Abstract
Absence of the blood coagulation inhibitor thrombomodulin (Thbd) from trophoblast cells of the mouse placenta causes a fatal arrest of placental morphogenesis. The pathogenesis of placental failure requires tissue factor, yet is not associated with increased thrombosis and persists in the absence of fibrinogen. Here, we examine the role of alternative targets of coagulation that might contribute to the placental failure and death of Thbd−/− embryos. We demonstrate that genetic deficiency of the protease-activated receptors, Par1 or Par2, in the embryo and trophoblast cells does not prevent the death of Thbd−/− embryos. Similarly, genetic ablation of the complement pathway or of maternal immune cell function does not decrease fetal loss. In contrast, Par4 deficiency of the mother, or the absence of maternal platelets, restores normal development in one-third of Thbd-null embryos. This finding generates new evidence implicating increased procoagulant activity and thrombin generation in the demise of thrombomodulin-null embryos, and suggests that platelets play a more prominent role in placental malfunction associated with the absence of thrombomodulin than fibrin formation. Our findings demonstrate that fetal prothrombotic mutations can cause localized activation of maternal platelets at the feto-maternal interface in a mother with normal hemostatic function.
Introduction
Thrombomodulin (Thbd) is a multifunctional membrane glycoprotein that forms a high-affinity complex with the blood coagulation protease, thrombin, and serves as a key regulator of the protein C pathway.1-4 Binding to Thbd inhibits thrombin's interactions with fibrinogen, coagulation factor V (fV), and platelet thrombin receptors, and enhances thrombin's substrate specificity toward the activation of protein C zymogen and thrombin activatable fibrinolysis inhibitor (TAFI). Activation of protein C by the Thbd-thrombin complex is facilitated when protein C is bound by the endothelial protein C receptor (EPCR) coexpressed with Thbd on the same cell. Activated protein C (APC) can initiate at least 2 distinct reaction pathways. After dissociation with EPCR, APC inactivates coagulation factors Va and VIIIa through limited proteolysis, thereby inhibiting further thrombin generation and activation of the blood coagulation system. This mechanism forms the basis of APC's anticoagulant activity. On the other hand, APC can remain bound to EPCR, and the EPCR-APC complex can activate G protein–coupled protease-activated receptors Par1, Par2, and possibly Par3.5-7 Activation of these signaling-competent receptors elicits a spectrum of responses in various cell types.8,9 Coexpression of Thbd, EPCR, and PAR receptors on a given cell therefore enables the anticoagulant as well as the cell-signaling functions of the protein C system.
Genetic disruption of Thbd or EPCR results in intrauterine death in mice at day 8.5 of embryonic development, secondary to a complete arrest of placental organogenesis.10,11 In addition to the developing vascular endothelium, both receptors are highly expressed on specialized zygote-derived cells of the placenta, the trophoblast cells.12,13 These give rise to the bulk of the fetal-derived cell mass of the placenta, and fulfill an endothelial cell-like role in the regulation of hemostasis in the placental vascular bed.14 Cell type–selective restoration of Thbd or EPCR expression in trophoblast cells restores normal placental development of Thbd- and EPCR-knockout embryos, respectively,15,16 demonstrating that the critical cell type on which either receptor must be expressed is not the vascular endothelium, but trophoblast cells. A loss of Thbd function is associated with a failure of trophoblast cells to maintain proliferation in the ectoplacental cone and gives rise to a population of differentiated derivatives that establish the bulk of the mature placenta.17
The pathogenic mechanism that disrupts placental development of embryos lacking Thbd or EPCR is still unclear. In particular, it remains unknown whether the critical role of these receptors for normal organogenesis of the placenta involves their function in the context of anticoagulation, cell signaling, or both. In humans and mice, the hemochorial type of placentation exposes fetal trophoblast cells to direct contact with maternal blood. Trophoblast cells exhibit an endothelial cell–like ability to control activation of coagulation in the vascular bed of the placenta.14 However, in striking contrast to endothelial cells, trophoblast cells constitutively express the initiator of coagulation, tissue factor (TF),14,17-20 and genetic suppression of TF activity on trophoblast cells restores normal development of mice lacking Thbd or EPCR.16,17 This suggests that the predominant function of Thbd and EPCR on trophoblast cells is to provide an anticoagulant mechanism, which counteracts unfettered, TF-initiated coagulation activation in the placenta. Additional evidence for APC-mediated anticoagulation as the critical Thbd function on trophoblast cells stems from the observation that maternal APC resistance secondary to the Leiden mutation in coagulation fV, when combined with reduced Thbd function on trophoblast cells secondary to fetal Thbd-Pro mutation, replicates the phenotype of Thbd-null embryos.21 Platelet depletion or ablation of Par4 from the mother completely restores the development of Thbd-Pro embryos, indicating that Par4-mediated activation of maternal platelets is the likely mechanism responsible for fetal loss in this model. However, fetal loss of Thbd-Pro embryos is not associated with overt placental thrombosis or an increased frequency of histologically dedectable platelet thrombi. These observations suggest that placental injury in Thbd-Pro embryos carried by mothers with fV Leiden is not caused by thrombosis, but by some other mechanism involving thrombin-activated platelets. Taken together, these data support the notion that the critical function of Thbd in placental development is to sustain APC-dependent anticoagulation function of the protein C pathway, which prevents placental injury by thrombin-activated platelets.
