells were then washed twice and resuspended in phosphate-buffered saline with 2% inactivated fetal calf serum before transplantation into mice. For each experiment, age- and sex-matched mice were used.
Cell sorting
AML cells were stained with phycoerythrin-conjugated anti-CD34 and phycoerythrin-cyanin 7-conjugated anti-CD38 before resuspension in a 4,6 diamidino-2-phenylindole containing solution of 2% fetal calf serum with phosphate-buffered saline. Sorting was performed on a BD Aria. Gates were set up to exclude nonviable cells and debris. Purity checks were performed to ensure sort quality. For each AML sample sorted, the entire CD34+CD38− fraction went into 1 mouse (except for sample 8, which was transplanted into 2 mice, one of which developed autologous lymphoma; this mouse was excluded from analysis as bone marrow was replaced by lymphoma cells). The CD34+CD38+ fraction of each sample was split between 1 to 4 mice.
Assessment of nucleophosmin mutation within engrafted mice
Statistics
Generalized linear models based on the negative binomial distribution were used to assess statistical significance of the difference between engraftment percentages in groups of mice receiving cells incubated with different antibodies.
Results
Anti-CD38 antibodies inhibit engraftment of both normal and leukemic cells in NOD/SCID mice
We investigated the effect of exposing mononuclear cells (from cord blood or AML) to antibody before transplantation into mice. Initially, antibodies against antigens expressed on repopulating cells were studied.1,6,9,19 Antibodies to CD45 (P < .001; Figure S1) and CD34 (P < .001; Figures S1,1A) inhibited engraftment of cord blood in the NOD/SCID mouse, whereas antibodies to CD13 (P = .27) and CD123 (P = .21) did not (Figure 1A). Neither antibodies to CD13 (P = .69) nor CD123 (P = .18) inhibited engraftment of AML (Figure 1B).
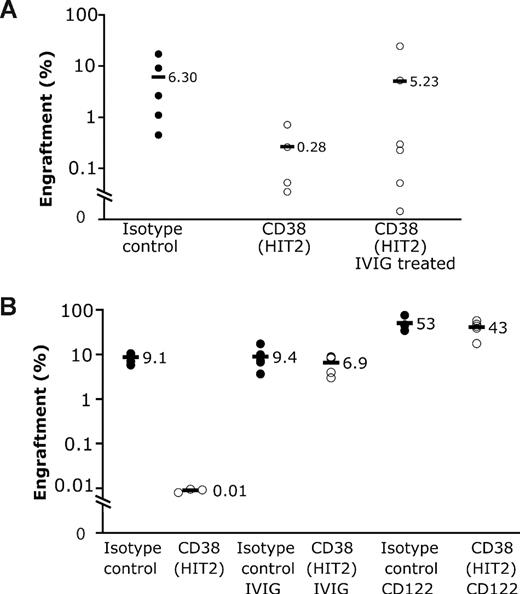
Effect of antibody on engraftment of human cells. Human mononuclear cells were incubated with either test antibody or isotype control before washing and transplantation into mice. After 6 weeks, the percentage of human cells in the bone marrow was determined. Horizontal bars represent mean engraftment percentage. (A) A total of 3.5 × 106 cord blood mononuclear cells were injected per mouse. There was a significant difference between control and anti-CD34 antibody (P < .001) and anti-CD38 antibody (P < .001). (B) A total of 5 × 106 AML cells (sample 1) were transplanted per mouse. Only anti-CD38 antibody significantly inhibited engraftment (P < .001). Blasts from sample 1 express CD13 and CD123 but lack CD34.
