Abstract
Many intracellular pathogens infect macrophages and these pathogens require iron for growth. Here we demonstrate in vitro that the intracellular growth of Chlamydia psittaci, trachomatis, and Legionella pneumophila is regulated by the levels of intracellular iron. Macrophages that express cell surface ferroportin, the only known cellular iron exporter, limit the intracellular growth of these bacteria. Hepcidin is an antimicrobial peptide secreted by the liver in response to inflammation. Hepcidin binds to ferroportin mediating its internalization and degradation. Addition of hepcidin to infected macrophages enhanced the intracellular growth of these pathogens. Macrophages from flatiron mice, a strain heterozygous for a loss-of-function ferroportin mutation, showed enhanced intracellular bacterial growth independent of the presence of exogenous hepcidin. Macrophages, from wild-type or flatiron mice, incubated with the oral iron chelator deferriprone or desferasirox showed reduced intracellular bacterial growth suggesting that these chelators might be therapeutic in chronic intracellular bacterial infections.
Introduction
Hypoferremia is a hallmark of inflammation and results from increased secretion of the liver hormone hepcidin (for review, see Nemeth and Ganz1 ). Hepcidin is secreted by hepatocytes in response to inflammatory stimuli, notably interleuklin-6 (IL-6).2-4 Hepcidin binds to the iron exporter ferroportin (Fpn), leading to its internalization and degradation.5 Fpn is the only identified cellular iron exporter and is present on macrophages, absorptive enterocytes, and placenta. Loss of cell surface Fpn results in hypoferremia, which, if persistent, leads to iron-limited erythropoiesis.6 The hypoferremia of inflammation is thought to represent an antibacterial defense response, as iron acquisition proteins are virulence factors for many species of bacteria.7
The loss of cell surface Fpn also leads to increased cellular iron, particularly in macrophages that are continuously obtaining iron from senescent red blood cells. The hypoferremia of inflammation may limit the growth of extracellular bacteria but may promote the growth of intracellular bacteria. Support for this hypothesis has been suggested by studies in Salmonella-infected macrophages, where addition of hepcidin promoted the growth of bacteria.8,9 We show that the growth of 3 other species of intracellular bacteria, Chlamydia psittaci, C trachomatis, and Legionella pneumophila, is promoted by hepcidin-mediated down-regulation of Fpn. Chlamydia spp are obligate intracellular pathogens that have a unique developmental cycle. The infectious forms of Chlamydia spp are termed elementary bodies. After infecting host cells, the elementary bodies differentiate (2-4 hours) within the inclusion membrane to the metabolically active bacteria, termed reticulate bodies, which are noninfectious. The reticulate bodies replicate within the expanding inclusion membrane for approximately 2 to 3 days, differentiate back to elementary bodies, and are released extracellularly (for review, see Dautry-Varsat et al10 ). L pneumophila is a facultative intracellular pathogen that is internalized into a pha-gosome that remains separated from lysosomes, but recruits endoplasmic reticulum–derived vesicles for the expanding phagosomal membrane and mitochondria for nutrients (for review, see De Buck et al11 ). Both Chlamydia and Legionella require iron for replication and expansion.12,13 We determined that macrophages with impaired Fpn activity, isolated from flatiron mice (a strain heterozygous for a loss-of-function ferroportin mutation), show increased susceptibility to intracellular bacterial growth. Further, we show in vitro that the growth of these intracellular bacteria can be suppressed by treatment with the clinically used oral iron chelators deferriprone and desferasirox.
Methods
L pneumophila was obtained from Dr M. S. Swanson (University of Michigan Medical School, Ann Arbor). C psittaci and C trachomatis were obtained from Dr P. Wyrick (Eastern Tennessee State University, Johnson City). Anti-Chlamydia antibody was generated in our laboratory and used as previously described.14 Anti–L pneumophila antibody was purchased from Abcam (Cambridge, MA).
Cells and media
HEK293T Fpn, a stable cell line in which Fpn–green fluorescent protein (GFP) expression is regulated by the ecdysone promoter, has been described previously.5 The stable HEK293T Fpn cells express Fpn-GFP only when the cells are grown in the presence of 10 μM ponasterone A, an insect steroid hormone that activates the ecdysone promoter. Murine bone marrow macrophages were isolated from mouse femurs and cultured as described.15 Cells were iron loaded by addition of ferric ammonium citrate (FAC; 10 μM iron).
Intracellular growth and infection efficiency in macrophages
L pneumophila, a virulent thymine auxotroph, was cultured in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES; Sigma-Aldrich, St Louis, MO)–buffered yeast extract broth supplemented with 100 μg thymidine/mL (AYET) at 37°C with agitation. For infections, macrophages were plated at a density of 50 000 cells on 35-mm tissue culture dishes in 1.5 mL RPMI-FBS and incubated with FAC for 2 days. Freshly cultured L pneumophila were added to macrophages at a multiplicity of infection (MOI) of 10. After 2 hours at 37°C, extracellular bacteria were removed by washing 3 times with 1.5 mL RPMI-FBS containing antibiotics (0.06 mg/mL penicillin, 0.1 mg/mL streptomycin; Sigma-Aldrich) and the cells were placed in RPMI-FBS supplemented with 100 μg/mL thymidine for an additional 18 to 48 hours. L pneumophila intracellular growth was measured at 24 hours after infection by pooling culture supernatants, and lysates were prepared by trituration of monolayers with sterile water and then plated (10 or 25 μL) on CYET plates. As a control, we confirmed that washing macrophage plates removed extracellular bacteria by plating the last wash onto CYET plates and counting CFUs. Greater than 95% of the bacteria was removed or killed with this treatment. L pneumophila colony-forming units (CFUs) were determined by growing macrophage cell lysates for 3 days at 37°C on the solid medium ACES-buffered charcoal-yeast extract agar supplemented with 100 μg thymidine/mL (CYET). For Chlamydia infections, HEK293T cells were induced to express Fpn-GFP for 18 hours and then incubated with C psittaci elementary bodies for 2 hours as previously described14 ; extracellular bacteria were removed, and 18 to 24 hours after infection cells were fixed and processed for immunofluorescence. Bone marrow macrophages were plated at a density of 50 000 cells on 35-mm tissue culture dishes in 1.5 mL RPMI-FBS and grown either with or without FAC for 2 days. Cells were then incubated with C psittaci or C trachomatis for 2 hours and extracellular bacteria removed; 18 to 24 hours after infection, cells were fixed and processed for immunofluorescence or lysed in 150 mM NaCl, 10 mM EDTA, 10 mM Tris (pH 7.4), 1% Triton X-100, and a protease inhibitor cocktail (Roche, Palo Alto, CA; lysis buffer) for Western blot analysis or for ferritin analysis.