The role of platelets in the developmental failure of Thbd-null embryos is unknown. Although Thbd-null embryos exhibit placental defects almost identical to Thbd-Pro embryos in mothers with fV Leiden, anticoagulation therapy with heparin or complete elimination of fibrinogen does not ameliorate placental defects in Thbd-null mice.17 We therefore considered TF-initiated pathogenic mechanisms other than fibrin clot formation, which might be related to platelet activation or to the anti-inflammatory or cell-signaling activities of the Thbd–protein C pathway. In the current work, we address whether excessive activation of Pars expressed on trophoblast cells or platelets contributes to the death of Thbd-null embryos. Our results fail to support the hypothesis that inadvertent Par activation on trophoblast cells mediates failed placentation and death of Thbd-null embryos. Rather, we find that fetal loss of approximately one-third of embryos is prevented by the genetic absence of maternal platelets or of the thrombin receptor Par4 from the mother. Notably, the greatest loss of Thbd-null embryos continues to occur in the absence of maternal platelets. We further address the mechanism of platelet-dependent and -independent fetal loss of Thbd-null embryos.
Methods
Mice
Animal experiments were conducted following standards and procedures approved by the Animal Care and Use Committee of the Medical College of Wisconsin. Thbd−/−, Nfe2−/−, Rag1−/−, γc−/−, Rag2−/−γc−, Par1−/−, Par2−/−, and Par4−/− mice have been described.12,22-25 These were maintained on a C57BL/6 genetic background and genotyped as previously described. C3 and C5 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The status of immune cell depletion in pregnant Thbd+/−Rag1−/−, Thbd+/−Rag2−/−, and Thbd+/− γc−/− females was confirmed by fluorescence-activated cell sorter (FACS) analysis of CD+ (B cells), B220+ (T cells), and NK1.1+ (natural killer [NK] cells) cells.
Histology
Embryonic development were assessed from days after coitum (dpc), assuming the midday of plug as 0.5 dpc and as previously described.17 Dissections were performed, and pictures of embryos were taken under a NikonSMZ-U microscope (Nikon, Melville, NY) equipped with a Spot insight color camera and Spotsoftware 3.2.4 (Diagnostic Instruments, Sterling Heights, MI). Images were taken with a 1× objective and the SMZ-U zoom set at 2×. The developmental progress of embryos was ascertained by comparing knockout embryos with Thbd-expressing littermates. Resorption was defined as the complete absence of embryo at the implantation site or the presence of tissue remnants without identifiable morphology. Developmental stage assessment and genotyping were performed as previously described.15,17 Activated platelets were identified with sc-6943 anti–mouse p-selectin polyclonal antibody used with ABC sc-2023 goat IgG staining systems (Santa Cruz Biotechnology, Santa Cruz, CA). In situ hybridization with Thbd-specific probes was performed as previously described.14
Complement inhibition and immune cell and platelet depletions
To determine the effect of complement inhibition on fetal loss, pregnant Thbd+/− females mated to Thbd+/− males were given subcutaneous bolus injections of 30 or 100 μg LMWH (enoxaparin; Aventis Pharmaceuticals, Bridgewater, NJ) daily from 5.5 dpc to 1 day before analysis of pregnancy. Pregnant Thbd−/− females were injected with polyclonal anti-asialo GM1 rabbit anti–mouse antibody (Wako Pure Chemical Industries, Chuo-Ku Osaka, Japan) on day 7.5 for depletion of NK cells and with purified anti–Ly-6G (Gr-1) rat anti–mouse antibody (Pharmingen, San Diego, CA) on day 8.5 for the depletion of granulocytes. For platelet depletion, a mixture of anti–glycoprotein (GP)Ibα rat monoclonal antibodies26 or polyclonal nonimmune IgG (control; R300 or C301; Emfret Analytics, Wurzburg, Germany) were injected via the tail vein at 4 μg/g body weight. Anti-GPIbα antibodies depleted 95% of the platelets within an hour and were counted manually and with an automated cell counter (ABX Diagnostics, Montpellier, France).
