Abstract
Hematopoietic stem cells (HSCs) regenerated in vivo display sustained differences in their self-renewal and differentiation activities. Variations in Steel factor (SF) signaling are known to affect these functions in vitro, but the cellular and molecular mechanisms involved are not understood. To address these issues, we evaluated highly purified HSCs maintained in single-cell serum-free cultures containing 20 ng/mL IL-11 plus 1, 10, or 300 ng/mL SF. Under all conditions, more than 99% of the cells traversed a first cell cycle with similar kinetics. After 8 hours in the 10 or 300 ng/mL SF conditions, the frequency of HSCs remained unchanged. However, in the next 8 hours (ie, 6 hours before any cell divided), HSC integrity was sustained only in the 300 ng/mL SF cultures. The cells in these cultures also contained significantly higher levels of Bmi1, Lnk, and Ezh2 transcripts but not of several other regulators. Assessment of 21 first division progeny pairs further showed that only those generated in 300 ng/mL SF cultures contained HSCs and pairs of progeny with similar differentiation programs were not observed. Thus, SF signaling intensity can directly and coordinately alter the transcription factor profile and long-term repopulating ability of quiescent HSCs before their first division.
Introduction
The hematopoietic system of the adult mouse is responsible for the daily production of billions of differentiated blood cells of various types. Because most of these cells have a limited lifespan and proliferative ability, they must be continuously generated from a population of more primitive cells that collectively have life-long self-sustaining ability. This function is restricted to a tiny subset of multipotent cells generally referred to as hematopoietic stem cells (HSCs). Historically, HSCs have been both defined and quantified retrospectively by their ability to generate clones containing both lymphoid and myeloid blood cells for at least 4 months when transplanted into irradiated recipients at limiting dilutions.1 Analyses of such mice have also allowed the long-term differentiation activity of individual HSCs to be characterized.2,3 Methods have now been developed for isolating purified populations in which 20% to 60% of the cells display this durability of reconstituting activity, indicating that intravenously injected HSCs can have very high seeding efficiencies in irradiated mice.4-7 The ability to isolate such highly purified HSC populations has also permitted a more direct and complete analysis of their in vivo differentiation activity in single-cell transplant experiments.6,8-11 More recently, serial transplants of such clonally repopulated mice have been performed. Together, these experiments have revealed that HSCs possess one of 4 distinct differentiation programs that are propagated over many generations in vivo.10,12 In addition, these studies have shown that extensive self-renewal ability in vivo is strongly associated with the display of either a balanced lympho-myeloid differentiation ability or a highly skewed generation of myeloid progeny.10
Intense interest in understanding the mechanisms that control HSC self-renewal has led to the identification of multiple genes that intrinsically regulate HSC properties. These include regulators of the cell cycle, such as Cdkn1a (p21)13 and Cdkn2c (p18)14 and members of the transcriptional control machinery, such as HoxA9,15 HoxB4,16 Phc1 (Rae28),17 Bmi1,18 Ezh2,19 Gfi1,20 Gata221 and Gata3,22 Etv6,23 Pcgf2 (Mel18),24 Mcl1,25 Meis1,15 and Runx1.21 In addition, growth factor receptors, such as GP13026 and KIT,27 and signaling factors, such as STAT3,28 STAT5,29 PTEN,30 and LNK,11 have been shown to affect the ability of HSCs to execute self-renewal divisions. These findings reinforce data suggesting that multiple external cues, including Steel factor (SF),31 thrombopoietin,32,33 transforming growth factor-β,34 flt3-ligand,35 and factors, such as interleukin-6 (IL-6) and IL-11,36,37 which signal through GP130, can modulate HSC self-renewal, proliferation, and viability independently. Nevertheless, only a few studies6,38 have provided definitive evidence of the directed extrinsic alteration of HSC self-renewal because this requires clonal analysis of highly purified HSCs stimulated under conditions where differences in cell death cannot account for differences in HSC recoveries.
Thus, both the heterogeneity of the HSC compartment itself and the mechanisms that regulate these cells appear to be more complex than initially anticipated. To investigate how these 2 issues may be interrelated, we first analyzed the in vivo differentiation programs displayed by single HSCs isolated by a simpler purification strategy that combines Rhodamine-123 (Rho) dye exclusion6 with Endothelial Protein C Receptor (EPCR or CD201) expression.39 The purpose of these experiments was to determine the extent to which the heterogeneity of HSCs observed previously might be dependent on the HSC purification strategy used. We then examined the timing and magnitude of changes in HSC activity that occur over a 4-day period in cultures initiated with a single cell maintained under different conditions of growth factor stimulation. The conditions chosen included different SF concentrations that do not affect either the viability or the initial mitogenic response of the cells when IL-11 is also included in the medium and that allow some preservation of HSC activity if the concentration of SF is sufficiently high.37 The results obtained reinforce the concept of an adult HSC population that is compartmentalized into subsets with distinct self-renewal and differentiation programs. They also demonstrate the rapidity with which external cues can alter the expression of key transcriptional regulators and the subsequent long-term repopulating and differentiation activity displayed by the cells in vivo.