Western blot analysis and immunofluorescence
Cellular proteins were extracted with lysis buffer and total protein concentrations were determined using BCA reagent (Pierce, Rockford, IL). Protein samples were separated on 4% to 20% acrylamide gels (BioRad, Hercules, CA) and transferred on Hybond-ECL (Amersham Biosciences, Piscataway, NJ). Fpn was detected using rabbit anti-Fpn antibody (1:1000), with peroxidase-conjugated goat anti–rabbit IgG as the secondary antibody (1:10 000; Jackson ImmunoResearch, West Grove, PA). Chlamydial infection was detected using rabbit anti–C psittaci antibody (1:500). Tubulin was detected using a mouse anti–alpha tubulin antibody (1:1000; GeneTex, San Antonio, TX). Chemiluminescent method was used for detection (Western Lightning; Perkin Elmer, Boston, MA).
For immunofluorescence, cells were fixed by methanol-flash for 5 minutes at −20°C, permeabilized in phosphate-buffered saline (PBS) containing 1% bovine albumin serum and 0.1% saponin, and incubated in rabbit anti-Fpn (1:100) along with either mouse anti–C psittaci antibody (1:100) or mouse anti–L pneumophila antibody (1:100; Abcam) for 60 minutes at room temperature, followed by Alexa 594–conjugated goat antirabbit antibody and Alexa 488–conjugated goat antimouse antibody (1:750; Molecular Probes, Eugene, OR) for 60 minutes at room temperature. Cells were visualized using an epifluorescence microscope (Olympus, Melville, NY) with a 60× oil-immersion objective. Images were acquired using Magnafire analysis software (Optronics, Goleta, CA).
Other procedures
Ferritin analysis was performed as previously described.16 Hepcidin (hep25) and hepcidin 20 (hep20) were a generous gift from Dr Tomas Ganz (University of California at Los Angeles) and were used at 1 μg/mL for 18 hours. DFO was purchased from Ciba-Geigy (Summit, NJ). Deferriprone and desferasirox were a generous gift from Dr Prem Ponka (McGill University, Montreal, QC). Total RNA was extracted from macrophages infected with C pneumophila using QIAshredder and RNeasy Mini kit (Qiagen, Chatsworth, CA). One-Step reverse-transcription–polymerase chain reaction (RT-PCR) kit (Invitrogen, Carlsbad, CA) was used with 500 ng RNA using the following conditions: RT: 50°C for 50 minutes; PCR: 94°C for 30 seconds; 60°C for 40 seconds; 72°C for 30 seconds; for 30 cycles using primers described in Maurer et al.17 RT-PCR was performed using primers for omcB, lcrH-1, and rs16 as an internal control. The products were run on 2% agarose gels, which were scanned and the bands quantified using Quantity One (BioRad). The results were plotted as bar graphs as relative density compared with rs16.
Results
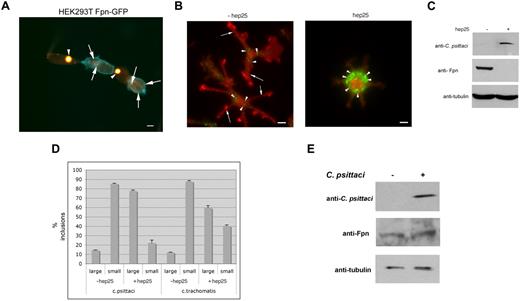
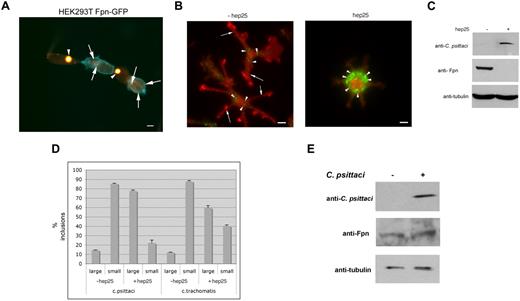
Expression of Fpn leads to cellular iron depletion and is predicted to limit the growth of intracellular bacteria. We tested this possibility by infecting HEK293T cells that express Fpn-GFP under the control of a ponasterone A–inducible promoter.5 Cells induced to express Fpn-GFP were infected with C psittaci for 2 hours and allowed to grow for an additional 18 hours. We found that the size of the inclusion body was inversely affected by the expression of Fpn-GFP. Cells that did not express Fpn-GFP showed extremely large inclusion bodies (Figure 1A arrowheads), while cells that expressed Fpn-GFP showed dramatically smaller inclusion bodies (Figure 1A arrows). Large inclusions are the result of a productive infection. Previous studies have shown that the expanding chlamydial inclusion is not derived from host proteins.14,18 No Fpn-GFP was observed on the inclusion membrane (data not shown) indicating that host proteins were sorted away from the expanding inclusion membrane.