Results
Fetal loss persists in the absence of Par1 or Par2 from trophoblast cells
To experimentally test whether inadvertent activation of Par1 or Par2 contributes to the fetal loss of Thbd-null embryos, we generated Thbd+/− mice with superimposed PAR deficiency and examined the effect of a loss of PAR function on the intrauterine survival of Thbd−/− embryos. In the absence of Thbd alone, embryos are growth arrested at E8.5 (embryonic day 8.5 of development, corresponding to Theiler stage 13) and are resorbed by E9.5.10,12 Par1 deficiency alone (in the presence of normal Thbd function) causes approximately 50% lethality around E10, secondary to defective blood vessel function in the yolk sac.24,27 Pregnancies from matings between Par1+/−Thbd+/− and Par1−/−Thbd+/− mice were examined at E10.5. Approximately half of the Par1−/− embryos exhibited the characteristic developmental defects described earlier,24,27 and no normal Thbd−/− embryos were recovered (Table 1). Thus, Par1 deficiency in trophoblast and embryo did not improve the survival of Thbd−/− embryos. Corresponding intercrosses were established between mice lacking Thbd and Par2. The absence of Par2 from trophoblast cells and other fetal tissues did not improve survival of Thbd−/− embryos (Table 1).
Maternal Par4 deficiency results in partial rescue of Thbd−/− mice
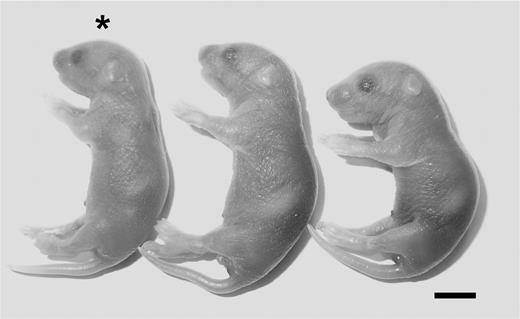
Maternal Par4 deficiency completely prevents the placental defects leading to the loss of ThbdPro/Pro embryos (with reduced Thbd function) carried by homozygous fV Leiden mother.21 To test whether maternal Par4 deficiency also rescues placental development of Thbd−/− embryos (with absent Thbd function), we examined the development of Thbd-null embryos carried by Par4−/−Thbd+/− mothers. In contrast to the cumulative analysis of more than 500 offspring from Thbd+/− intercrosses, mating of Par4−/−Thbd+/− females to Thbd+/− males yielded a small number of viable Par4+/−Thbd−/− neonates of normal size and appearance. Of 64 liveborn pups, 6 were Par4+/−Thbd−/−, corresponding to approximately one-third of the yield expected from a complete rescue (P = .004; Table 2; Figure 1), indicating persistent intrauterine loss of two-thirds of the Thbd−/− embryos. Thbd−/− pups died in the perinatal period prior to weaning for undetermined causes; no pups genotyped at weaning were Thbd−/− (Par4−/−Thbd+/− intercrosses; n = 54; no Par4−/−Thbd−/−, analysis at 3 to 4 weeks).
Phenotype of Thbd−/− neonate in Par4−/− mothers. *Thbd−/− neonate shown with its littermates. Scale bar represents 5 mm.
Phenotype of Thbd−/− neonate in Par4−/− mothers. *Thbd−/− neonate shown with its littermates. Scale bar represents 5 mm.