Methods
Mice
Bone marrow donors were 8- to 12-week-old C57Bl/6J-CD45.1 or CD45.2 mice. All transplant recipients were CD45-congenic C57Bl/6J-W41/W41 (W41/W41) mice previously given a sublethal dose of irradiation (360 cGy x-rays at 350 cGy per minute). All mice were bred and maintained in the British Columbia Cancer Research Centre animal facility in microisolator cages and provided with sterile food, water, and bedding. All animal procedures were done in accordance with the Canadian Council on Animal Care with specific project and protocol approval from the University of British Columbia Animal Care Committee.
Purification, culture, and transplantation of CD45midlin−Rho−SP cells and CD45midRho−EPCR++ cells
Bone marrow cells were harvested in 2% (vol/vol) fetal bovine serum-supplemented Hank's Balanced Salt Solution (collectively called HF). Red blood cells were lysed, and the cells were then washed and suspended at 1.0 to 2.5 × 106 cells/mL in prewarmed Iscove modified Dulbecco medium (StemCell Technologies, Vancouver, BC) supplemented with 10 mg/mL of bovine serum albumin, 10 μg/mL of insulin, and 200 μg/mL of transferrin (BIT; StemCell Technologies), 100 units/mL of penicillin, 100 μg/mL of streptomycin (StemCell Technologies), and 10−4 M of β-mercaptoethanol (Sigma-Aldrich, St Louis, MO) (referred to as serum-free medium (SFM)). Lineage marker-negative (lin−) CD45midRhodamine (Rho)− side population (SP) cells were then isolated as described.6,10,40 CD45midRho−EPCR++ cells were isolated after adding 0.1 μg/mL of Rho (Invitrogen, Carlsbad, CA) to the cells in SFM and incubating the suspension for 30 minutes at 37°C, before washing in cold HF, and incubating for a further 10 minutes in 10% rat serum (Sigma-Aldrich) plus 2.4G2 antimouse FcR antibody (StemCell Technologies). The cells were then stained for 30 minutes with allophycocyanin (APC)-conjugated anti-CD45 (BD Biosciences, San Jose, CA) and phycoerythrin (PE)–conjugated anti-EPCR (StemCell Technologies). Cells were finally washed and suspended in HF containing 0.1 μg/mL of propidium iodide (PI, Sigma-Aldrich). PI−CD45midRho−EPCR++ cells were sorted using the single-cell deposition unit on a FACSVantage or FACSDiVa (BD Biosciences) into the individual wells of a round-bottom 96-well plate containing 100 to 200 μL of SFM. Each plate was then visually inspected to establish the presence of a single viable cell in each well.6,40 Some single cells were then harvested directly and injected intravenously into sublethally irradiated Ly-5 congenic W41/W41 recipients.6,40 Other cells were first cultured for different periods of time with various concentrations of purified recombinant mouse SF (StemCell Technologies) as indicated and human IL-11 (Genetics Institute, Cambridge, MA) before assessment.
Analysis of transplanted mice
Peripheral blood samples were collected from the tail vein of mice 8, 16, and 24 weeks after transplantation. Red blood cells were lysed with ammonium chloride for 10 minutes on ice and the white blood cells (WBCs) were stained with antibodies for both donor and recipient CD45 allotypes (using anti-CD45.1-APC and anti-CD45.2-fluorescein isothiocyanate-labeled antibodies) as well as anti–Ly6g-PE/anti-Mac1-PE for myeloid (GM) cells, anti–B220-PE for B cells, and anti–CD5-PE for T cells (CD45.2-fluorescein isothiocyanate purified and conjugated in the Terry Fox Laboratory; CD45.1-APC from eBioscience, San Diego CA; all other antibodies from BD Biosciences). All double-negative or double-positive CD45.1 and CD45.2 events were excluded from further analysis. To calculate repopulation levels, the contributions of the donor CD45 allotype to the populations of circulating GM, B, T, and total WBCs were calculated. Recipients with more than 1% donor-derived WBCs at 16 and/or 24 weeks after transplantation were considered to be repopulated with HSCs; α (myeloid-biased), β (balanced lympho-myeloid), γ (lymphoid-biased and still multilineage at 4 months), and δ (lymphoid-biased but no longer multilineage at 4 months) subtypes of HSCs were discriminated by calculating the relative ratios of the donor contributions to the GM versus B plus T lineages measured at 4 months after transplantation.10