Expression of Fpn limits the growth of Chlamydia. (A) HEK293Fpn-GFP cells were incubated with FAC for 24 hours and induced to express Fpn-GFP (green) using ponasterone A. Eighteen hours after induction, cells were incubated with C psittaci at an MOI of 100 bacteria/cell for 2 hours, extracellular bacteria removed by extensive washing, and cells placed in growth medium for specified times. Twenty-four hours after infection, cells were fixed and processed for immunofluorescence using a mouse anti–C psittaci antibody followed by an Alexa 594–conjugated goat anti–mouse IgG (red). Cells that were successfully induced showed Fpn-GFP (green) at the plasma membrane and had small inclusions. Cells that did not express Fpn-GFP had large inclusions. Arrowheads denote large inclusions and arrows denote small inclusions. Bar represents 10 μm. (B) Bone marrow macrophages isolated from C57/B6 mice were iron loaded and infected with C psittaci for 2 hours as in panel A. Following infection, cells were incubated with or without 1 μg/mL hep25 for 18 hours. Cells were methanol fixed and processed for immunofluorescence using rabbit anti-Fpn and mouse anti–C psittaci followed by an Alexa 594–conjugated goat anti–rabbit IgG (red) and an Alexa 488–conjugated goat anti–mouse IgG (green). Arrowheads denote C psittaci and arrows denote plasma membrane Fpn. Bar represents 10 μm. (C) Cells treated as in panel B were solubilized in lysis buffer and applied to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Chlamydial infection was assessed by Western blot using mouse anti–C psittaci followed by peroxidase-conjugated goat anti–mouse IgG. Fpn levels were assessed using rabbit anti-Fpn followed by peroxidase-conjugated goat anti–rabbit IgG. Tubulin levels were detected using mouse antitubulin followed by peroxidase-conjugated goat anti–mouse IgG as a loading control. (D) Large (greater than 1 μ) and small inclusions were quantified from cells infected with C psittaci or C trachomatis. More than 200 cells were analyzed for inclusion size for each sample. Error bars represent the standard deviation of the mean of 3 independent experiments. (E) Macrophages were incubated with C psittaci for 2 hours, extracellular bacteria removed, and cells incubated in growth media for an additional 18 hours. Cells were solubilized in lysis buffer and applied to SDS-PAGE, and Fpn levels assessed as in panel D.
Expression of Fpn limits the growth of Chlamydia. (A) HEK293Fpn-GFP cells were incubated with FAC for 24 hours and induced to express Fpn-GFP (green) using ponasterone A. Eighteen hours after induction, cells were incubated with C psittaci at an MOI of 100 bacteria/cell for 2 hours, extracellular bacteria removed by extensive washing, and cells placed in growth medium for specified times. Twenty-four hours after infection, cells were fixed and processed for immunofluorescence using a mouse anti–C psittaci antibody followed by an Alexa 594–conjugated goat anti–mouse IgG (red). Cells that were successfully induced showed Fpn-GFP (green) at the plasma membrane and had small inclusions. Cells that did not express Fpn-GFP had large inclusions. Arrowheads denote large inclusions and arrows denote small inclusions. Bar represents 10 μm. (B) Bone marrow macrophages isolated from C57/B6 mice were iron loaded and infected with C psittaci for 2 hours as in panel A. Following infection, cells were incubated with or without 1 μg/mL hep25 for 18 hours. Cells were methanol fixed and processed for immunofluorescence using rabbit anti-Fpn and mouse anti–C psittaci followed by an Alexa 594–conjugated goat anti–rabbit IgG (red) and an Alexa 488–conjugated goat anti–mouse IgG (green). Arrowheads denote C psittaci and arrows denote plasma membrane Fpn. Bar represents 10 μm. (C) Cells treated as in panel B were solubilized in lysis buffer and applied to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Chlamydial infection was assessed by Western blot using mouse anti–C psittaci followed by peroxidase-conjugated goat anti–mouse IgG. Fpn levels were assessed using rabbit anti-Fpn followed by peroxidase-conjugated goat anti–rabbit IgG. Tubulin levels were detected using mouse antitubulin followed by peroxidase-conjugated goat anti–mouse IgG as a loading control. (D) Large (greater than 1 μ) and small inclusions were quantified from cells infected with C psittaci or C trachomatis. More than 200 cells were analyzed for inclusion size for each sample. Error bars represent the standard deviation of the mean of 3 independent experiments. (E) Macrophages were incubated with C psittaci for 2 hours, extracellular bacteria removed, and cells incubated in growth media for an additional 18 hours. Cells were solubilized in lysis buffer and applied to SDS-PAGE, and Fpn levels assessed as in panel D.
We confirmed the effect of Fpn-GFP expression on C psittaci growth using macrophages, as these cells express high levels of endogenous Fpn in response to iron.19 Cultured bone marrow macrophages from C57/B6 mice were incubated with ferric ammonium citrate (FAC, 10 μM iron) to increase their iron content. The cells were then infected with C psittaci for 2 hours, the extracellular bacteria were removed, cells were grown for 24 hours in the presence or absence of hepcidin, and bacterial growth was assayed by immunofluorescence microscopy and Western analysis using anti–C psittaci antibodies. At the multiplicity of infection used, greater than 95% of cells showed the presence of inclusion bodies (Figure 1B arrowheads). Fpn was present on the plasma membrane (Figure 1B arrows) but was not found surrounding the bacterial inclusions. The antimicrobial peptide hepcidin binds to Fpn inducing its internalization and degradation.5,20 The internalization of Fpn results in cellular iron retention. Addition of hepcidin led to the loss of Fpn at the plasma membrane and a dramatic increase in C psittaci organisms. The percentage of infected cells did not change, but there was an increase in both the size of the inclusion body and the number of inclusion bodies/infected cell. Changes in Fpn levels in response to hepcidin were confirmed by immunoblot (Figure 1C). The number of large and small C psittaci inclusions was quantified (Figure 1D). Similar results were seen when macrophages were infected with C trachomatis, albeit the change after hepcidin treatment was not as dramatic as that seen in cells infected with C psittaci. The concentration of hepcidin used in these experiments (500 nM) is consistent with measurements of either serum or urine hepcidin in iron-loaded or infected individuals.5
Previous studies suggested that macrophages increase the expression of Fpn mRNA when exposed to Salmonella typhimurium.9 To determine whether C psittaci infection affected Fpn levels, cultured bone marrow macrophages not exposed to iron were infected with C psittaci for 2 hours. Extracellular bacteria were removed and cells incubated for an additional 18 hours and Fpn levels assessed by Western analysis. As expected, Fpn levels in macrophages not exposed to iron were lower than those exposed to iron (compare Figure 1C and 1E). No change in Fpn levels was observed upon C psittaci infection (Figure 1E). Similarly, no change in Fpn levels was seen when macrophages were exposed to L pneumophila (data not shown).