Effect of antibody on engraftment of human cells. Human mononuclear cells were incubated with either test antibody or isotype control before washing and transplantation into mice. After 6 weeks, the percentage of human cells in the bone marrow was determined. Horizontal bars represent mean engraftment percentage. (A) A total of 3.5 × 106 cord blood mononuclear cells were injected per mouse. There was a significant difference between control and anti-CD34 antibody (P < .001) and anti-CD38 antibody (P < .001). (B) A total of 5 × 106 AML cells (sample 1) were transplanted per mouse. Only anti-CD38 antibody significantly inhibited engraftment (P < .001). Blasts from sample 1 express CD13 and CD123 but lack CD34.
We then assessed the effect of antibodies against markers thought not to be on repopulating cells.1 Neither antibodies to CD3 nor CD20 (P = .43) inhibited engraftment of cord blood (data not shown). However, anti-CD38 antibody (HIT2 clone) inhibited engraftment of cord blood cells in 4 separate experiments (P < .001) with a mean 16-fold (± 5) reduction in the percentage engraftment (Figure 1A). The AT13/5 (P < .001; Figure 2A) and HB7 (P = .02; data not shown) clones of CD38 also reduced engraftment significantly.
Inhibitory effect of anti-CD38 antibody is mediated by Fc portion. Cord blood (A) or AML (B) mononuclear cells were incubated with whole antibody or F(ab′)2 fragments of anti-CD38 antibody (clone AT13/5) before washing and injection into mice. After 6 weeks, the percentage of human cells in the bone marrow was determined. Horizontal bars represent mean engraftment percentage per condition. (A) A total of 5 × 106 cord blood cells were injected per mouse. There was no significant difference in engraftment between control and F(ab′)2 groups (P = .45), whereas there were significant differences between the anti-CD38 antibody and F(ab′)2 groups (P < .001). (B) A total of 6 × 106 AML cells (sample 4) were injected per mouse. There was a significant difference between anti-CD38 antibody and F(ab′)2 groups (P < .001).
Inhibitory effect of anti-CD38 antibody is mediated by Fc portion. Cord blood (A) or AML (B) mononuclear cells were incubated with whole antibody or F(ab′)2 fragments of anti-CD38 antibody (clone AT13/5) before washing and injection into mice. After 6 weeks, the percentage of human cells in the bone marrow was determined. Horizontal bars represent mean engraftment percentage per condition. (A) A total of 5 × 106 cord blood cells were injected per mouse. There was no significant difference in engraftment between control and F(ab′)2 groups (P = .45), whereas there were significant differences between the anti-CD38 antibody and F(ab′)2 groups (P < .001). (B) A total of 6 × 106 AML cells (sample 4) were injected per mouse. There was a significant difference between anti-CD38 antibody and F(ab′)2 groups (P < .001).
The effect of CD38 antibody was rapid; the number of PKH26 bright cord blood cells was reduced in the bone marrow just 16 hours after injection (P < .001; Figure S2).
Accessory cells do not reverse the inhibitory effects of CD38
Given that repopulating cells are thought to be CD38-negative, we suspected that the effect must be by killing accessory cells.20 However, addition of 106 irradiated cord blood mononuclear cells to CD38-coated mononuclear cells after washing anti-CD38 antibody from the cells did not reverse the inhibitory action. There was no significant difference between cells treated with CD38 only and cells treated with CD38 before addition of accessory cells at 6 (P = .9) or 11 weeks after transplantation (P = .2; data not shown).
Inhibitory effect of CD38 antibody is dependent on Fc receptors
To determine whether the effect of anti-CD38 antibody was mediated by the Fc portion of the antibody, human cells were incubated with either anti-CD38 antibody or F(ab′)2 fragments of the same antibody and injected into mice. Engraftment was significantly higher for cells treated with the F(ab′)2 fragment than whole antibody (P < .001; Figure 2).