Quantitative real-time PCR analysis
A PicoPure kit (Arcturus Bioscience, Mountain View, CA) was used to extract RNA from cells harvested from cultures initiated 16 hours previously with 70 to 200 CD45midRho−EPCR++ adult mouse bone marrow cells placed in 200 μL of SFM containing 20 ng/mL of IL-11 and 1, 10, or 300 ng/mL of SF. The extracted RNA was reverse-transcribed into cDNA using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen Canada, Burlington, ON), and then quantitative real-time (Q-RT)-PCR analyses were performed using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). Primers were as follows: Gapdh (NM_008084) forward: AACTTTGGC-ATTGTGGAAGG and reverse: ATGCAGGGATGATGTTCTGG; Bmi-1 (NM_007552.3) forward: AAACCAGACCACTCCTGAACA and reverse: TCTTCTTCTCTTCATCTCATTTTTGA; Ezh2 (NM_007971.1) forward: CGCTCTTCTGTCGACGATGTTTT and reverse: GTTGGGTGTTGCATGGAAGG; Gata2 (NM_008090.3) forward: TGACTATGGCAGCAGTCTCTTC and reverse: ACACACTCCCGGCCTTCT; Gata3 (NM_008091.2) forward: GGTATCCTCCGACCCACCAC and reverse: CCAGCCAGGGCAGAGATCC; Gfi1 (NM_010278.2) forward: CGAGATGTGCGGCAAGACC and reverse: ACAGTCAAAGCTGCGTTCCT; Hoxa9 (NM_010456.2) forward: GTTCCAGCGTCTGGTGTTTT and reverse: ACAATGCCGAGAATGAGAGC; Sh2b3 (Lnk, NM_008507.3) forward: CAACACACACAAGGCTGTCA and reverse: CCTGTGCACAAGAACTACATCTG; Meis1 (NM_010789.2) forward: GCACAGGTGACGATGATGAC and reverse: AGGGTGTGTTAGATGCTGGAA; Runx1 (NM_009821) forward: AAGACCCTGCCCATCGCTTT and reverse: TGCCATGACGGTGACCAGAG.
Statistical analysis
L-Calc software (StemCell Technologies) was used to quantify the number of HSCs present by limiting dilution analysis of pooled 10-day clones. In Table 1, the range defined by plus or minus 1 SEM is shown in parentheses underneath the calculated frequency of HSCs present. Fisher exact test (available in the open source statistics package R [www.r-project.org]) was used to determine whether or not the frequency of HSCs calculated in individual 8-hour, 16-hour, and 1- to 2-day doublet cells, and 4-day clones was significantly different from the input frequency of HSCs.
HSC yields are reduced in cultures containing a low concentration of SF
| Cytokine condition . | Dose of cells injected* . | No. of positive mice/no. of mice tested . | No. of HSCs detected after 10 days (± SEM)† . |
|---|---|---|---|
| 300 ng/mL SF | 7 | 5/5 | 27 |
| + 20 ng/mL IL-11 | 2 | 3/8 | (19-39) |
| 0.7 | 0/6 | ||
| 10 ng/mL SF | 7 | 1/5 | 2 |
| + 20 ng/mL IL-11 | 2 | 0/5 | (0.8-6) |
| 0.7 | 0/3 |
| Cytokine condition . | Dose of cells injected* . | No. of positive mice/no. of mice tested . | No. of HSCs detected after 10 days (± SEM)† . |
|---|---|---|---|
| 300 ng/mL SF | 7 | 5/5 | 27 |
| + 20 ng/mL IL-11 | 2 | 3/8 | (19-39) |
| 0.7 | 0/6 | ||
| 10 ng/mL SF | 7 | 1/5 | 2 |
| + 20 ng/mL IL-11 | 2 | 0/5 | (0.8-6) |
| 0.7 | 0/3 |
Each dose of transplanted cells was sampled from a large pool of 10-day clones generated in either 300 ng/mL or 10 ng/mL SF in combination with 20 ng/mL IL-11. The cell doses are expressed as “starting equivalents” (day 0 = ∼ 30 HSCs). Because each experiment was initiated with 100 starting cells, a cell dose of 7 represents 7% of all the cells from the pooled clones.
L-Calc software (StemCell Technologies) was used to calculate the mean frequency of HSCs in the pooled 10-day clones from limiting dilution transplant results. The starting HSC frequency (3/10 = 30%) was determined from single-cell transplants of freshly isolated CD45midlin−Rho−SP cells.