Thbd−/− embryos do not progress beyond E8.5, and are invariably resorbed by E9.5. We analyzed embryos at E9.5 from crosses of Par4−/−Thbd+/− females and Thbd+/− males. These yielded several E9.5 Par4+/−Thbd−/− embryos that had progressed beyond E8.5 or Theiler stage 13 (Total number of embryos, n = 50; 11 Par4+/−Thbd−/−; Table 2; Figure 2A-D). The E9.5 Thbd−/− embryos in Par4-deficient mothers were of heterogeneous size, ranging from severely runted to normal (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), and only approximately half had beating hearts at the time of dissection. Thus, in the Par4−/− mother, most Thbd−/− embryos at E9.5 had progressed beyond Theiler stage 13, but only a fraction of these (approximately one-third) appeared grossly normal in size and had beating hearts. Simultaneous deletion of fetal and maternal Par4 (embryos derived from Par4−/−Thbd+/− intercrosses) also rescued Thbd−/− embryos at E9.5, but did not further increase their yield (Table 2).
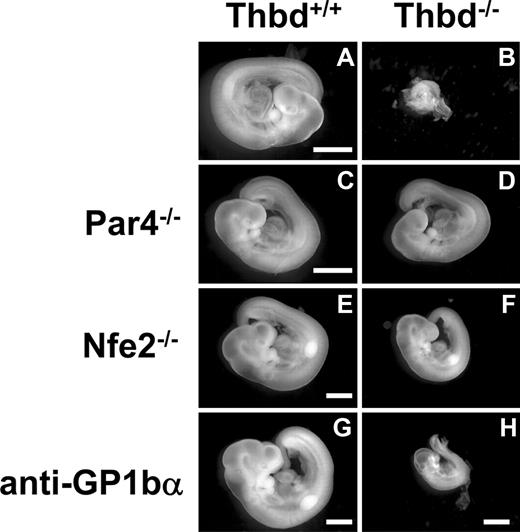
Phenotype of Thbd−/− embryos in Thbd+/− mothers. Thbd-expressing embryos (A,C,E,G) are shown with their respective Thbd−/− littermates (B,D,F,H). Embryos in panels A through D and E through H were photographed at E9.5 and E10.5, respectively. Thbd−/− embryos are resorbed by E9.5 (B). In mothers lacking the thrombin receptor Par4 (C,D), and in mothers lacking platelets due to Nfe2 deficiency (E,F) or anti-GP1bα antibody–induced depletion (G,H), Thbd−/− embryos completed axonal rotation (“turning”), a hallmark of transition from E8.5 to E9.5 stage of development. Embryos were photographed in water using equipment described in “Histology.” Scale bars represent 1 mm.
Phenotype of Thbd−/− embryos in Thbd+/− mothers. Thbd-expressing embryos (A,C,E,G) are shown with their respective Thbd−/− littermates (B,D,F,H). Embryos in panels A through D and E through H were photographed at E9.5 and E10.5, respectively. Thbd−/− embryos are resorbed by E9.5 (B). In mothers lacking the thrombin receptor Par4 (C,D), and in mothers lacking platelets due to Nfe2 deficiency (E,F) or anti-GP1bα antibody–induced depletion (G,H), Thbd−/− embryos completed axonal rotation (“turning”), a hallmark of transition from E8.5 to E9.5 stage of development. Embryos were photographed in water using equipment described in “Histology.” Scale bars represent 1 mm.
These findings demonstrate that maternal Par4 deficiency somewhat prolongs the intrauterine survival of most Thbd−/− embryos, but overcomes fetal loss of approximately one-third of Thbd−/− embryos. Notably, fetal loss of most Thbd−/− mice persists in the absence of maternal Par4.
Partial rescue of Thbd−/− mice by depletion of maternal platelets
Mice lacking the transcription factor Nfe2 lack functional platelets.25 Nfe2−/− and Thbd+/− mice were interbred to generate Thbd+/− females with superimposed Nfe2−/− deficiency (Nfe2−/−Thbd+/−). A total of 2 live Thbd−/− neonates were obtained of 37 analyzed at term (Nfe2−/−Thbd+/− interbreeding; n = 37; 14 Nfe2−/−Thbd+/+, 21 Nfe2−/−Thbd+/−, 2 Nfe2−/−Thbd−/−). These numbers reflect a rescue of approximately 20% of Thbd−/− embryos in an Nfe2−/− mother, similar to the results obtained with Par4−/− mothers. When pregnancies from Nfe2−/−Thbd+/− females mated to Thbd+/− males were examined at E10.5, 3 Thbd−/− embryos that had progressed beyond Theiler stage 13 were noted of a total of 21 embryos (5 would be expected from this cross). These were smaller in size than Thbd-expressing littermates, resembling the appearance of Thbd−/− embryos in Par4−/− mothers (Figure 2E,F). Immunodepletion of maternal platelets with anti-mouse GP1bα antibodies also allowed Thbd−/− embryos to progress beyond E8.5 (Figure 2G,H).
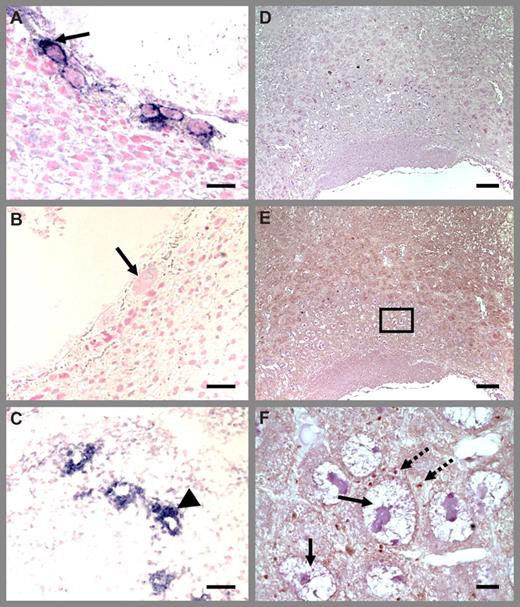
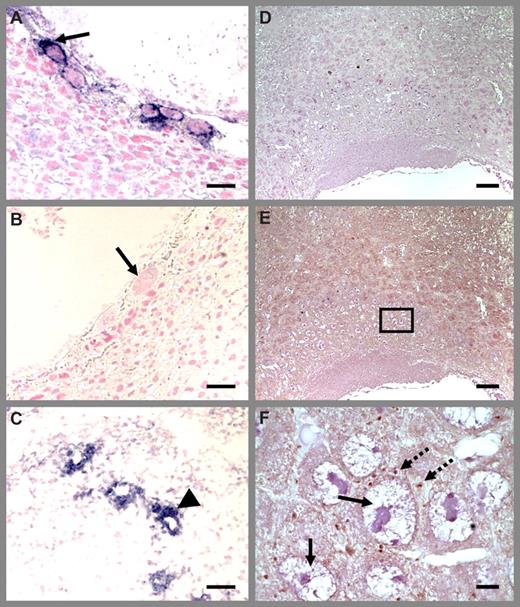
Immunohistologic detection with P-selectin antibodies documented the presence of activated platelets in the deciduas and maternal blood spaces between trophoblast giant cells in E8.5 placenta (Figure 3) and also in spiral arteries (data not shown). Platelets were dispersed or present in small nonoccluding clusters, with occasional platelet-fibrin aggregates. Comparison of the distribution of platelets between the placentas corresponding to Thbd-null and Thbd-expressing embryos did not reveal any striking differences in the incidence or size of platelet aggregates, as detected by P-selectin staining. These results are consistent with the previously reported absence of blood clots or increased fibrin deposition in the Thbd−/− placentas.28
Platelet localization in Thbd−/− placenta using P-selectin antibodies. Thbd-expressing (A) and -null (B) placentas were identified by in situ hybridization using antisense RNA probes. Blue color indicates Thbd expression. The null units express maternally derived Thbd in spiral arteries (C). Diffuse P-selectin stain (brown color) was observed at low magnification (E) (nonimmune IgG control in panel D), identified to correspond to platelets at higher magnification (F). Platelets are abundantly present in maternal blood spaces surrounding trophoblast giant cells. Platelet distribution was similar in Thbd−/− (E,F) and Thbd-expressing (not shown) utero-placental units. Slides were counterstained with nuclear fast red (A-C) or hematoxylin (D-F), mounted in Permount (Fisher Scientific, Pittsburgh, PA) and examined under a Nikon Eclipse E600 microscope (Nikon, Melville, NY). Pictures were taken using a 10×/0.3 (A-E) or 100× oil/1.3 (F) objective with a spot camera (Diagnostic Instruments, Sterling Heights, MI). Solid arrows indicate trophoblast cells; dashed arrows indicate platelets; arrowheads indicate spiral arteries. Panel F corresponds to the boxed region in panel E. Scale bars represent 0.05 mm (A-C), 0.1 mm (D), and 0.01 mm (E,F).