Inhibitory effect of CD38 antibody is reduced by treating mice with immunosuppressive antibodies
It is not feasible to make F(ab′)2 fragments of all antibodies relevant to hematopoiesis. A more generally applicable approach is required. We therefore treated mice with immunosuppressive antibodies to try to reverse the antibody-mediated clearance. IVIG has been used to treat immune thrombocytopenia in humans and in mouse models.14 Anti-CD122 antibody binds to the interleukin-2 receptor and depletes natural killer cells and some macrophages and monocytes.13
IVIG reversed the inhibitory effect of anti-CD38 in both normal (Figure 3A) and AML samples (Figure 3B). Anti-CD122 not only reversed this effect but also significantly increased the level of engraftment obtained with AML (Figure 3B). This is supported by previously published data reporting a significant increase of cord blood engraftment in anti-CD122–pretreated immunodeficient mice, revealing a new population of so-called rapid SCID-repopulating cells in the CD34+CD38+ cell fraction.15
Additional immunosuppression reverses inhibitory effect of CD38. IVIG or anti-CD122 antibody were administered to mice to attempt to reverse antibody-mediated clearance of cells. (A) IVIG was administered to mice before injection of 6 million cord blood cells previously incubated with either control or anti-CD38 antibody. There was a significant difference between anti-CD38 antibody and anti-CD38 antibody plus IVIG groups (P < .001), whereas there was no significant difference between control and anti-CD38 antibody plus IVIG groups (P = .09). (B) Treatment of mice with either IVIG or anti-CD122 antibody resulted in the reversal of the inhibitory effect of anti-CD38 antibody on engraftment of AML cells (sample 1; 5.7 × 106 per mouse; P < .001).
Additional immunosuppression reverses inhibitory effect of CD38. IVIG or anti-CD122 antibody were administered to mice to attempt to reverse antibody-mediated clearance of cells. (A) IVIG was administered to mice before injection of 6 million cord blood cells previously incubated with either control or anti-CD38 antibody. There was a significant difference between anti-CD38 antibody and anti-CD38 antibody plus IVIG groups (P < .001), whereas there was no significant difference between control and anti-CD38 antibody plus IVIG groups (P = .09). (B) Treatment of mice with either IVIG or anti-CD122 antibody resulted in the reversal of the inhibitory effect of anti-CD38 antibody on engraftment of AML cells (sample 1; 5.7 × 106 per mouse; P < .001).
Inhibitory effect of CD38 persists in more immunodeficient mice
We tested whether the inhibitory effect of CD38 antibody would be preserved in more immunodeficient variants of the NOD/SCID mouse. In NOD/SCID/β2m−/− mice, in which natural killer cells are genetically depleted, engraftment of cord blood cells was reduced by 4-fold compared with 16-fold with NOD/SCID mice (P = .06; Table S1). In NOD/SCID/β2m−/− mice, the engraftment of AML sample 3 was abolished by the HIT2 and AT13/5 clones of CD38 (P < .001; Figure 4A), whereas the engraftment of sample 1 was reduced by 3-fold (P = .08; Table S1).
Effect of CD38 antibody in NOD/SCID/β2m−/− and NOD/SCID/IL2rγ−/− mice. AML cells were incubated with either isotype control or test antibody and transplanted into mice. (A) A total of 5.5 × 106 AML cells (sample 3) were transplanted into NOD/SCID/β2m−/− mice. Both the HIT2 and AT13/5 clones of anti-CD38 antibody abolished engraftment of AML at 6 weeks. Blasts from sample 3 express CD33 and CD13. A total of 7.5 × 106 T-cell-deplete AML cells were transplanted into NOD/SCID/IL2rγ−/− mice from sample 4 (B) and sample 5 (C). Engraftment of both AML and normal human hematopoietic cells was noted in each mouse. The percentage of human cells with mutant NPM and wild-type (WT) NPM was determined quantitatively to assess percentage of normal and AML cells. ● and ○ represent the percentage of total bone marrow that was leukemic. ▴ and ▵ represent the percentage of bone marrow that was normal human hematopoietic cells. Note that for each mouse there is a circle and a triangle. For each AML sample, there was a significant reduction in the percentage of AML in mice receiving cells incubated with CD38 antibody (P = .001), whereas there was no significant reduction in normal hematopoietic cells from these same mice (P > .1).