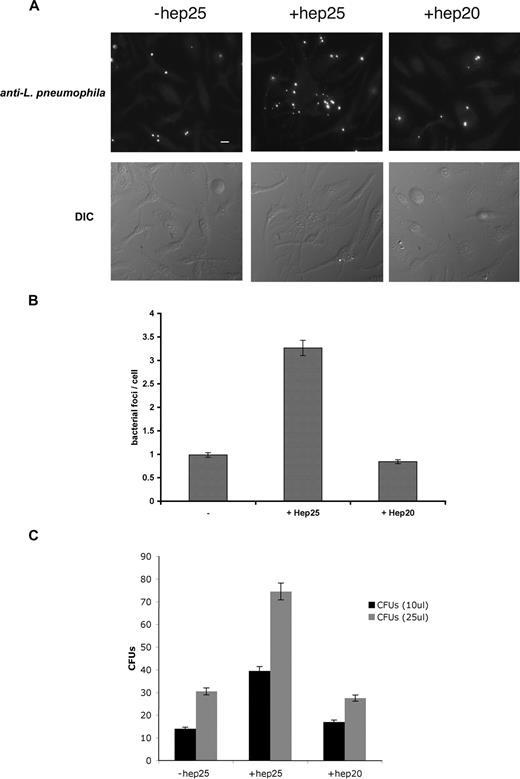
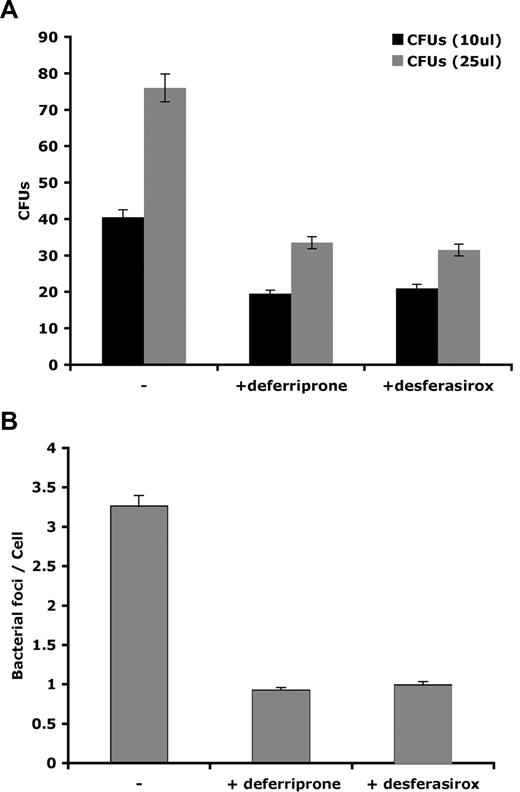
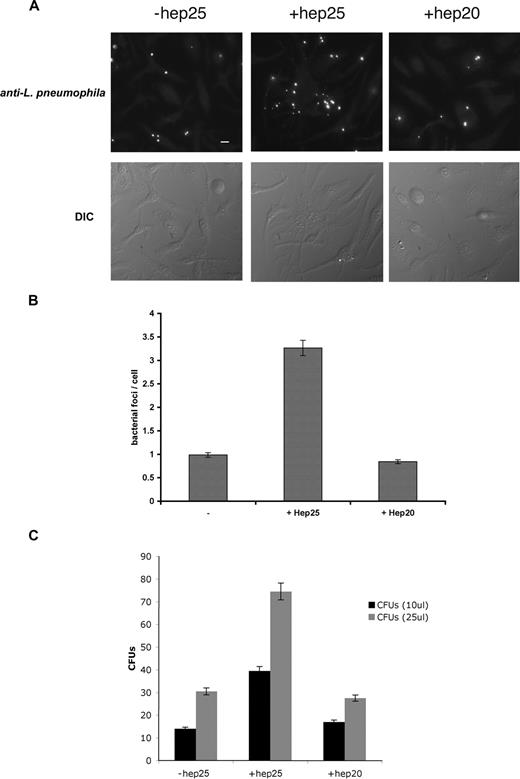
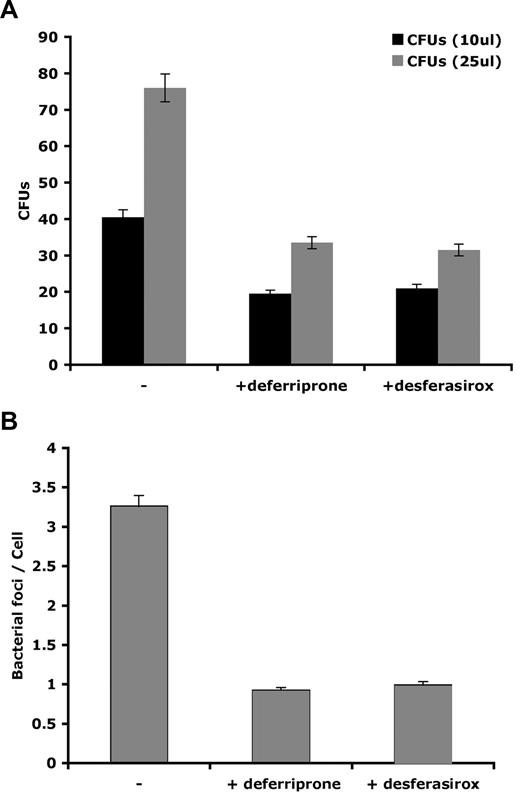
Hepcidin-mediated down-regulation of Fpn also promoted the intracellular growth of L pneumophila. Macrophages from A/J mice, a strain susceptible to L pneumophila infection,21 were incubated with FAC and infected with L pneumophila, and infection was assessed by immunofluorescence (Figure 2A), bacterial foci/cell (Figure 2B), and bacterial colony assay (Figure 2C). Once macrophages were infected, the addition of the active form of hepcidin (hep25) resulted in a significant increase in productive bacterial growth. A truncated form of hepcidin lacking the first 5 amino acids, hep20 that does not bind to Fpn,5 did not affect bacterial growth.