Results
Different patterns of in vivo repopulation are obtained by individual HSCs isolated using different purification strategies
We have previously shown that 4 distinct differentiation patterns are revealed when single CD45midlin−Rho−SP cells from normal adult bone marrow are assayed in vivo and their progeny tracked longitudinally.6,10,40 However, the Hoechst 33342 staining protocol used to obtain these cells is both cumbersome and associated with some toxicity,41 and subsequent studies suggested it might be replaced by selecting for cells expressing very high levels of EPCR39 (top 0.1% of total mouse bone marrow cells, Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). To characterize the CD45midRho−EPCR++ population in terms of HSC purity and subtype, we transplanted 27 sublethally irradiated W41/W41 mice each with a single CD45-congenic cell of this phenotype in a total of 3 experiments and then analyzed the subsequent contribution of the input cell to GM, B, and T cells in the peripheral blood (Figure 1A,B). The clones derived from 18 (66%) of these single-cell transplants contributed at least 1% of the total circulating WBCs at one or more time points during the 6 months of follow-up. Of these 18 clones, 13 (48% of the original 27) were clearly multilineage at some time point and reached an overall level of more than 1% of all the WBCs at 4 months after transplantation (Figure 1C,D). The 4 types of repopulation patterns observed previously (ie, α = myeloid-biased, β = balanced lympho-myeloid, γ = lymphoid-biased and still multilineage at 4 months, and δ = lymphoid-biased but no longer multilineage at 4 months)10 were also seen in the mice injected with single CD45midRho−EPCR++ cells. These patterns are illustrated in Figure 2 where the data are shown both as clonal contributions to all 3 lineages combined (Figure 2A-D) and also as proportional contributions to each of the 3 lineages monitored (Figure 2E-H). Importantly, the frequency of each of the 4 subtypes within the CD45midRho−EPCR++ fraction was similar to that previously described for single CD45midlin−Rho−SP cells (Figure 1E,F)10 consistent with the idea that these represent significant subsets of the entire dormant HSC population. Cells isolated using either purification strategy can thus be considered equivalent and, accordingly, results obtained from either source of cells in subsequent experiments were pooled.
Approximately half of all CD45midRho−EPCR++ cells are HSCs, and these segregate into the same distribution of subtypes as CD45midlin−Rho−SP HSCs. (A) The selection of a Rho− population as defined by cells unstained with Rho. If the Rho− cells are plotted in a CD45 vs EPCR plot (B), it is evident that the majority of the remaining EPCR++ cells are CD45mid and comprise a distinct population that represents only 0.11% of the Rho− fraction for a total of 0.005% of the total adult mouse bone marrow cells. When 3 independent transplantation experiments were performed and the results were pooled, the frequency of HSCs was approximately 50% (C). Representative fluorescence-activated cell sorter plots for a repopulated mouse transplanted 16 weeks earlier with a single CD45midRho−EPCR++ cell are shown in panel D. By comparing the ratio of donor-derived B, T, and GM cells in the peripheral blood at 16 weeks, mice were determined to have been repopulated by one of the 4 previously defined HSC subtypes.10 The pie charts in panels E and F show the percentage of each subtype that is represented in each HSC population.
Approximately half of all CD45midRho−EPCR++ cells are HSCs, and these segregate into the same distribution of subtypes as CD45midlin−Rho−SP HSCs. (A) The selection of a Rho− population as defined by cells unstained with Rho. If the Rho− cells are plotted in a CD45 vs EPCR plot (B), it is evident that the majority of the remaining EPCR++ cells are CD45mid and comprise a distinct population that represents only 0.11% of the Rho− fraction for a total of 0.005% of the total adult mouse bone marrow cells. When 3 independent transplantation experiments were performed and the results were pooled, the frequency of HSCs was approximately 50% (C). Representative fluorescence-activated cell sorter plots for a repopulated mouse transplanted 16 weeks earlier with a single CD45midRho−EPCR++ cell are shown in panel D. By comparing the ratio of donor-derived B, T, and GM cells in the peripheral blood at 16 weeks, mice were determined to have been repopulated by one of the 4 previously defined HSC subtypes.10 The pie charts in panels E and F show the percentage of each subtype that is represented in each HSC population.
All 4 patterns of HSC differentiation are observed in the recipients of single CD45midRho−EPCR++ cells. (A-D) Examples of individual mice repopulated with freshly isolated cells where colored areas represent donor WBCs of GM (red), B-cell (blue), and T-cell (yellow) lineages as a percentage of all WBCs over time after transplant. Data are stacked such that the sum of each lineage contribution represents the percentage donor contribution to all WBCs. (E-H) The separate donor contributions to the individual GM (red), B-cell (blue), and T-cell (yellow) lineages shown as bars. The percentage donor contribution to the total WBCs is shown as a gray area behind the bars.
All 4 patterns of HSC differentiation are observed in the recipients of single CD45midRho−EPCR++ cells. (A-D) Examples of individual mice repopulated with freshly isolated cells where colored areas represent donor WBCs of GM (red), B-cell (blue), and T-cell (yellow) lineages as a percentage of all WBCs over time after transplant. Data are stacked such that the sum of each lineage contribution represents the percentage donor contribution to all WBCs. (E-H) The separate donor contributions to the individual GM (red), B-cell (blue), and T-cell (yellow) lineages shown as bars. The percentage donor contribution to the total WBCs is shown as a gray area behind the bars.