Effect of CD38 antibody in NOD/SCID/β2m−/− and NOD/SCID/IL2rγ−/− mice. AML cells were incubated with either isotype control or test antibody and transplanted into mice. (A) A total of 5.5 × 106 AML cells (sample 3) were transplanted into NOD/SCID/β2m−/− mice. Both the HIT2 and AT13/5 clones of anti-CD38 antibody abolished engraftment of AML at 6 weeks. Blasts from sample 3 express CD33 and CD13. A total of 7.5 × 106 T-cell-deplete AML cells were transplanted into NOD/SCID/IL2rγ−/− mice from sample 4 (B) and sample 5 (C). Engraftment of both AML and normal human hematopoietic cells was noted in each mouse. The percentage of human cells with mutant NPM and wild-type (WT) NPM was determined quantitatively to assess percentage of normal and AML cells. ● and ○ represent the percentage of total bone marrow that was leukemic. ▴ and ▵ represent the percentage of bone marrow that was normal human hematopoietic cells. Note that for each mouse there is a circle and a triangle. For each AML sample, there was a significant reduction in the percentage of AML in mice receiving cells incubated with CD38 antibody (P = .001), whereas there was no significant reduction in normal hematopoietic cells from these same mice (P > .1).
Anti-CD38 also inhibited the engraftment of human cells from 2 AML samples (4 and 5) in NOD/SCID/IL2rγ−/− mice (these are supposed to be the most permissive mouse strain) (P < .01; Table S1). We noted that, in these mice transplanted with AML, a large CD45+CD33+ population as well as a smaller CD45+CD19+ population was detected. To assess whether these human cells were normal or leukemic, we sorted each population and quantitatively analyzed the exon 12 of the nucleophosmin (NPM) gene. The CD45+CD19+ population contained wild-type NPM. The majority of the CD45+CD33+ cells contained mutant NPM with a small amount of wild-type NPM (89% ± 12% mutant NPM for sample 4 and 80% ± 1% mutant NPM for sample 5). The data indicate that, in this mouse strain, chimeric engraftment is present with both AML and normal hematopoietic cells within the same mouse both derived from the same patient sample. Interestingly, in this model, the anti-CD38 had a greater effect on leukemic cells (4.8- to 11-fold reduction in engraftment) than on normal cells (1.7- to 1.8-fold reduction) within the same mouse (Figure 4B,C). These data not only indicate that anti-CD38 still had an inhibitory effect on AML samples in NOD/SCID/IL2rγ−/− mice but also that caution must be taken when analyzing the engraftment of AML samples in this mouse strain as chimeric engraftment might be present.
Intrabone marrow injection reduces the inhibitory effect of CD38 antibody
We assessed whether direct intrabone marrow injection might abrogate the inhibitory effect given that peripheral clearance of antibody-coated cells by the spleen is well recognized in humans. CD38 reduced the engraftment of AML sample 1 by 5-fold (P = .05) when the sample was given by intrabone marrow injection (data not shown) but more than 100-fold (P < .001) when given by intravenous injection (Figure 1B).