Platelet localization in Thbd−/− placenta using P-selectin antibodies. Thbd-expressing (A) and -null (B) placentas were identified by in situ hybridization using antisense RNA probes. Blue color indicates Thbd expression. The null units express maternally derived Thbd in spiral arteries (C). Diffuse P-selectin stain (brown color) was observed at low magnification (E) (nonimmune IgG control in panel D), identified to correspond to platelets at higher magnification (F). Platelets are abundantly present in maternal blood spaces surrounding trophoblast giant cells. Platelet distribution was similar in Thbd−/− (E,F) and Thbd-expressing (not shown) utero-placental units. Slides were counterstained with nuclear fast red (A-C) or hematoxylin (D-F), mounted in Permount (Fisher Scientific, Pittsburgh, PA) and examined under a Nikon Eclipse E600 microscope (Nikon, Melville, NY). Pictures were taken using a 10×/0.3 (A-E) or 100× oil/1.3 (F) objective with a spot camera (Diagnostic Instruments, Sterling Heights, MI). Solid arrows indicate trophoblast cells; dashed arrows indicate platelets; arrowheads indicate spiral arteries. Panel F corresponds to the boxed region in panel E. Scale bars represent 0.05 mm (A-C), 0.1 mm (D), and 0.01 mm (E,F).
Thus, genetic or immunologic depletion of maternal platelets produces results similar to maternal Par4 deficiency. While these data establish that platelets modify the penetrance of fetal loss of Thbd−/− embryos, we were unable to correlate absence of Thbd with increased incidence of platelet-rich thrombi, recapitulating prior results obtained from the analysis of ThbdPro/Pro embryos carried by fV Leiden mothers.
Embryonic lethality persists in immune cell–depleted mice
In contrast to platelet deficiency, genetic or pharmacologically induced depletion of T cells, B cells, NK cells, or granulocytes had no effect on the survival of Thbd−/− embryos. Treatment of Thbd+/− females with granulocyte- and/or NK cell–depleting antibodies resulted in 95% reduction in peripheral blood granulocyte count (1% ± 0.32% vs 18.7% ± 1.21%: treated vs controls), and 50% reduction in NK1.1+ cells in the spleen (2% ± 0.27% vs 4.5% ± 0.40%: treated vs controls). Embryos were collected at 10.5 dpc from treated females mated to Thbd+/− males. Of 20 embryos examined in granulocyte-depleted mothers, no normal Thbd−/− embryos were obtained, significantly differing from 5 expected based on Mendelian inheritence (P = .01). Likewise, no normal Thbd−/− embryos were found among 59 embryos examined from NK cell–depleted mothers (approximately 14 expected). Simultaneous depletion of maternal granulocytes and NK cells also did not yield normal Thbd−/− embryos (29 embryos analyzed, 7 expected). All Thbd−/− embryos retrieved from these experiments were in advanced stages of decay. Crosses between Thbd+/− mice and Rag1−/−, γc−/−, and Rag2−/−γc−/− mice (lacking lymphocytes, NK cells, or both, respectively) were used to address the role of NK cells and lymphocytes in fetal loss. Thbd+/−Rag1−/−, Thbd+/−γc−/−, and Thbd+/−Rag2−/− γc−/− females generated by mouse breeding were mated to Thbd+/− males, and the progeny was analyzed at 9.5 dpc. No normal or growth-arrested Thbd−/− embryos were obtained from these experiments, demonstrating that the genetic absence of lymphocytes and/or NK cells in the mother does not prevent midgestational death and rapid resorption of Thbd−/− embryos (Table 3).
Together, these data are congruent with the conclusion that the partial rescue of Thbd−/− embryos in Par4-deficient mothers may be attributed to the disruption of Par4 function in maternal platelets, with no important contribution of immune cells.
Fetal loss of Thbd-null embryos does not involve complement C3 or C5
Akin to fetal loss caused by Thbd deficiency, fetal loss induced by injection of human antiphospholipid (APL) antibodies into pregnant mice is prevented by genetic reduction of tissue factor activity.29 Activation of the complement pathway has been identified as a key pathogenic mechanism in APL-induced model of fetal loss.30-32 Thbd modulates complement activation through TAFI-dependent inactivation of C3a and C5a33-35 via direct complement inhibition mediated by the lectin-like domain of Thbd,36 and potentially through inhibiting C3b binding to activated platelets.37 Complement activation secondary to the loss of Thbd function could in theory account for a platelet-independent component of fetal loss of Thbd−/− embryos, and/or link platelet activation to a complement-mediated pathogenesis of fetal loss.