Expression of Fpn limits the growth of L pneumophila. (A) Bone marrow macrophages isolated from A/J mice were iron loaded as described in Figure 1, infected with L pneumophila for 2 hours, and incubated with or without hepcidin (hep25) or hep20 for 18 hours. Following hepcidin treatment, cells were fixed and processed for immunofluorescence using mouse anti–L pneumophila followed by Alexa 488–conjugated goat anti–mouse IgG. Bar represents 10 μm. (B) Quantification of intracellular L pneumophila expressed as bacteria foci/cell calculated from panel A where 10 fields of cells were examined per sample with approximately 10 to 15 cells present per field. The error bars represent the standard deviation of the mean from 3 separate experiments. (C) Cells treated as in panel A were lysed to release intracellular bacteria and lysates plated on CYET at either 10 or 25 μL. Bacterial colonies (CFUs) grown at 37°C were quantified 3 days after plating. The error bars represent the standard deviation of the mean from 3 separate experiments.
Expression of Fpn limits the growth of L pneumophila. (A) Bone marrow macrophages isolated from A/J mice were iron loaded as described in Figure 1, infected with L pneumophila for 2 hours, and incubated with or without hepcidin (hep25) or hep20 for 18 hours. Following hepcidin treatment, cells were fixed and processed for immunofluorescence using mouse anti–L pneumophila followed by Alexa 488–conjugated goat anti–mouse IgG. Bar represents 10 μm. (B) Quantification of intracellular L pneumophila expressed as bacteria foci/cell calculated from panel A where 10 fields of cells were examined per sample with approximately 10 to 15 cells present per field. The error bars represent the standard deviation of the mean from 3 separate experiments. (C) Cells treated as in panel A were lysed to release intracellular bacteria and lysates plated on CYET at either 10 or 25 μL. Bacterial colonies (CFUs) grown at 37°C were quantified 3 days after plating. The error bars represent the standard deviation of the mean from 3 separate experiments.
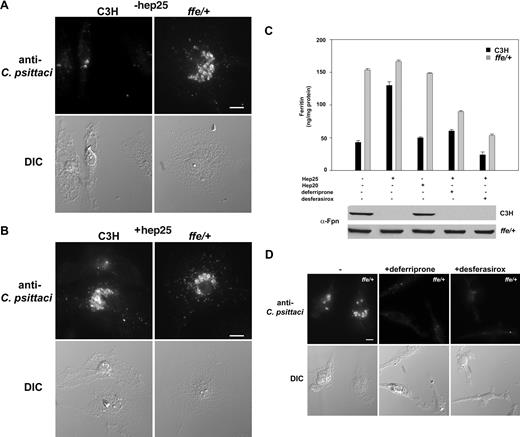
To show that the iron-exporting activity of Fpn limited bacterial growth, we examined C psittaci growth in macrophages isolated from the flatiron mutant mouse line. flatiron mice have a missense mutation (H32R) in one allele of Fpn leading to missorting of Fpn and its retention in intracellular compartments.22 Fpn (H32R) acts as a dominant negative affecting the ability of wild-type Fpn to export iron. This results in a severe reduction in the amount of iron exported. The flatiron mouse shows iron loading in Kupffer cells similar to patients with ferroportin disease,23 and macrophages cultured from the flatiron mouse show a dramatically impaired ability to export iron. flatiron (ffe/+) mouse macrophages supported a robust C psittaci infection compared with macrophages isolated from wild-type C3H animals (Figure 3A). The size of inclusions was unchanged in flatiron macrophages incubated with hep25 compared with wild-type macrophages, which show increased inclusion bodies in cells incubated with hep25 (Figure 3B). Changes in intracellular iron levels were confirmed by ferritin analysis (Figure 3C).
The Fpn disease mouse model ffe/+ macrophages show increased C psittaci infections. (A,B) Wild-type (C3H) and ffe/+ mouse bone marrow macrophages were incubated with iron for 48 hours, infected with C psittaci for 2 hours, incubated with or without hep25 for 18 to 24 hours, and processed for C psittaci immunofluorescence as in Figure 1. (C) Ferritin levels were determined from cells infected as in panel A and then incubated with hep25, hep20, hep25 and deferriprone, or hep25 and desferasirox. Fpn levels were detected by Western blot using a rabbit anti–mouse Fpn followed by a peroxidase conjugated goat anti–mouse IgG. Error bars represent SD. (D) Bone marrow macrophages from ffe/+ mice treated as in panel C were processed for immunofluorescence. Bar represents 10 μm. Experiments were performed a minimum of 3 times and the error bars represent the standard deviation of the mean.
The Fpn disease mouse model ffe/+ macrophages show increased C psittaci infections. (A,B) Wild-type (C3H) and ffe/+ mouse bone marrow macrophages were incubated with iron for 48 hours, infected with C psittaci for 2 hours, incubated with or without hep25 for 18 to 24 hours, and processed for C psittaci immunofluorescence as in Figure 1. (C) Ferritin levels were determined from cells infected as in panel A and then incubated with hep25, hep20, hep25 and deferriprone, or hep25 and desferasirox. Fpn levels were detected by Western blot using a rabbit anti–mouse Fpn followed by a peroxidase conjugated goat anti–mouse IgG. Error bars represent SD. (D) Bone marrow macrophages from ffe/+ mice treated as in panel C were processed for immunofluorescence. Bar represents 10 μm. Experiments were performed a minimum of 3 times and the error bars represent the standard deviation of the mean.
These results indicate that it is the iron-exporting activity of Fpn that modulates bacterial growth. This conclusion leads to the speculation that iron chelators might suppress the growth of bacteria that results from hepcidin-mediated Fpn down-regulation. To test this hypothesis, hep25-treated wild-type macrophages (C3H) or flatiron macrophages infected with C psittaci were incubated with iron chelators. We specifically focused on 2 clinically used oral iron chelators deferriprone (L1) and desferasirox (ICL670A), as these chelators are expected to be membrane permeable whereas deferrioxamine (DFO) is less permeable.24 We confirmed that these chelators were able to remove iron stored in macrophages by measuring cellular ferritin levels. Addition of FAC to flatiron macrophages leads to an increase in the iron storage protein ferritin. Ferritin levels persist when external iron is removed, as the flatiron defect in Fpn prevents iron export.22 Addition of deferriprone or desferasirox to iron-loaded flatiron macrophages decreased ferritin levels (Figure 3C). Similar results were seen for wild-type macrophages that had been preexposed to hepcidin. Addition of deferriprone or desferasirox to flatiron macrophages infected with C psittaci did not reduce the percentage of cells infected but did affect the degree of infection. The size of the C psittaci inclusion body was reduced in chelator-treated macrophages (Figure 3D).