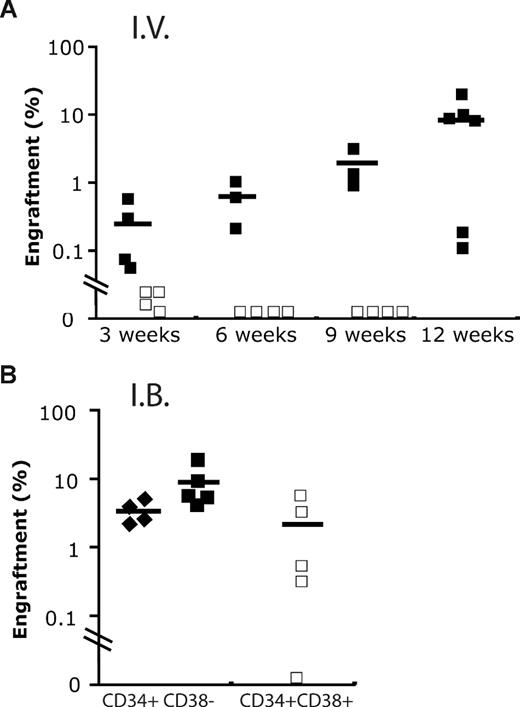
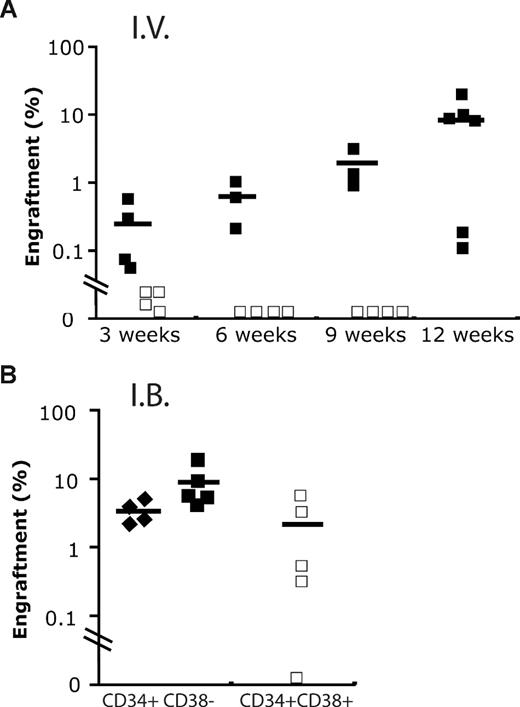
We also compared the repopulating potential present in CD34+CD38+ cord blood cells using either intrabone marrow or intravenous injection. Although short-term repopulation was seen with the intravenous route, by 9 weeks no human cells were detectable. By contrast, multilineage hematopoiesis was seen up to 12 weeks when cells were injected intrabone (Figure 5). Of note, far fewer CD34+CD38− cord blood cells were required to engraft mice. These data are in accordance with a previous study reporting the presence of a repopulating activity in the CD34+CD38+ cord blood cells up to 12 weeks. Nevertheless, this fraction was unable to give rise to secondary transplantation.3
Engraftment of cord blood fraction by intravenous and intrabone marrow routes. (A) Human engraftment kinetics produced by 2400 CB Lin−CD34+CD38− (+/−; ■) and 48 000 Lin−CD34+CD38+ (+/+; □) cells, respectively, when administrated intravenously (I.V.) into NOD/SCID−β2−/− mice. (B) Although similar doses of +/+ cells were able to engraft in immunodeficient mice at 9 to 12 weeks using intrabone marrow injection (I.B.), the level of engraftment produced was still lower compared with 600+/− cells (♦).
Engraftment of cord blood fraction by intravenous and intrabone marrow routes. (A) Human engraftment kinetics produced by 2400 CB Lin−CD34+CD38− (+/−; ■) and 48 000 Lin−CD34+CD38+ (+/+; □) cells, respectively, when administrated intravenously (I.V.) into NOD/SCID−β2−/− mice. (B) Although similar doses of +/+ cells were able to engraft in immunodeficient mice at 9 to 12 weeks using intrabone marrow injection (I.B.), the level of engraftment produced was still lower compared with 600+/− cells (♦).