To assess the potential role of complement activation in fetal loss of Thbd-null embryos, pregnant females carrying Thbd−/− embryos were subjected to a low-molecular-weight heparin (LMWH) treatment regimen, identical to that used to prevent APL antibody-induced fetal loss.32 Daily injections of 30 or 100 μg LMWH did not prevent the loss of Thbd−/− embryos. Of a total of 37 embryos examined, 27 were normal and 10 were resorbed, and none of the normal embryos were Thbd−/− (Thbd+/− intercrosses; 5 pregnancies; n = 27, none Thbd−/−; P = .003). Likewise, a superimposed simultaneous loss of complement C5 function in the mother and the fetus did not rescue Thbd−/− embryos (C5−/−Thbd+/− intercrosses; n = 23; 4 Thbd+/+, 19 Thbd+/−, none Thbd−/−; P = .005). Equivalent results were obtained with C3-deficient mice.
These data argue against a significant contribution of complement activation to the pathogenic mechanism causing fetal loss of Thbd−/− mice.
Discussion
Previous investigations into the etiology of fetal loss of Thbd-deficient embryos suggested that the essential role of the Thbd–protein C system for murine placental development is not readily accounted for by the anticoagulation function of the protein C pathway and raised the possibility that Thbd deficiency might modify the function of placental trophoblast cells through altered PAR activation.17 The goals of the current study were to test this hypothesis and to examine other potential effectors of Thbd deficiency.
To assess whether PAR activation is necessary for the Thbd embryonic phenotype, we determined the fate of Thbd−/− embryos in the absence of Par1 or Par2 expression on fetal trophoblast cells. The persistent loss of Thbd−/−Par1−/− and of Thbd−/−Par2−/− embryos demonstrates that increased activation of Par1 on the trophoblast cell surface, perhaps secondary to a switch from APC to thrombin as the dominant Par1 agonist, cannot satisfactorily explain the loss of Thbd−/− embryos. In addition, these results clarify that disruption of Par2-mediated signaling initiated by trophoblast-associated TF does not replicate the beneficial effect of TF deficiency on the survival of Thbd-null embryos. Any deleterious effects associated with loss of APC-specific signaling via PARs in Thbd−/− embryos would of course not be ameliorated by Par deficiency, and a contribution by loss of possible APC-specific effects on trophoblast PAR activation to placental malfunction cannot be ruled out by these experiments.
Examination of the effects of Par4 deficiency provided a surprise. We observed that Par4 deficiency of the mother or lack of maternal platelets allowed approximately one-third of Thbd−/− embryos to develop to term. Rescue of a significant fraction of Thbd−/− embryos by elimination of Par4 or the mother's platelets is a dramatic finding (Thbd-null embryos almost never survive to birth). This observation contrasts with the complete absence of rescue in response to pharmacologic anticoagulation of the mother, and to the genetic absence of fibrinogen.17 It therefore generates new evidence implicating increased procoagulant activity and thrombin generation in the demise of Thbd-null embryos, despite the observed absence of overt thrombosis. It also suggests that platelet activation plays a more prominent role in the prothrombotic outcome of defects in protein C anticoagulation pathway in the placental vascular bed than fibrin formation. Of note, in the systemic circulation defects in this pathway are more commonly associated with venous thrombosis, in which anticoagulation treatment is thought to be more effective than antiplatelet treatment.
Although the current work does not formally prove that the platelet-dependent effects on placental development are due to platelet Par4 activation, locally increased thrombin generation at the Thbd-deficient trophoblast surface and secondary activation of the platelet thrombin receptor Par4 is a conceivable mechanism linking the loss of Thbd to altered platelet function. How platelets affect placental development is unclear. Histologic analysis of Thbd−/− placentas failed to reveal increased accumulation of platelet aggregates in affected pregnancies, consistent with previous findings and the recent analysis of the fV Leiden model.21,28 These data suggest that the platelet-mediated fetal loss of Thbd−/− embryos does not involve overt thrombosis, defined as large platelet aggregates or platelet-fibrin thrombi. The previously documented lack of rescue of Thbd by the complete absence of maternal and embryonic fibrinogen supports this conclusion. Overall, given that our data implicate Par4-dependent platelet activation in the death of Thbd−/− embryos, small and rapid turnover platelet thrombi may be involved, but it seems unlikely that stable occlusive thrombi that disrupt blood flow within the placenta cause the death of Thbd−/− embryos.