SF concentration can rapidly alter key HSC properties without affecting cell viability or cell-cycle progression
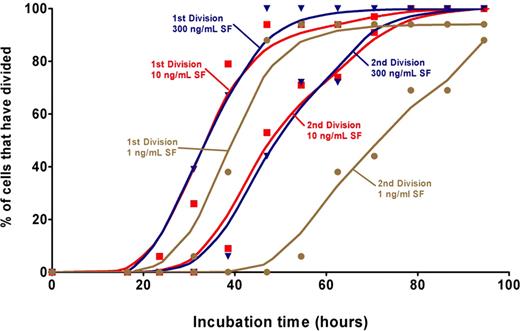
To determine how quickly exposure to different concentrations of SF might alter the ability of HSCs to sustain their stem cell properties and the relationship of such effects to their viability and mitogenic responses, single CD45midlin−Rho−SP or CD45midRho−EPCR++ cells were cultured individually for defined periods in SFM supplemented with 20 ng/mL of IL-11 and 1, 10, or 300 ng/mL of SF. These concentrations of SF were chosen based on previous experiments indicating that they should stimulate very poor, low, and optimal HSC self-renewal divisions in vitro, respectively.37 In a first series of experiments, 130 such cultures were visually examined every 4 to 6 hours to determine whether and when any cells died and the timing of any divisions that took place based on the first appearance of 2 cells and then 3 or 4 cells. A cumulative plot of the data obtained is shown in Figure 3. In the cultures containing 300 ng/mL of SF, no divisions occurred before 22 hours of incubation, but more than 90% of the original cells completed a first mitosis within the following 20 hours (ie, between 22 and 42 hours after the cells were placed at 37°C). The division kinetics of the cells incubated in the 10 ng/mL SF condition were indistinguishable from those displayed by the cells incubated in the 300 ng/mL SF condition, and no cell death was observed in any of the 114 wells containing cells maintained under either of these 2 conditions. Cultures containing 1 ng/mL SF showed a slightly delayed entry into the first and second division. Dead cells first became evident in these latter cultures after the second division and 35% to 40% of the clones were no longer viable after 4 days of culture.
Kinetics of division of CD45midlin−Rho−SP cells cultured in different SF concentrations. A total of 130 cells (n = 56 for 300 ng/mL, n = 58 for 10 ng/mL, and n = 16 for 1 ng/mL) were deposited individually into 96-well plates, visually confirmed to be single cells, and then observed every 4 to 6 hours to determine their kinetics of division. A cell was scored as having undergone a first division when a second cell could be observed in the well and a second division when a third cell could be seen. There was less than 1% cell death when the cells were cultured in either 10 or 300 ng/mL SF. A Lowess spline curve was generated in GraphPad Prism (version 4.03) using 248 values estimated based on the marked values in the time course and is shown for each of the first and second divisions under each condition.
Kinetics of division of CD45midlin−Rho−SP cells cultured in different SF concentrations. A total of 130 cells (n = 56 for 300 ng/mL, n = 58 for 10 ng/mL, and n = 16 for 1 ng/mL) were deposited individually into 96-well plates, visually confirmed to be single cells, and then observed every 4 to 6 hours to determine their kinetics of division. A cell was scored as having undergone a first division when a second cell could be observed in the well and a second division when a third cell could be seen. There was less than 1% cell death when the cells were cultured in either 10 or 300 ng/mL SF. A Lowess spline curve was generated in GraphPad Prism (version 4.03) using 248 values estimated based on the marked values in the time course and is shown for each of the first and second divisions under each condition.
An additional 100 single cells were cultured for 10 days in either 10 ng/mL or 300 ng/mL of SF (plus 20 ng/mL of IL-11). At this time, there was no obvious difference in the sizes of the clones generated. However, when the pooled cells from each set of cultures were assayed for the number of HSCs present using a limiting dilution transplant protocol, the cultures containing 300 ng/mL of SF showed full maintenance of the input number, whereas the cultures containing 10 ng/mL of SF showed that the HSC numbers had declined approximately 13-fold (Table 1).
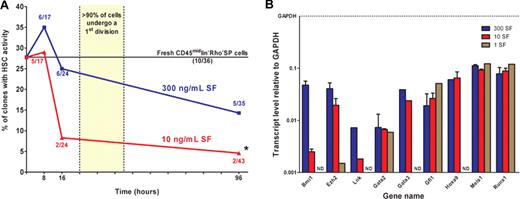
We then compared the frequency of clones that retain HSC activity during the first 4 days in culture when maintained in 300 ng/mL versus 10 ng/mL of SF (plus 20 ng/mL of IL-11). For these experiments, another 160 single-cell cultures were initiated. Then 8, 16, or 96 hours later, each single cell, or the clone it had generated, was harvested individually and transplanted into an irradiated mouse to determine whether one or more HSCs were present. As shown in Figure 4A, the proportion of cells still detectable as HSCs remained unchanged for the first 16 hours in the cultures containing 300 ng/mL of SF and, even after 4 days, the frequency of clones containing at least one HSC was still 50% of the frequency of HSCs in the original suspension. In the cultures containing 10 ng/mL of SF, the number of HSCs also did not change during the first 8 hours of incubation (Fisher exact test, n = 17, P = 1.0) but, within the next 8 hours, their numbers decreased to one-third of the input value (Fisher exact test, n = 24, P = .10) and remained significantly reduced thereafter (Fisher exact test, n = 43, P = .009). These findings raise the intriguing possibility that changes in SF stimulation can directly and very rapidly regulate the functional integrity of HSCs in vitro while they are still in a quiescent state, without affecting their immediate viability or the timing of their subsequent proliferative response.