Similar results were observed in hepcidin-treated macrophages infected with L pneumophila. Addition of deferriprone or desferasirox reduced L pneumophila levels as assayed by colony formation (Figure 4A) and immunofluorescence followed by the quantification of bacterial foci/cell (Figure 4B). The effect of these chelators on reducing cellular iron did not affect hepcidin-mediated Fpn degradation as seen by Western blot (data not shown). These results indicate that these chelators can remove iron directly from macrophages by a mechanism independent of Fpn.
Iron chelators deferriprone and desferasirox remove iron stored in macrophages limiting intracellular L pneumophila growth. Bone marrow macrophages isolated from A/J mice were iron loaded as in Figure 1, infected with L pneumophila for 2 hours, and incubated with hepcidin with or without iron chelators deferriprone or desferasirox for 18 hours. Cells were either (A) lysed and lysates plated on CYET for 3 days and CFUs determined or (B) processed for immunofluorescence and bacterial foci/cell determined as described in Figure 2. Experiments were repeated a minimum of 3 times. Error bars represent the standard deviation of the mean.
Iron chelators deferriprone and desferasirox remove iron stored in macrophages limiting intracellular L pneumophila growth. Bone marrow macrophages isolated from A/J mice were iron loaded as in Figure 1, infected with L pneumophila for 2 hours, and incubated with hepcidin with or without iron chelators deferriprone or desferasirox for 18 hours. Cells were either (A) lysed and lysates plated on CYET for 3 days and CFUs determined or (B) processed for immunofluorescence and bacterial foci/cell determined as described in Figure 2. Experiments were repeated a minimum of 3 times. Error bars represent the standard deviation of the mean.
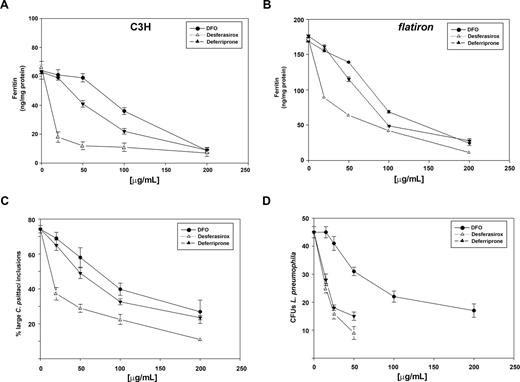
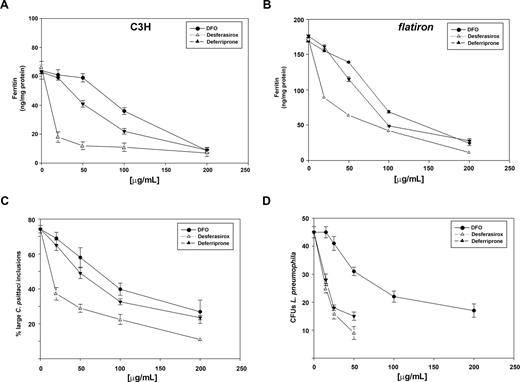
Until oral iron chelators became available, chelation therapy was restricted to the injectable iron chelator deferrioxamine (DFO). The limitations of DFO therapy are that it requires injections and DFO is poorly membrane permeable.25 We compared the ability of DFO, deferriprone, and desferasirox to chelate intracellular iron in wild-type (C3H) and flatiron mouse macrophages. Desferasirox was the most efficient at reducing intracellular iron (ferritin) levels in wild-type macrophages (Figure 5A) and in the ferroportin disease mouse model flatiron (Figure 5B). Iron chelation was effective in wild-type cells where ferritin levels were reduced from 64 ng/mg protein to 10 ng/mg protein. In flatiron macrophages, where ferritin levels were initially higher (170 ng/mg protein), desferasirox was again the most efficient at decreasing intracellular iron stores. The ability to chelate intracellular iron was also reflected in iron-dependent intracellular pathogen growth. Flatiron macrophages infected with C psittaci showed predominantly large intracellular bacterial inclusions (Figure 3). Incubation with different concentrations of iron chelators resulted in a reduced number of large inclusions (Figure 5C). Similarly, incubation of L pneumophila–infected macrophages with different concentrations of iron chelators resulted in lower numbers of CFUs (Figure 5D). Again, desferasirox was more effective in limiting intracellular pathogen growth than deferriprone, which was more effective than DFO.
Oral iron chelators desferasirox and deferriprone are more effective than DFO in chelating intracellular iron and limiting intracellular C psittaci and L pneumophila growth. Macrophages from either (A) wild-type mice (C3H) or (B) flatiron mice were grown in FAC for 48 hours and then incubated in the presence of different concentrations of DFO (•), desferasirox (△), or deferriprone (▲) for 18 hours. Cells were lysed and ferritin levels determined as described in “Methods.” (C) Flatiron macrophages grown in FAC for 48 hours were infected with C psittaci for 2 hours, extracellular bacteria washed away, and cells incubated in the presence of different concentrations of DFO (•), desferasirox (△), or deferriprone (▲). Twenty-fours hours after infection cells were fixed and processed for immunofluorescence, and the percentage of cells with large inclusions was determined as in Figure 1. (D) A/J macrophages grown in FAC for 48 hours were infected with L pneumophila for 2 hours, extracellular bacteria washed away, and cells incubated in the presence of different concentrations of DFO (•), desferasirox (△), or deferriprone (▲). Cells were lysed and lysates plated on CYET for 3 days and CFUs determined. Experiments were repeated a minimum of 3 times. Error bars represent the standard deviation of the mean.
Oral iron chelators desferasirox and deferriprone are more effective than DFO in chelating intracellular iron and limiting intracellular C psittaci and L pneumophila growth. Macrophages from either (A) wild-type mice (C3H) or (B) flatiron mice were grown in FAC for 48 hours and then incubated in the presence of different concentrations of DFO (•), desferasirox (△), or deferriprone (▲) for 18 hours. Cells were lysed and ferritin levels determined as described in “Methods.” (C) Flatiron macrophages grown in FAC for 48 hours were infected with C psittaci for 2 hours, extracellular bacteria washed away, and cells incubated in the presence of different concentrations of DFO (•), desferasirox (△), or deferriprone (▲). Twenty-fours hours after infection cells were fixed and processed for immunofluorescence, and the percentage of cells with large inclusions was determined as in Figure 1. (D) A/J macrophages grown in FAC for 48 hours were infected with L pneumophila for 2 hours, extracellular bacteria washed away, and cells incubated in the presence of different concentrations of DFO (•), desferasirox (△), or deferriprone (▲). Cells were lysed and lysates plated on CYET for 3 days and CFUs determined. Experiments were repeated a minimum of 3 times. Error bars represent the standard deviation of the mean.
Iron chelators may have direct toxic effects toward bacteria independent of iron chelation. We tested this possibility by infecting macrophages from flatiron mice with C psittaci for 2 hours and incubating the cells with DFO, desferasirox, or deferriprone for 24 hours. Cells incubated with DFO, desferasirox, or deferriprone showed no significant differences in the size of inclusion bodies compared with cells not treated with the chelators (Figure 6A). As seen before, iron-loaded macrophages showed large inclusions reflecting increased numbers of C psittaci. When the iron-loaded cells were incubated with iron chelators, the size of the inclusions was reduced. To determine whether the decrease in inclusion body size was due to reduced levels of available iron, we preincubated desferasirox, the most effective iron chelator, with increasing amounts of iron prior to addition to the iron-loaded cells. The iron-chelating effects on bacterial growth could be overcome by saturating the ability of desferasirox to chelate iron as determined by the increase in the size of the inclusion bodies (Figure 6A right panel). The amount of iron in macrophages under these experimental conditions was determined by measuring ferritin levels (Figure 6B).
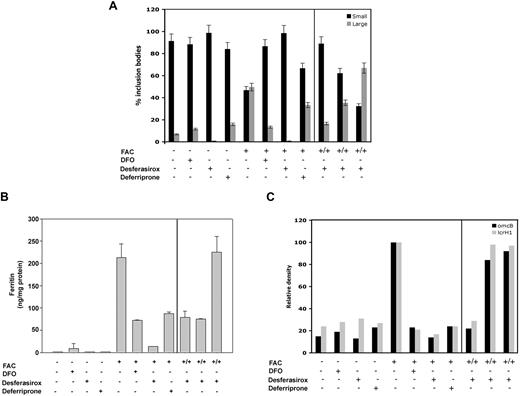
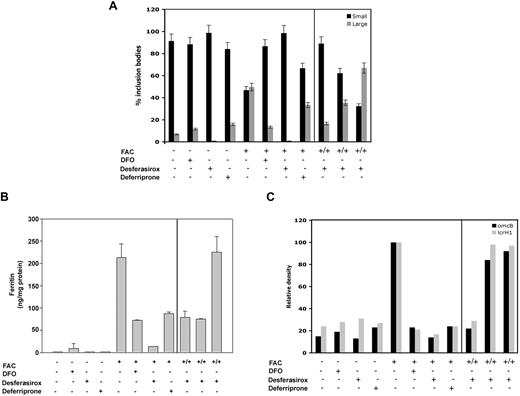
Iron chelators limit the growth of Chlamydia by reducing intracellular iron. Macrophages from flatiron mice were grown in the presence (+) or absence (−) of FAC for 18 hours. Cells were infected with C psittaci for 2 hours, extracellular bacteria removed, and cells incubated in the presence of DFO, desferasirox, deferriprone, or desferasirox that was preincubated with 10 to 100 μM iron as FAC (+/+). Twenty-four hours after infection, cells were processed for immunofluorescence and large and small inclusions quantified as in Figure 1 (A), lysed for ferritin analysis as in Figure 5 (B), or lysed for RT-PCR analysis of C psittaci genes omcB, lcrH-1, and rs16 (C) as described in “Other procedures.” Error bars represent the standard deviation of the mean.
Iron chelators limit the growth of Chlamydia by reducing intracellular iron. Macrophages from flatiron mice were grown in the presence (+) or absence (−) of FAC for 18 hours. Cells were infected with C psittaci for 2 hours, extracellular bacteria removed, and cells incubated in the presence of DFO, desferasirox, deferriprone, or desferasirox that was preincubated with 10 to 100 μM iron as FAC (+/+). Twenty-four hours after infection, cells were processed for immunofluorescence and large and small inclusions quantified as in Figure 1 (A), lysed for ferritin analysis as in Figure 5 (B), or lysed for RT-PCR analysis of C psittaci genes omcB, lcrH-1, and rs16 (C) as described in “Other procedures.” Error bars represent the standard deviation of the mean.
The data confirm that growth of intracellular pathogens is dependent on iron availability in macrophages. We examined the effect of cellular iron levels on C psittaci iron-responsive genes to confirm the direct effect of iron levels on pathogen virulence. RT-PCR was performed on RNA extracted from infected flatiron macrophages using primers for omcB (outer membrane protein) and lcrH-1 (type III secretory system chaperone), 2 known iron-responsive genes.17 Both omcB and lcrH-1 showed increased expression in iron-loaded macrophages compared with a non–iron-responsive internal RNA control, rs16 (Figure 6C). The expression of these genes was decreased when infected macrophages were treated with iron chelators, indicating that iron depletion has direct effect on C psittaci virulence. Together, these results suggest that iron chelation therapy might be efficacious in the treatment of intracellular bacterial infections.
Discussion
Iron acquisition genes are virulence factors for most species of bacteria including those that live as intracellular parasites (for review, see Schaible and Kaufmann7 ). Bacteria are internalized into membranous compartments (endosomes/phagosome) and then subsequently trafficked to the lysosome for degradation. Intracellular pathogens have evolved specific mechanisms to survive within this intracellular environment. Some bacteria such as Salmonella persist in the endocytic pathway, others escape the endo/lysosomal system and exist in the cytosol (Listeria monocytogenes). Other bacteria remain within a membranous envelope that may be a modified version of the endoplasmic reticulum (L pneumophila) or a membranous compartment generated by the bacteria (Chlamydia; for review, see Gruenberg and van der Goot26 ). Regardless of the specific compartment in which they exist, all of these intracellular bacteria require iron to develop a productive infection. The mechanism of iron acquisition by intracellular bacteria such as Legionella, Chlamydia, or Mycobacteria is undefined, but it is reasonable to assume that the cytosolic iron pool is the source. Conditions that limit host cell iron acquisition will affect the growth of intracellular bacteria.
Expression of the mammalian iron exporter Fpn would limit the growth of intracellular bacteria by depleting cytosolic iron. Expression of Fpn can deplete cytosolic iron leading to the loss of the iron-storage protein ferritin.5,27,28 If expression of Fpn is not regulated, cellular iron deprivation can lead to cell death. Under normal conditions Fpn activity is limited by several mechanisms. First, Fpn mRNA has a 5′ iron-responsive element (IRE) that limits translation of the mRNA.27,29 Under low iron conditions, protein levels are reduced. This mode of regulation, however, may be diminished in macrophages engaged in erythrophagocytosis. Macrophages in the spleen and liver are continuously ingesting iron through the internalization of senescent red blood cells. Pulmonary macrophages, particularly from individuals living in industrialized areas or smokers, are also being continuously exposed to iron loads.30 In addition, macrophages play an important role in the removal of aged polymorphonuclear monocytes and apoptotic cells, all potential sources of iron. Under these conditions Fpn expression is expected to be high.
The second mode of Fpn regulation is through its interaction with hepcidin, a peptide hormone synthesized by the liver in response to increased hepatic iron and to inflammatory stimuli. Hepcidin binds to Fpn, inducing its internalization and degradation.5,20 The removal of Fpn from the cell surface leads to decreased iron export and increased cellular iron retention. Secretion of hepcidin in response to systemic bacterial infection mediates the hypoferremia of inflammation, which if persistent will lead to anemia.2,31,32 Fpn is a multimer and the H32R mutant protein found in the flatiron mouse participates in multimer formation with the wild-type protein. The H32R mutant acts as a dominant negative, severely reducing the amount of wild-type protein on the cell surface.22,33 This results in iron retention in macrophages, similar to that seen in patients with ferroportin disease.23 We determined that macrophages from flatiron mice show enhanced bacterial growth independent of hepcidin levels compared with macrophages from wild-type C3H mice. This suggests that patients with ferroportin disease might be more susceptible to intracellular bacterial infections. Incubation of C psittaci– or L pneumophila–infected mouse macrophages with iron chelators deferriprone or desferasirox reduced the level of bacterial infections supporting the hypothesis that productive intracellular bacterial infections require intracellular iron.
We have demonstrated that macrophage iron retention promotes the intracellular growth of L pneumophila, C psittaci, and C trachomatis, but Dill and Raulston reported that limited changes in intracellular iron content did not affect the growth of Chlamydia.34 The studies of Dill and Raulston, using our established cell line HEK293T expressing Fpn-GFP, suggested that the levels of Fpn-GFP expressed under an inducible promoter were not capable of reducing iron below a threshold needed to affect chlamydial growth. It is unclear why in that study expression of Fpn did not lead to decreased ferritin levels. In our studies, the HEK293T cells are iron loaded prior to inducing the expression of Fpn-GFP. Greater than 95% of cells express Fpn-GFP and greater than 90% of the cells are infected with Chlamydia, but the expansion of the chlamydial inclusion was limited when Fpn-GFP was expressed. Our observations in macrophages in which the effect of hepcidin is on endogenous Fpn are consistent with the results in the HEK293T expressing Fpn-GFP cells. Others have shown that macrophage iron retention promotes the intracellular growth of Salmonella and Mycobacterium.8,9 These results indicate that inflammation leading to hepcidin secretion will promote intracellular bacterial growth. These data support the epidemic pathogenic selection hypotheses, which state that increased macrophage iron export should decrease intracellular bacterial growth.35
The hallmark of bacterial infections such as Chlamydia and Mycobacteria is persistent inflammation, which by increasing hepcidin expression would lead to macrophage iron retention and increased intracellular bacterial growth. Recent studies have shown that Pseudomonas aeruginosa, acting through TLR-4, can induce hepcidin expression in macrophages.36 Expression of hepcidin in cultured macrophages was also reported following infection with Mycobacterium avium and Mycobacterium tuberculosis.37 Further, infection of macrophages with P aeruginosa resulted in a decrease in Fpn mRNA. We have not observed changes in Fpn levels in macrophages infected with C psittaci or L pneumophila. Conditions that inhibit hepcidin expression or deplete macrophage iron levels might be therapeutic as they would limit or reduce intracellular bacterial growth. Studies using cultured cells have shown that addition of DFO leads to decreased growth for a number of intracellular bacteria. The ability of DFO to cross membranes, however, is problematic. Patient compliance is also problematic, as DFO is not absorbed by the gut and must be injected. Recently, a new generation of oral iron chelators has been developed that have been approved for human use. We demonstrated that these chelators, deferriprone and desferasirox, can remove iron from iron-loaded macrophages supporting the view that these chelators are membrane permeable. We have also shown that the chelators can reduce intracellular growth of Chlamydia and Legionella in hepcidin-treated macrophages. These results suggest that these iron chelators might be efficacious in the treatment of persistent bacterial infections.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Prem Ponka (McGill University, Montreal, QC) for the iron chelators deferriprone and desferasirox, Dr Tomas Ganz (University of California, Los Angeles) for hepcidin, Dr David Haile (University of Texas, San Antonio) for Fpn antibody, Dr Priscilla Wyrick (Eastern Tennessee State University, Johnson City) for Chlamydia strains, Dr Michelle Swanson (University of Michigan, Ann Arbor) for L pneumophila, and Dr Lee Niswander (University of Colorado Health Sciences Center, Denver) for the flatiron mice.
This work was supported by National Institutes of Health grants HL26922 and DK079047 (J.K.).
National Institutes of Health
Authorship
Contribution: P.N.P. performed experiments and wrote the paper; I.D.D. performed experiments and edited the paper; N.D. performed experiments; I.Z. provided the flatiron mouse model and edited the paper; J.K. analyzed the data and wrote the paper; and D.M.W. performed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Diane McVey Ward, Department of Pathology, School of Medicine, University of Utah, Salt Lake City, UT 84132; e-mail: diane.mcveyward@path.utah.edu.