We considered alternate mechanisms by which Par4-mediated platelet activation may increase the incidence of fetal loss. Platelets may modulate inflammatory reactions and immune responses through regulated expression of adhesion and immune receptors, release of inflammatory mediators and cytokines,38 recruitment of leukocytes,39,40 and lymphocyte trafficking,41 as well as through their ability to activate the complement system via P-selectin–mediated C3b binding.37 Such platelet effects on inflammation and complement activation could potentially interact in a synergistic or additive manner with similar effects of Thbd deficiency, and thereby explain how platelet activation modulates the penetrance of fetal loss: Thbd deficiency may compound complement activation secondary to the loss of TAFI-dependent inactivation of C3a and C5a,33-35 or due to the lack of direct complement inhibition mediated by the lectin-like domain.36 Likewise, APC exerts profound anti-inflammatory effects that are based on cell signaling initiated by the EPCR-APC complex.9 However, an exhaustive study to examine the involvement of immune cells and the complement pathway failed to produce any evidence for a significant contribution of complement, inflammation, or platelet-leukocyte interactions in the fetal loss of Thbd−/− embryos, be it platelet dependent or platelet independent. Importantly, the current study demonstrates that platelets modify the penetrance of developmental failure of Thbd-null embryos, and provides evidence that this biologic function of platelets is not based on the well-recognized mechanisms underpinning their function in hemostasis or those involving the recruitment of inflammatory cells. A role of platelet-released products in altering trophoblast physiology via cell-signaling mechanisms can be envisaged, but remains to be investigated.
It is notable that the majority of fetal loss of Thbd−/− embryos persists in the complete absence of maternal platelets or Par4. Activation of maternal platelets at the feto-maternal interface, therefore, only partly explains fetal loss of Thbd−/− embryos, and the targets of coagulation activation that mediate loss of the majority of Thbd−/− embryos remain unclear. Given that elimination of both fibrin(ogen) and Par4-dependent platelet activation is necessary to mimic the hemorrhagic phenotype of prothrombin deficiency, we cannot exclude the possibility that elimination of both fibrin(ogen) and Par4 would fully rescue Thbd−/− embryos. Unfortunately, this experiment is not possible because Fib−/−Par4−/− mice exsanguinate in the perinatal period.42 It therefore remains unclear if all of the fetal loss of Thbd−/− embryos can be explained by the inability of fetal trophoblast cells to sustain the anticoagulant function of the protein C pathway. The absence of signaling function of APC, or the absence of some other Thbd function unrelated to APC generation, are other possible mechanisms of the majority of fetal loss of Thbd−/− embryos. Presented data demonstrate that the majority of fetal loss does not involve loss of Thbd-dependent inhibition of complement activity or any other immune cell–mediated mechanism. Work has been initiated to determine whether selective reconstitution of the protein C pathway with anticoagulation-only or signaling-only APC variants43 can bypass the developmental block of Thbd-null embryos.
Although mutations associated with a severe reduction of Thbd or EPCR function are extremely rare in humans, the insights gained from the current study are likely to be relevant for human reproduction. In human pregnancies, platelets associate closely with endovascular trophoblasts within the lumen of spiral arteries,44 and exaggerated platelet activation and impaired trophoblast development are prominent features of preeclampsia and HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome.45,46 Our studies in mice demonstrates that fetal prothrombotic gene defects can cause localized activation of maternal platelets in a mother with normal hemostatic function, and that platelet activation can translate into impaired placental development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Barbara Fleming for preparing histologic sections.
This work was supported by National Institutes of Health grants HL44907, HL65590 (S.R.C.), and HL60655 (H.W.); Deutsche Forschungsgemeinschaft grants IS-67/2-2 and IS-67/4-2 (B.I.); and the Ziegler Family Chair for Research (H.W.).
National Institutes of Health
Authorship
Contribution: R.S. and H.W. designed and performed experiments, interpreted data, and wrote the manuscript; L.S. and S.R.C. designed the Par1, Par2, and Par4 experiments, conducted Par1 and Par2 experiments, and wrote the manuscript; B.I. conducted granulocyte depletion and Rag2γc breeding experiments; and M.Z. maintained animal colonies and coordinated breeding experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rashmi Sood, Medical College of Wisconsin, 8701 Watertown Plank Rd, CRI, TBRC, Milwaukee, WI 53226; e-mail: rsood@mcw.edu; and Hartmut Weiler, Blood Center of Wisconsin, 8727 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: hartmut.weiler@bcw.edu.