Time course of changes in HSC activity under different concentrations of SF. (A) Single cells or their clonal progeny were injected after 8, 16, or 96 hours of culture in 20 ng/mL IL-11 plus either 300 ng/mL (blue) or 10 ng/mL (red) SF. Each data point represents the percentage of positive transplants (number of positive mice as a proportion of the total analyzed) for each condition and time point assessed. The straight line across the graph represents the starting HSC content as determined by single-cell transplants of freshly isolated CD45midlin−Rho−SP or CD45midRho−EPCR++ cells. *Value significantly different (P < .05) from the input HSC frequency. (B) Q-RT-PCR analyses of transcript levels in extracts of cells harvested from 16-hour cultures initiated with CD45midRho−EPCR++ cells and maintained in 20 ng/mL IL-11 plus 300 ng/mL (blue bars), 10 ng/mL (red bars), or 1 ng/mL SF (brown bars). All values normalized to Gapdh. ND indicates not detected. Values shown are the mean plus or minus SEM values obtained in 2 or 3 independent experiments, with each measurement derived from triplicate assays. Data without error bars are from a single experiment.
Time course of changes in HSC activity under different concentrations of SF. (A) Single cells or their clonal progeny were injected after 8, 16, or 96 hours of culture in 20 ng/mL IL-11 plus either 300 ng/mL (blue) or 10 ng/mL (red) SF. Each data point represents the percentage of positive transplants (number of positive mice as a proportion of the total analyzed) for each condition and time point assessed. The straight line across the graph represents the starting HSC content as determined by single-cell transplants of freshly isolated CD45midlin−Rho−SP or CD45midRho−EPCR++ cells. *Value significantly different (P < .05) from the input HSC frequency. (B) Q-RT-PCR analyses of transcript levels in extracts of cells harvested from 16-hour cultures initiated with CD45midRho−EPCR++ cells and maintained in 20 ng/mL IL-11 plus 300 ng/mL (blue bars), 10 ng/mL (red bars), or 1 ng/mL SF (brown bars). All values normalized to Gapdh. ND indicates not detected. Values shown are the mean plus or minus SEM values obtained in 2 or 3 independent experiments, with each measurement derived from triplicate assays. Data without error bars are from a single experiment.
To determine whether this loss of HSC functional activity might be correlated with differences in the expression of critical genes associated with HSC self-renewal control, we set up parallel bulk cultures of CD45midRho−EPCR++ cells using the same conditions of 20 ng/mL of IL-11 plus 1, 10, or 300 ng/mL of SF and then analyzed the RNA extracted from them 16 hours later by Q-RT-PCR. No significant differences were seen in the levels of transcripts for Gata2, Gata3, Gfi1, Hoxa9, Meis1, or Runx1 in cells incubated in any of the different concentrations of SF tested. However, transcript levels for both Bmi-1 and Ezh2, 2 members of the group of repressive Polycomb transcription factor genes, were significantly higher in the cultures containing 300 ng/mL of SF (Figure 4B). A similar finding was also noted for Lnk, a gene encoding a negative regulator of receptor tyrosine kinase receptor signaling.
Most HSC self-renewal divisions stimulated by SF and IL-11 in vitro are asymmetric
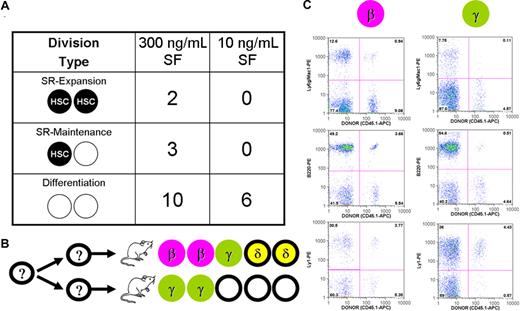
In a final series of experiments, we investigated the type of repopulating cells generated from the first division of 21 CD45midlin−Rho−SP cells maintained in 20 ng/mL of IL-11 plus either 300 ng/mL of SF (n = 15) or 10 ng/mL of SF (n = 6). Single-cell cultures were visually monitored to determine the timing of the first division and then, between 4 and 8 hours later, the 2 daughter cells were harvested, separated, and each transplanted into a separate irradiated recipient. Assessment of the repopulation patterns obtained showed that 5 of the 15 progeny pairs generated under the high SF condition contained daughter HSCs (ie, maintenance of the starting frequency of 1 in 3), whereas none of the 6 pairs generated under the 10 ng/mL of SF condition did, again indicative of a significant (Fisher exact test, P = .048) rapid reduction in HSCs under this condition (Figure 5A). Interestingly, even in the presence of 300 ng/mL of SF, both progeny were HSCs in only 2 cases and were then not of the same differentiation subtype. In the other 3 positive pairs, only one of the 2 progeny was an HSC (Figure 5B). An example of the 4-month repopulation data obtained from one of the 2 pairs where both progeny were HSCs is shown in Figure 5C. Taken together, these results demonstrate the highly preferential execution of asymmetric divisions by HSCs in vitro under the conditions examined.