Some SL-ICs express CD38
Given the pronounced inhibitory effect of anti-CD38 antibody on engraftment of AML, we suspected that some SL-ICs express CD38. To test this hypothesis, we transplanted immunodeficient mice with CD34+CD38+ and CD34+CD38− fractions from 7 AML samples but used measures to abrogate the inhibitory effect of anti-CD38 antibody. The most immunodeficient strains of mice available were used, and additional immunosuppressive treatment was administered in the form of IVIG14 or anti-CD122 antibody.15 In addition, the intrabone marrow route was used for most experiments. Mice were killed 8 to 11 weeks after transplantation. The mean purity of the CD34+CD38+ fraction was 92.3% (± 6%). The phenotypes are shown in Figure S3. Results are summarized in Table 2. Engraftment was seen from all 7 AML samples from the CD34+CD38+ fraction, with AML developing in 19 of 21 mice. By contrast, no leukemic engraftment was seen after transplantation of CD34+CD38− cells in 4 of 7 samples (samples 3, 4, 7, and 10). CD34+CD38− cells from one of these 4 samples (sample 4) gave rise to multilineage engraftment consistent with engraftment of normal hematopoietic cells. The NPM gene in these cells was wild-type (by contrast, the engrafted cells from the CD34+CD38+ fraction contained mutated NPM). Of the mice transplanted with CD34+CD38− cells from the 3 other samples (samples 6, 8, and 9), one died before analysis (sample 6), one was engrafted by AML (sample 8), and one had chimeric engraftment (sample 9). Indeed, in this sample, both CD45+CD33+ and CD45+CD19+ cells of approximately equal proportions were present. Nevertheless, it has not been possible to confirm whether the myeloid cells were normal or leukemic as sample 9 had a normal karyotype and no mutations identified.
Of note, 2 samples (samples 3 and 4) that were inhibited more than 100-fold by CD38 antibody had SL-ICs limited to the CD34+CD38+ fraction (Table S1).
Discussion
We have demonstrated that anti-CD38 antibody has a profound negative effect on the engraftment of human cord blood and AML repopulating cells in NOD/SCID mice. The data support the notion that CD38-coated human cells are cleared by innate immune cells through a process that is dependent on the Fc portion of the antibody. The inhibitory effect of CD38 was less marked in mouse strains having a genetic deficit in natural killer cells than in NOD/SCID mice, suggesting that natural killer cells have a role in this clearance. Although the effect of CD38 was reduced in more immunodeficient strains, it was still significant, indicating that other innate effectors are also involved.
Different AML specimens appear to display different vulnerabilities to killing by different immune effectors. For example, sample 3 was eliminated by cells other than natural killer cells as anti-CD38 antibody completely abolished engraftment in the NOD/SCID/β2m−/− that lacks natural killer activity. Sample 1, on the other hand, was susceptible to natural killer cell–mediated killing as there was a far greater inhibitory effect in NOD/SCID compared with NOD/SCID/β2m−/− mice.
It has been suggested that intrabone injection is more efficient than intravenous injection because of failure of some repopulating cells to home to the bone marrow.15 An alternative explanation is that intravenously injected repopulating cells are cleared in the periphery by immune cells. The spleen is a key organ in the immune-mediated clearance of antibody-coated cells in humans, and splenectomy is sometimes performed to relieve autoimmune cytopenias.21 The spleen may be mediating the effect of the anti-CD38 antibody (although other peripheral organs such as the lung or liver may also be involved) as injection of cells directly into the bone marrow partially abrogates the inhibitory effect. Another possible mechanism is that anti-CD38 antibody induces clumping of cells in vitro. Once transplanted, clumped cells may lodge in the spleen or lung, thus failing to reach the marrow.
The presence of a surface antigen on a repopulating cell did not guarantee that an antibody against that antigen would inhibit engraftment. Other factors must determine whether the antibody has an inhibitory effect. The isotype of the antibody cannot be critical given that almost all the antibodies assessed were mouse IgG1 κ and yet several had little or no effect on engraftment. We have tried to establish the reason why anti-CD38 antibody is so active compared with the other antibodies using antibody-dependent cytotoxicity assays. Although anti-CD38 antibody had a statistically significant effect compared with isotype control, anti-CD38 was not more active than other antibodies, such as anti-CD13. There was no effect of anti-CD38 antibody alone or after cross-linking, and so, an interaction with immune cells is required.
Consistent with our data, it has been reported that treating NOD/SCID mice with anti–natural killer cell antibody allows engraftment of normal CD34+CD38+ cells at 12 weeks.3 These cells, however, did not have the ability to generate a graft in secondary transplantations and are likely to equate to multipotent progenitors rather than HSCs.3 A similar population was capable of engrafting primary but not secondary recipients in preimmune sheep.2 These cells were probably eliminated by antibody-mediated immune clearance in other studies.1
The finding that SL-ICs were found in the CD34+CD38+ fraction from all 7 sorted AML samples is novel. The potent inhibitory effect of anti-CD38 antibody is the probable explanation why this was not seen in previous studies. The data from inhibitory experiments back up the sorting experiments; one advantage these have over the sorting experiments is that these avoid the subjectivity that is inherent in selecting negative and positive populations. We have not yet tested whether these CD34+CD38+ cells can initiate leukemia in secondary transplantations, and it is possible that these CD34+CD38+ cells are leukemic progenitors with limited self-renewal capacity (analogous to normal multipotent progenitors). Nevertheless, it should be pointed out that, in samples 3 and 4, all SL-IC seems to be restricted to the CD34+CD38+ fraction, suggesting, at least in these patients, that these cells might have self-renewal potential.
We observed no leukemic engraftment from the CD34+CD38− fraction of 4 samples. This appears to conflict with previous studies6,7 suggesting that SL-ICs are restricted to the CD34+CD38− fraction. We think that the likely explanation for this apparent discrepancy lies in the heterogeneity of AML. There is a large variation in the expression of CD34 and CD38; in this study, the median percentage of CD34+CD38− was 0.076% (range, 0.0044%-8.1%) for the sorted AMLs; whereas in the Bonnet and Dick study,6 the median was approximately 10-fold higher at 0.75% (range, 0.02%-2%). The 4 samples with lowest CD34+CD38− expression had SL-ICs restricted to the CD34+CD38+ fraction. When one looks at the expression profiles of the samples with the smallest CD34+CD38− fraction (samples 3 and 10, Figure S3), it can be seen that this fraction is negligible; thus, it is perhaps unsurprising that there are no SL-ICs detectable within it. With further study, it may be possible to identify the likely phenotype of SL-ICs in a particular AML based on the expression profile of CD34 and CD38.
There are 2 caveats that may explain why CD34+CD38− cells did not engraft: (1) We assessed engraftment of sorted fractions at 8 to 11 weeks and had no leukemia engraftment from 4 of 7 leukemia samples injected with CD34+CD38− cells; it is possible that CD34+CD38− cells may initiate leukemia only at later time points or only in secondary transplantations, as has been suggested using tracking experiments.22 (2) There may be a minimum threshold dose of cells required to initiate AML in immunodeficient mice. (In previous studies, AML was initiated after transplantation of 1000-5000 CD34+CD38− cells.6,7 ) In 6 of 8 experiments, we transplanted a dose of CD34+CD38− cells that was equivalent to a greater dose of mononuclear cells than the dose of CD34+CD38+ cells (Table 2). However, the absolute dose of CD34+CD38− cells transplanted was in the thousands for most experiments. It is possible that some SL-ICs reside within this fraction but are excluded as a result of inefficiencies of the xenotransplant model.
The identity of the normal cell from which leukemia originates has been the source of much recent debate. The analysis of the phenotypes of SL-ICs has been used to determine the origin of different types of acute lymphoblastic leukemia.23 The similarity of AML SL-ICs and normal repopulating cells (ie, CD34+CD38−) led some authors to hypothesize that AML is derived from an HSC.6 Others argue that the features of myeloid differentiation that define AML point to a progenitor origin. In support of this, it has been demonstrated that mouse myeloid progenitors can be transformed by the MLL oncogene.24 One would predict that SL-ICs derived from a normal progenitor would have phenotypic features of progenitors with expression of CD34 and CD38. Of relevance, sample 3, which contained the translocation t(11;19)(q23;p13.1) involving the MLL oncogene, had SL-ICs detectable only within the CD34+CD38+ fraction (Table 2).
Recent data suggest that the situation is probably more complicated. Barabe et al used the MLL oncogene to transform human cord blood cells into acute leukemia.25 This work suggested that leukemic progenitors acquire self-renewal capacity and become a second generation of SL-ICs (the first generation comprising SL-ICs derived from HSCs). In patients, similar events may occur; this is a possible explanation for the presence of 2 types of SL-ICs in some samples (eg, sample 8): one with a primitive (CD34+CD38−) phenotype and one with a progenitor (CD34+CD38+) phenotype. Barabe et al25 reported that the second-generation SL-ICs came to predominate as the leukemia evolved. The same thing may happen in some patients. By the time of diagnosis, second-generation SL-ICs with a progenitor phenotype (CD34+CD38+) may predominate and SL-ICs with a primitive phenotype (CD34+CD38−) may no longer be detectable. This explanation is an alternative to the direct transformation of normal myeloid progenitors. Further study will be needed to better delineate this important question.
Of note, the effect of anti-CD38 antibody is much greater for AML than for cord blood. In addition, the anti-CD38 antibody had a greater effect on leukemic grafts than on normal grafts where both were present in the same NOD/SCID/IL2rγ−/− mice (both leukemic and normal cells were derived from the same patient sample; Figure 4B,C). This differential effect may allow CD38 antibodies to be used therapeutically, particularly for cases of AML where all SL-ICs reside in the CD34+CD38+ fraction.
In conclusion, we demonstrate for the first time the elimination of repopulating cells by an antibody directed against an antigen thought not to be on repopulating cells. Any groups using the NOD/SCID mouse need to be aware of potential bias introduced by the use of antibodies. Unless the effects of antibodies are neutralized, sorted fractions that contain repopulating cells may not repopulate mice because the repopulating cells are eliminated by antibody-mediated clearance. This may lead to the incorrect assignment of the phenotype of repopulating cells and errors in determining the frequency of these cells. Moreover, we demonstrate that some SL-ICs express CD34 and CD38. This suggests that the SL-IC compartment is more heterogeneous than previously recognized. In combination with previous work, the current data suggest at least 3 phenotypes of SL-ICs: (1) CD34+CD38−, (2) CD34+CD38+, and (3) CD34−5. This heterogeneity not only indicates a potential differential origin or progression of the disease, but also has important implications for the development of new therapies to eradicate these cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who gave samples and Dr Michael Jenner for providing diagnostic information.
This work was supported by Cancer Research United Kingdom (D.B.). D.C.T. is supported by a Medical Research Council Clinician Scientist Fellowship.
Authorship
Contribution: D.C.T. designed and performed research, analyzed and interpreted data, performed statistical analysis, and wrote the manuscript; F.M.-M., D.J.P., K.A., and C.R. performed research; F.A.-A. performed research and analyzed and interpreted data; D.L. provided vital data; H.O. and J.C. provided vital materials; S.G.A. contributed vital analytic tools and materials; T.A.L. provided vital materials and data; J.G.G. interpreted data and wrote the manuscript; D.B. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dominique Bonnet, Haematopoietic Stem Cell Laboratory, CRUK London Research Institute, 44 Lincoln's Inn Fields, London WC2A 3PX, United Kingdom; e-mail: dominique.bonnet@cancer.org.uk or David Taussig, Medical Oncology, Room 310, John Vane Building, Chaterhouse Square ECIM6BQ, London, UK; e-mail: david.taussig@qmul.ac.uk.
References
Author notes
*F.M.-M. and F.A.-A. contributed equally to this study.