Asymmetry of HSC expansion and maintenance divisions stimulated by 20 ng/mL IL-11 plus either 10 ng/mL or 300 ng/mL SF. (A) Single-cell cultures of CD45midlin−Rho−SP cells were monitored every 4 hours to determine when a first division took place. Between 4 and 8 hours after the first mitosis, doublets were separated and each of the 2 cells injected into a separate recipient with the resulting division type shown. SR indicates self-renewal; SF, Steel factor. (B) The distribution of HSC subtypes is detailed in the cases where at least one of the 2 daughter cells repopulated an irradiated recipient. (C) Fluorescence-activated cell sorter plots for a pair of repopulated mice transplanted 16 weeks earlier with individual cells from one separated daughter pair.
Asymmetry of HSC expansion and maintenance divisions stimulated by 20 ng/mL IL-11 plus either 10 ng/mL or 300 ng/mL SF. (A) Single-cell cultures of CD45midlin−Rho−SP cells were monitored every 4 hours to determine when a first division took place. Between 4 and 8 hours after the first mitosis, doublets were separated and each of the 2 cells injected into a separate recipient with the resulting division type shown. SR indicates self-renewal; SF, Steel factor. (B) The distribution of HSC subtypes is detailed in the cases where at least one of the 2 daughter cells repopulated an irradiated recipient. (C) Fluorescence-activated cell sorter plots for a pair of repopulated mice transplanted 16 weeks earlier with individual cells from one separated daughter pair.
Discussion
Recent experiments using single-cell transplants have provided evidence of heterogeneity within the rare subset of quiescent adult mouse bone marrow cells that produce mature blood cells for extended periods of time (> 4 months) in irradiated recipients. This heterogeneity is manifested as a marked skewing (or not) in favor of either granulopoietic or lymphoid differentiation in vivo that is faithfully transmitted to derivative repopulating cells.10 This finding raises the important notion that in HSCs, self-renewal, cell- cycle entry and progression, and lineage restriction are independent processes that are regulated by mechanisms with at least some distinct features.
To further investigate the prevalence of heterogeneity among the HSCs present in adult mouse bone marrow, we performed a series of single-cell transplantations using a population that was isolated using a simpler but similarly powerful procedure (using only anti-CD45 and anti-EPCR antibodies in combination with Rho staining). Detailed characterization of the clonal differentiation patterns displayed by the CD45midRho−EPCR++ cells in irradiated recipients revealed the same 4 types of repopulation patterns (α, β, γ, and δ) previously identified in similar experiments in which the progeny of individually transplanted CD45midlin−Rho−SP cells were analyzed.10 Moreover, these 4 patterns were distributed in the same proportions in recipients of either type of purified cells. These results indicate that the CD45midlin−Rho−SP and CD45midRho−EPCR++ fractions of normal adult mouse bone marrow are highly overlapping populations. They also provide further evidence that the quiescent HSCs present in normal adult mouse bone marrow are heterogeneous with respect to the differentiation programs they exhibit on transplantation into irradiated hosts.
Quiescent HSCs rapidly lose the ability to efflux Rho when they are stimulated to divide,42 a phenotypic lability that extends to many markers used to purify quiescent adult HSCs (eg, CD34, MAC1, AA4.1, and CD38, and the ability to efflux Hoechst 33342).42-47 Interestingly, in preliminary experiments, we have found that the EPCR++ phenotype exhibited by HSCs is stable regardless of their activation/cycling status (D.G.K., C.J.E., unpublished findings, 2007). Thus, high EPCR expression on HSCs may more closely resemble the recently reported stable expression of the SLAM family antigen (CD150) on HSCs.48
We were also able to use the CD45midlin−Rho−SP and CD45midRho−EPCR++ purification strategies interchangeably to determine the proportion of HSCs that would execute at least 2 self-renewal divisions when cultured under “optimal” cytokine conditions in vitro (300 ng/mL of SF plus 20 ng/mL of IL-11).6,37 Visual monitoring of clone development in vitro showed that every input cell completed at least 2 divisions within 4 days. Functional assessment of 4-day clones showed that the frequency of clones containing at least one HSC was approximately half the frequency of HSCs in the original population. Indeed, this would probably be an underestimate because any HSCs in S/G2/M would not have been detected.49,50 Thus, continuous exposure to a high concentration of SF in combination with IL-11 appears sufficient to allow many input HSCs to execute multiple self-renewal divisions over a 4-day period.
To address this question more directly, we analyzed the individual first division G1 progeny of 15 input cells cultured under the same conditions. The results showed that one or both of the members of 5 of these progeny pairs (30%) was an HSC, again, a similar proportion to the frequency of HSCs in the starting cells. However, characterization of the types and levels of WBCs produced in vivo by each of the 2 cells in these 5 progeny pairs showed that they all appeared to be the product of asymmetric divisions (ie, 3 pairs contained only one HSC and the other 2 pairs contained 2 HSCs, but each member of the pair was a different subtype). These findings are consistent with a more rapid loss in vitro of the HSC subtypes that retain extensive self-renewal activity in vivo10 and a stochastic distribution of self-renewal events among individual multipotent cells proliferating in vitro.51 At the same time, they appear inconsistent with the reported symmetry of the first 2 divisions of CD34−KSL cells in vitro52 and of the clonally amplified HSCs seen after 10 days in vitro.10 Clearly, additional experiments will be required to determine more precisely when and how symmetry is established when HSCs are stimulated under different conditions in vitro.
Our experiments also provide new information about the effects of exposing HSCs and/or their clonal derivatives in vitro to different SF concentrations. For this comparison, single cells from the same HSC-enriched populations were incubated in IL-11 plus a level of SF (1 or 10 ng/mL) that was predicted to give a markedly decreased output of HSCs, as suggested by previous results using 10-day cultures of less purified bone marrow cells.37 Assessment of the cells cultured in the 10 ng/mL SF condition confirmed the predicted loss of HSC activity within 10 days. More detailed time course studies further revealed that the loss of HSC activity induced by inadequate SF stimulation did not become manifest until after 8 hours but was evident within the ensuing 8 hours. From the subsequent kinetics of division seen, it can be readily inferred that most of the cells would not have exited G0 until after the first 16 hours of incubation. Thus, loss of HSC function probably occurred in most affected cells before they had entered the cell cycle. Interestingly, these effects also occurred in the absence of any evidence of effects on their immediate viability or mitogenic responses. Previous evidence of a similarly rapid induction of differentiation in the absence of proliferation by pluripotent hematopoietic cells was noted many years ago in studies of the FDCP-mix(bcl-2) cell line model.53 The findings presented here now show that this is also a property of normal adult HSCs.
The ability of HSCs to undergo such abrupt changes in their function raises intriguing questions about the nature of the molecular events involved and provides a novel experimental system for the further analysis of this process. Here we performed an initial survey of a small number of transcripts associated with HSC integrity. Strikingly, even this early dataset revealed a significant rapid and specific reduction in transcripts for Bmi1 and Ezh2 that accompanied the biologic changes in HSC function apparent within 16 hours of incubation under low SF conditions. Both Bmi1 and Ezh2 are of interest because they have been strongly implicated as regulators of the adult HSC self-renewal program.18,19,54 Our findings also extend those demonstrating that HSCs are detectable among one or both of the first division progeny of Lnk−/− cells incubated in cultures containing 50 ng/mL of SF.11,55 Because LNK is a negative regulator of SF-activated KIT signaling,55 deletion of Lnk might be expected to enhance HSC output in cultures containing a concentration of SF that is suboptimal for wild-type HSCs. Curiously, we found that Lnk transcripts were lower in cells maintained in the lower SF conditions. This result suggests that our current picture of how HSC self-renewal is controlled by known regulators may be incomplete, including the predicted consequence of reduced Lnk transcripts. Accordingly, it will be important in future studies to evaluate multiple elements of the self-renewal machinery, as well as measuring changes at the level of the encoded proteins and their activity.
In conclusion, this study provides further support for a model of HSC control in which there are elements regulating self-renewal activity that are different from those controlling lineage restriction, quiescence, or survival. The rapidity with which HSCs can be induced to differentiate under defined signaling conditions now provides a novel framework for delineating in molecular terms precisely how these elements achieve the biologic readouts used to define HSC function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Flow Cytometry Facility of the Terry Fox Laboratory and the Animal Resource Centre of the BC Cancer Agency, S. Russell for technical assistance, and Stem Cell Technologies and Genetics Institute for gifts of reagents.
This work was supported by grants from the National Cancer Institute of Canada (with funds from the Terry Fox Run). D.G.K. received Studentships from the Stem Cell Network, the Canadian Institutes of Health Research, and the Michael Smith Foundation for Health Research. B.J.D. received Studentships from the National Cancer Institute of Canada and the Stem Cell Network.
Authorship
Contribution: D.G.K. and C.J.E. designed experiments (B.J.D. provided input); D.G.K. carried out HSC isolation, transplants, flow analysis, and cultures (B.J.D. provided assistance); E.M. and D.G.K. performed transcript analysis; D.G.K., J.C., and E.M. performed peripheral blood collection and analysis; and C.J.E. and D.G.K. wrote the paper (B.J.D. provided input).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Connie J. Eaves, Terry Fox Laboratory, 675 West 10th Avenue, Vancouver, BC, Canada V5Z 1L3; e-mail: ceaves@bccrc.ca.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal