Abstract
In β-thalassemia, the mechanism driving ineffective erythropoiesis (IE) is insufficiently understood. We analyzed mice affected by β-thalassemia and observed, unexpectedly, a relatively small increase in apoptosis of their erythroid cells compared with healthy mice. Therefore, we sought to determine whether IE could also be characterized by limited erythroid cell differentiation. In thalassemic mice, we observed that a greater than normal percentage of erythroid cells was in S-phase, exhibiting an erythroblast-like morphology. Thalassemic cells were associated with expression of cell cycle–promoting genes such as EpoR, Jak2, Cyclin-A, Cdk2, and Ki-67 and the antiapoptotic protein Bcl-XL. The cells also differentiated less than normal erythroid ones in vitro. To investigate whether Jak2 could be responsible for the limited cell differentiation, we administered a Jak2 inhibitor, TG101209, to healthy and thalassemic mice. Exposure to TG101209 dramatically decreased the spleen size but also affected anemia. Although our data do not exclude a role for apoptosis in IE, we propose that expansion of the erythroid pool followed by limited cell differentiation exacerbates IE in thalassemia. In addition, these results suggest that use of Jak2 inhibitors has the potential to profoundly change the management of this disorder.
Introduction
β-Thalassemia, one of the most common congenital anemias, arises from partial or complete lack of β-globin synthesis. β-Thalassemia major, also known as Cooley anemia,1 is the most severe form of this disease, and is characterized by ineffective erythropoiesis (IE) and extramedullary hematopoiesis (EMH), requiring regular blood transfusions to sustain life.1-5 In β-thalassemia intermedia, where a larger amount of β-globin is synthesized, the clinical picture is milder and the patients do not require frequent transfusions. The ineffective production of red blood cells in both forms of the disease has been attributed to erythroid cell death during the maturation process mediated by apoptosis or hemolysis. It was proposed that accumulation of alpha-globin chains leads to the formation of aggregates, which impair erythroid maturation triggering apoptosis.6-13 Ferrokinetic studies done in 1970 suggested that 60% to 80% of the erythroid precursors in β-thalassemia major die in the marrow or extramedullary sites.14 However, several observations call into question the view that cell death is the only cause of IE in β-thalassemia.
First, the number of apoptotic erythroid cells in thalassemic patients is low compared with that anticipated by ferrokinetic studies.14,15 In fact, only 15% to 20% of bone marrow (BM) erythroid precursors (CD45−/CD71+) present apoptotic features in aspirates from affected patients.6,8,16 Second, hemolytic markers in young β-thalassemic patients are normal or only slightly increased, unless the patients suffer from splenomegaly or the liver has been damaged by iron overload or viral infections.17 Third, the original ferrokinetic studies18-21 do not exclude that the majority of the iron administered to patients affected by IE could be directly stored by liver parenchymal cells rather than being used by erythroid cells.22-26 This would explain the ferrokinetic studies without invoking massive erythroid apoptosis or hemolysis.
Given the controversies in the literature over the cause of IE, we have undertaken a detailed investigation of this process in 2 mouse models that mimic β-thalassemia intermedia (th3/+) and major (th3/th3). In th3/+ mice, both the βminor and βmajor genes have been deleted from one chromosome.27,28 Adult th3/+ mice exhibit hepatosplenomegaly, anemia, and aberrant erythrocyte morphology comparable with that found in patients affected by β-thalassemia intermedia. Mice completely lacking adult β-globin genes (th3/th3) die late in gestation,27 limiting their utility as a model of β-thalassemia major. To circumvent this problem, we undertook bone marrow transplantation, wherein hematopoietic fetal liver cells (HFLCs) were harvested from th3/th3 embryos at embryonic day 14.5 (E14.5) and injected into lethally irradiated syngeneic wild-type (wt) adult recipients.29 Hematologic analyses of engrafted mice performed 6 to 8 weeks after transplantation revealed severe anemia due not to pancytopenia but rather to low red blood cell (RBC) and reticulocyte counts together with massive splenomegaly and extensive EMH.29,30 These animals could be rescued and the hematologic parameters, splenomegaly, and EMH normalized by lentiviral-mediated β-globin gene transfer29,30 or by blood transfusion,22 supporting the notion that their phenotype is specifically due to erythroid impairment. In this way, we established the first adult mouse model of β-thalassemia major.29
The principal regulator of both basal and stress erythropoiesis is erythropoietin (Epo).31-33 Interaction of Epo with the Epo receptor (EpoR) induces, through Jak2 and Stat5, multiple signaling pathways designed to prevent apoptosis and to support erythroid proliferation.34-36 The severity of the anemia in Stat5-deficient mice correlates with the relative loss of Bcl-XL expression.37-39 Bcl-XL prevents apoptosis during the final stages of erythroid differentiation rather than at the erythroid colony-forming unit (CFU-E) or proerythroblast stage as shown by several groups.40,41 Therefore, up-regulation of Bcl-XL mediated by Epo is expected to protect erythroid cells primarily during the final stages of differentiation. Thus, abnormal Epo levels as well as increased synthesis or posttranslational modification of cell cycle–associated proteins could play a crucial role in regulating the proliferation and apoptosis of erythroid cells in β-thalassemia.41,42
The present results provide 5 new major findings. First, the mechanism leading to a disproportionate number of proliferating erythroid cells in β-thalassemia is associated with expression of cell cycle–promoting and survival factors that mitigate apoptosis. Second, although our data do not exclude a role for apoptosis in triggering IE, they suggest that controlling maturation of erythroid precursors plays an important role in this process. Third, our observations point to additional factors, intrinsic and/or extrinsic, that limit erythroid differentiation in β-thalassemia. Fourth, we have demonstrated that inhibition of Jak2 has a profound effect both in vitro and in vivo, limiting erythroid cell proliferation and reversing splenomegaly in thalassemic mice. Finally, some of the animal results have been corroborated in human blood and spleen specimens from patients, suggesting that our findings can be extended to the human disease.
Methods
Purification of erythroid cells from the spleen
Spleens from wt, th3/+, and th3/th3 mice were harvested and mechanically dissociated into single-cell suspensions. Murine mononuclear cells were then isolated by centrifugation using Lympholyte-M density gradients (Cedarlane Laboratories, Westbury, NY) following the manufacturer's instructions. Cells were incubated on ice for 15 minutes with a cocktail containing 10 μg each of nonerythroid fluorescein isothiocyanate (FITC)–conjugated antibodies (GR-1, MAC1, CD4, CD8, CD11b, and CD49; BD PharMingen, San Diego, CA). After washing, the cells were incubated for 15 minutes at 4°C with anti-FITC microbeads (Miltenyi Biotech, Auburn, CA). The cell suspension was placed on a magnetic column and the eluted erythroid cells were kept for RNA extraction, protein analysis, in vitro culture with carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) staining, or flow cytometric analysis. This study received approval from the Weill Medical College of Cornell University institutional animal care and use committee and International Review Board.
Primary splenic erythroid cell cultures and CFSE staining
CFSE was added to the cells to give a final concentration of 1.25 μM. After 10 minutes at 37°C, further dye uptake was prevented by addition of 5 volumes of cold medium and incubated in ice for 5 minutes. The cells were then washed 3 times and seeded at 107 cells/mL in Iscove modified Dulbecco medium with 30% fetal bovine serum (Hyclone, South Logan, UT), 1% deionized bovine serum albumin (BSA), 100 IU/mL penicillin, 100 μg/mL streptomycin (Mediatech, Manassas, VA), 0.1 mM β-thioglycerol (mTG; Sigma-Aldrich, St Louis, MO), and 0.1 mM recombinant human erythropoietin (rHuEpo, 10 U/mL; Amgen, Thousand Oaks, CA). Aliquots of the cells were then cultured in the presence and absence of 100 μM colcemid with and without AG490 (100 μM; Calbiochem-EMD Biosciences, San Diego, CA) or TG101209 (TargeGen, San Diego, CA), 2 Jak2 inhibitors.
Phospho-Jak2 analysis
One million cells per genotype were fixed and permeabilized (Fix and Perm Kit; Invitrogen, Grand Island, NY) as per the manufacturer's instructions. Cells were incubated for 30 minutes with 0.05 μg phospho-Jak2 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or with 0.05 μg isotype control (Santa Cruz Biotechnology). The cells were washed twice with 1% BSA–phosphate-buffered saline and then incubated for 30 minutes at room temperature in the dark with 0.05 μg of a secondary antibody (Jackson ImmunoResearch, West Grove, PA). After washing twice, the cells were immediately analyzed using flow cytometry. For the peptide competition assay, 0.05 μg phospho-Jak2 polyclonal antibody was incubated for 2 hours at room temperature with a 5-fold concentration of blocking peptide in Medium B of the Fix and Perm Kit. The peptide-antibody solution was then added to the fixed cells and they were incubated as described in this paragraph.
Results
Increased numbers of immature and proliferating erythroid cells in β-thalassemia
Using HFLCs derived from wt, th3/+, and th3/th3 embryos, we generated groups of mice that underwent transplantation with the same genetic background. As a control for the transplantation procedure, we compared these animals to same-sex th3/+ and wt mice that had not undergone transplantation, observing no statistical differences in their respective patterns of erythropoiesis (n ≥ 3 per genotype, data not shown). Two months after transplantation, th3/+ and th3/th3 mice revealed anemia (Table 1) together with splenomegaly, hepatic EMH, and iron overload as shown previously.22,29,30,43,44 We also examined the spleen and the BM by fluorescence activated cell sorting (FACS), comparing the fraction of cells in discrete erythroid populations using markers for the transferring receptor (CD71) and erythroid specificity (Ter119). In thalassemic mice, there was both a relative and absolute expansion of the immature erythroid progenitor cell fraction compared with cells in the final stages of erythroid differentiation and maturation (orthochromatic cells, reticulocytes, and erythrocytes; Figures S1,S2, available on the Blood website; see the Supplemental Materials link at the top of the online article; Table 1). A skewed FACS profile indicative of IE was already visible in th3/th3 embryos at E16.5, as indicated by the absence of more mature cells (Figure S3 red oval). This indicated that engraftment of HFLCs into healthy adult animals faithfully transferred the genetic defect leading to IE. Animals that received a transplant of th3/th3 HFLCs showed low reticulocyte counts and loss of mature erythroid cells (CD71−/Ter119+) by FACS, suggesting that the disappearance of erythroid cells in these animals occurs prior to or simultaneous with the generation of reticulocytes. Cytospins (Figure 1A) of purified erythroid cells (as shown in Figure S2) clearly showed that the thalassemic samples were composed of a homogeneous population characterized by larger and more immature cells. This finding indicates either elimination of late-stage erythroid cells or an imbalance between cell proliferation and cell differentiation.
Hematologic parameters in β-thalassemia
| Hematologic parameter . | wt . | th3/+ . | th3/th3 . |
|---|---|---|---|
| HB level, g/dL | 14.4 ± 0.92 | 8.80 ± 1.10 | 2.80 ± 0.81 |
| Total RBC count, 106/μL | 9.60 ± 0.60 | 7.90 ± 1.10 | 2.40 ± 0.60 |
| Total reticulocyte count, 105/μL | 2.80 ± 0.54 | 22.4 ± 4.64 | 2.05 ± 0.74 |
| Total BM CD71+/Ter119+, × 108 | 2.58 ± 0.09 | 4.04 ± 1.68 | 1.82 ± 0.83 |
| Total BM CD71−/Ter119+, × 108 | 0.73 ± 0.29 | 0.23 ± 0.08 | 0.18 ± 0.08 |
| Total spleen CD71+/Ter119+, × 108 | 0.21 ± 0.09 | 7.58 ± 3.38 | 14.40 ± 5.04 |
| Total spleen CD71−/Ter119+, × 108 | 0.71 ± 0.43 | 1.00 ± 0.35 | 1.17 ± 0.73 |
| Total BM+ spleen CD71+/Ter119+, × 108 | 2.95 ± 0.99 | 11.80 ± 5.04 | 15.0 ± 6.13 |
| Total BM+ spleen CD71−/Ter119+, × 108 | 1.51 ± 0.44 | 1.26 ± 0.39 | 1.52 ± 0.88 |
| % (CD71+/Ter119+)/(Total Ter119+) | 4.97 ± 2.48 | 58.70 ± 0.94 | 80.20 ± 4.73 |
| Hematologic parameter . | wt . | th3/+ . | th3/th3 . |
|---|---|---|---|
| HB level, g/dL | 14.4 ± 0.92 | 8.80 ± 1.10 | 2.80 ± 0.81 |
| Total RBC count, 106/μL | 9.60 ± 0.60 | 7.90 ± 1.10 | 2.40 ± 0.60 |
| Total reticulocyte count, 105/μL | 2.80 ± 0.54 | 22.4 ± 4.64 | 2.05 ± 0.74 |
| Total BM CD71+/Ter119+, × 108 | 2.58 ± 0.09 | 4.04 ± 1.68 | 1.82 ± 0.83 |
| Total BM CD71−/Ter119+, × 108 | 0.73 ± 0.29 | 0.23 ± 0.08 | 0.18 ± 0.08 |
| Total spleen CD71+/Ter119+, × 108 | 0.21 ± 0.09 | 7.58 ± 3.38 | 14.40 ± 5.04 |
| Total spleen CD71−/Ter119+, × 108 | 0.71 ± 0.43 | 1.00 ± 0.35 | 1.17 ± 0.73 |
| Total BM+ spleen CD71+/Ter119+, × 108 | 2.95 ± 0.99 | 11.80 ± 5.04 | 15.0 ± 6.13 |
| Total BM+ spleen CD71−/Ter119+, × 108 | 1.51 ± 0.44 | 1.26 ± 0.39 | 1.52 ± 0.88 |
| % (CD71+/Ter119+)/(Total Ter119+) | 4.97 ± 2.48 | 58.70 ± 0.94 | 80.20 ± 4.73 |
Data are means plus or minus SD.
In β-thalassemic mice, there is a decreased number of differentiated cells, a small increase of apoptotic and hemolytic markers, and elevated production of Epo. (A) In cytospins of purified splenic erythroid cells, distinctive types of cells can be seen representing different stages of maturation. More mature cells are characterized by a smaller size, decreased cytoplasmic basophilia, and an increase in nuclear pyknosis. (May-Grünwald Giemsa stain; magnification, 400×.) These observations were corroborated by FACS analyses (Figures S1,S2). Apoptosis was investigated (B) by CC3 assay on spleen sections; (C) by TUNEL assay on BM and spleen sections; (D) by CC3 assay on purified erythroid cells; and (E) by annexin-V assay on fresh spleen and BM cells (data not shown; n ≥ 3 per genotype). For panels A through D, images were captured on a Nikon Eclipse E800 microscope (Melville, NY), with a Retiga Exi camera (Qimaging, Burnaby, BC) and a Plan Fluor 40×/0.75 numeric aperture objective, then acquired using the IPLab 3.65a software (Scanalytics, Fairfax, VA). Brightness/contrast and color balance were adjusted using Adobe Photoshop 7.0.1 (Adobe Systems, San Jose, CA). The levels of hemolytic markers (F) bilirubin and (G) LDH were also investigated (n ≥ 6 per genotype). In panels E-G, th3/+ and th3/th3 mice are indicated as +/− and −/−, respectively. A nonparametric t test was used for statistical analysis. Increased Epo levels inversely correlate with those of hemoglobin in thalassemic mice. Measurements were made of (H) Epo levels in mice 2 months after BMT and (I) Epo and Hb levels in mice up to 1 year of age. In panel H, a nonparametric t test was used for statistical analysis; n ≥ 3 per genotype; P = .037 (*) and P = .001 (***), respectively, for th3/+ and th3/th3 mice compared with wt animals. In this panel th3/+ and th3/th3 mice are indicated, respectively, as +/− and −/−. In panel I, increased Epo levels inversely correlate with Hb in thalassemic mice. Epo levels were measured in random mice up to 1 year of age or 1 year after BMT in wt (□, n = 17) and th3/+ (▲, n = 18) mice. Pearson r test was used to determine the degree of linear association or the correlation coefficient between the Hb and Epo levels (wt, nonsignificant, P = .087; th3/+, P = .027). Error bars represent SD.
In β-thalassemic mice, there is a decreased number of differentiated cells, a small increase of apoptotic and hemolytic markers, and elevated production of Epo. (A) In cytospins of purified splenic erythroid cells, distinctive types of cells can be seen representing different stages of maturation. More mature cells are characterized by a smaller size, decreased cytoplasmic basophilia, and an increase in nuclear pyknosis. (May-Grünwald Giemsa stain; magnification, 400×.) These observations were corroborated by FACS analyses (Figures S1,S2). Apoptosis was investigated (B) by CC3 assay on spleen sections; (C) by TUNEL assay on BM and spleen sections; (D) by CC3 assay on purified erythroid cells; and (E) by annexin-V assay on fresh spleen and BM cells (data not shown; n ≥ 3 per genotype). For panels A through D, images were captured on a Nikon Eclipse E800 microscope (Melville, NY), with a Retiga Exi camera (Qimaging, Burnaby, BC) and a Plan Fluor 40×/0.75 numeric aperture objective, then acquired using the IPLab 3.65a software (Scanalytics, Fairfax, VA). Brightness/contrast and color balance were adjusted using Adobe Photoshop 7.0.1 (Adobe Systems, San Jose, CA). The levels of hemolytic markers (F) bilirubin and (G) LDH were also investigated (n ≥ 6 per genotype). In panels E-G, th3/+ and th3/th3 mice are indicated as +/− and −/−, respectively. A nonparametric t test was used for statistical analysis. Increased Epo levels inversely correlate with those of hemoglobin in thalassemic mice. Measurements were made of (H) Epo levels in mice 2 months after BMT and (I) Epo and Hb levels in mice up to 1 year of age. In panel H, a nonparametric t test was used for statistical analysis; n ≥ 3 per genotype; P = .037 (*) and P = .001 (***), respectively, for th3/+ and th3/th3 mice compared with wt animals. In this panel th3/+ and th3/th3 mice are indicated, respectively, as +/− and −/−. In panel I, increased Epo levels inversely correlate with Hb in thalassemic mice. Epo levels were measured in random mice up to 1 year of age or 1 year after BMT in wt (□, n = 17) and th3/+ (▲, n = 18) mice. Pearson r test was used to determine the degree of linear association or the correlation coefficient between the Hb and Epo levels (wt, nonsignificant, P = .087; th3/+, P = .027). Error bars represent SD.
Apoptosis and hemolysis levels are only slightly increased in β-thalassemia
Historically, IE in thalassemia has been attributed to increased cell death due to apoptosis or hemolysis of erythroid cells during the maturation process. Apoptosis in β-thalassemia has been investigated primarily using the annexin-V assay. We used this assay in our animal studies and observed some differences (Figure 1E), although they were not statistically significant. However, annexin-V also labels mature erythrocytes, and it has been shown that thalassemic cells have an abnormal exposure of phosphatidylserine.13,44 Therefore, this assay could also recognize the membranes of senescent and damaged erythroid cells that are not necessarily undergoing apoptosis. To evaluate only apoptosis in nucleated erythroid cells, we conducted more specific assays, involving cleaved-caspase-3 (CC3; Figure 1B) and TUNEL on the erythroid tissue specimens (Figure 1C). Both assays exhibited limited qualitative differences between thalassemic and healthy mice, lower than those observed with the annexin-V assay. Only quantitative analyses by CC3 (Figure 1D) staining of purified splenic cells revealed a small increase in the percentage of apoptotic cells in thalassemic mice compared with controls (from less than 1% in wt mice to 4% in th3/th3 animals).
Bilirubin and lactic acid dehydrogenase (LDH) levels, which are elevated if red cells hemolyze, were unchanged or only slightly increased in thalassemic compared with healthy mice (Figure 1F,G). In th3/+ mice, these observations indicated that limited hemolysis was present despite erythrocyte formation. In th3/th3 erythroid cells, the average amount of alpha-globin transcript was, on average, 3-fold less than that in wt animals (data not shown). Therefore, the low bilirubin and LDH levels in th3/th3 mice emphasize the limited maturation of their erythroid cells, the erythropoiesis blockade happening before the formation of fully hemoglobinized cells.
In summary, the immature morphology exhibited by thalassemic erythroid cells suggests that an altered cell cycle and limited cell differentiation could be responsible for the low levels of apoptosis and hemolysis seen in this disease compared with earlier predictions arising from ferrokinetic measurements.10,14
β-Thalassemia is characterized by an increased number of proliferating erythroid cells
Because the Epo levels in thalassemic animals were dramatically increased (Figure 1H,I), we investigated some of the downstream markers related to the cell cycle. High levels of Epo could explain the relatively low levels of apoptosis that we observed in thalassemic animals, since this cytokine prevents apoptosis and induces cell proliferation.46 On binding Epo, EpoR undergoes a conformational change that, through Jak2, triggers the signal transducers and activators of the transcription factor Stat5. As a consequence, several proteins involved in the cell cycle and in apoptosis modulation such as Bcl-XL might be up-regulated.41,47 Using Western blot analysis, we showed that larger amounts of the proteins EpoR, Bcl-XL, cyclin-dependent kinase 2 (Cdk2), and CycA (Figure 2A-D) were associated with purified th3/+ and th3/th3 erythroid cells compared with those from wt controls. These data suggest that an increased number of erythroid cells are proliferating and protected from apoptosis or that genes, which promote the cell cycle and attenuate apoptosis, are up-regulated in the erythroid cells of β-thalassemic mice.
Increased amount of antiapoptotic and cell cycle–related proteins in purified wt and thalassemic erythroid cells. Representative Western blots performed on cells from wt (lanes 1,2), th3/+ (lanes 3,4), and th3/th3 (lanes 5,6) mice, and control cell lines (lane 7) probed with (A) Bcl-XL (control: NIH-3T3 cells), (B) CycA (control: Mel cells); (C) Cdk2 (control: Mel cells); and (D) EpoR (control: K562 cells). The upper band in panel A is described as the deamidated form of the protein. Bcl-XL deamidation has been shown to produce a complete loss of the antiapoptotic function of Bcl-XL.48 Similar ratios of the 2 bands are present in both normal and thalassemic mice. The membrane used for Bcl-XL was reprobed with CycA antibody. Specific antibodies against the phosphorylated and nonphosphorylated forms of the protein were used sequentially. In all cases, the same membranes were reprobed against β-actin as a loading control.
Increased amount of antiapoptotic and cell cycle–related proteins in purified wt and thalassemic erythroid cells. Representative Western blots performed on cells from wt (lanes 1,2), th3/+ (lanes 3,4), and th3/th3 (lanes 5,6) mice, and control cell lines (lane 7) probed with (A) Bcl-XL (control: NIH-3T3 cells), (B) CycA (control: Mel cells); (C) Cdk2 (control: Mel cells); and (D) EpoR (control: K562 cells). The upper band in panel A is described as the deamidated form of the protein. Bcl-XL deamidation has been shown to produce a complete loss of the antiapoptotic function of Bcl-XL.48 Similar ratios of the 2 bands are present in both normal and thalassemic mice. The membrane used for Bcl-XL was reprobed with CycA antibody. Specific antibodies against the phosphorylated and nonphosphorylated forms of the protein were used sequentially. In all cases, the same membranes were reprobed against β-actin as a loading control.
To further analyze cell cycling and differentiation, we investigated the number of proliferating cells. Ki-67 can be detected in all active phases of the cell cycle, whereas exit from the active cell cycle leads to a rapid down-regulation of its mRNA and protein expression.49,50 The Mcm3 protein is expressed both in proliferating cells and in those that have ceased to do so, but are not terminally differentiated.50 Both Ki-67 and Mcm3 staining gave similar results, showing that a larger number of erythroid cells in the splenic red pulp of th3/+ and th3/th3 animals were positive for these 2 markers than in wt mice (Figure 3A-C). Moreover, clonogenic assays also showed an increased number of erythroid progenitor cells in th3/+ and th3/th3 mice compared with controls (erythroid burst-forming units [BFU-Es] were increased 1.3- and 4.7-fold, respectively, in BM, and 40- and 600-fold, respectively, in spleen, whereas CFU-Es were increased 4.0- and 5.0-fold in BM, respectively, and 43- and 600-fold, respectively, in spleen; n = 3 per genotype). Similar results were obtained previously in another strain of mice affected by thalassemia intermedia.51,52 To assess whether Ki-67+ cells were proliferating, and to quantify the percentage and total number of erythroid cells in S-phase, we injected 5-bromo-2-deoxyuridine (BrdU) into healthy and thalassemic mice. Our data showed that a large number of BrdU+ cells were also Ki-67+ (Figure S4), and a higher proportion of purified nucleated erythroid cells were in S-phase (BrdU+) in thalassemic compared with healthy mice (22%, 30%, and 45%, respectively, in wt, th3/+, and th3/th3 mice; Figure 3D). Taken together, these data indicate that there is an increased number of erythroid cells in thalassemia that are proliferating (Ki-67 and BrdU assays) and that are not terminally differentiated (Mcm3 assay).
Increased number of cycling and undifferentiated erythroid cells in thalassemic versus healthy mice. Immunostaining for Ki-67 on spleen (A) and liver (B) specimens and for Mcm3 on liver (C) showed an increased number of cycling and undifferentiated cells in extramedullary sites of thalassemic mice (magnification, 400×). Mcm3 was also probed on spleen sections and the pattern was very similar to that observed for Ki-67 (data not shown). In particular, thalassemic liver sections showed an increased number of proliferating cells in areas associated with EMH. Cyclin-B1 staining (data not shown) confirmed that more proliferating cells are present in the spleens of thalassemic compared with wt mice. (D) Staining and analysis of cytospins of purified splenic erythroid cells after injection of BrdU in vivo showed that there is an increased percentage of cycling erythroid cells in β-thalassemic mice compared with healthy (20%, 30%, and 40% in wt, th3/+, and th3/th3, respectively; magnification, 400×). For panels A through D, images were captured on a Nikon Eclipse E800 microscope with a Retiga Exi camera (Qimaging) and a Plan Fluor 40×/0.75 numerical aperture objective, then acquired using the IPLab 3.65a software (Scanalytics). Brightness/contrast and color balance were adjusted using Abobe Photoshop 7.0.1 (Adobe Systems). (E) FACS analysis of CFSE-treated cells costained with antibodies to CD71 and Ter119. Erythroid cells from wt mice cultured in the presence of colcemid (purple line) or AG490 (blue line) showed little difference from untreated cells (pink line). Staining with 7-AAD, PI, and annexin-V excluded dead or apoptotic cells (n = 4 per genotype). After 48 hours, no further cell expansion was observed; instead there is a decline in cell number, indicating that these cells did not have an intrinsic self-sustaining ability to proliferate under these tissue culture conditions. (F) FACS analysis of freshly purified erythroid cells using an antibody that recognizes the phosphorylated form of Jak2 (green line). The blue line represents the cells stained with the isotype. As a control for the specificity of the antibody, the same cells were stained with the antibody after preincubation with the competitor peptide (red line, n = 3 per genotype).
Increased number of cycling and undifferentiated erythroid cells in thalassemic versus healthy mice. Immunostaining for Ki-67 on spleen (A) and liver (B) specimens and for Mcm3 on liver (C) showed an increased number of cycling and undifferentiated cells in extramedullary sites of thalassemic mice (magnification, 400×). Mcm3 was also probed on spleen sections and the pattern was very similar to that observed for Ki-67 (data not shown). In particular, thalassemic liver sections showed an increased number of proliferating cells in areas associated with EMH. Cyclin-B1 staining (data not shown) confirmed that more proliferating cells are present in the spleens of thalassemic compared with wt mice. (D) Staining and analysis of cytospins of purified splenic erythroid cells after injection of BrdU in vivo showed that there is an increased percentage of cycling erythroid cells in β-thalassemic mice compared with healthy (20%, 30%, and 40% in wt, th3/+, and th3/th3, respectively; magnification, 400×). For panels A through D, images were captured on a Nikon Eclipse E800 microscope with a Retiga Exi camera (Qimaging) and a Plan Fluor 40×/0.75 numerical aperture objective, then acquired using the IPLab 3.65a software (Scanalytics). Brightness/contrast and color balance were adjusted using Abobe Photoshop 7.0.1 (Adobe Systems). (E) FACS analysis of CFSE-treated cells costained with antibodies to CD71 and Ter119. Erythroid cells from wt mice cultured in the presence of colcemid (purple line) or AG490 (blue line) showed little difference from untreated cells (pink line). Staining with 7-AAD, PI, and annexin-V excluded dead or apoptotic cells (n = 4 per genotype). After 48 hours, no further cell expansion was observed; instead there is a decline in cell number, indicating that these cells did not have an intrinsic self-sustaining ability to proliferate under these tissue culture conditions. (F) FACS analysis of freshly purified erythroid cells using an antibody that recognizes the phosphorylated form of Jak2 (green line). The blue line represents the cells stained with the isotype. As a control for the specificity of the antibody, the same cells were stained with the antibody after preincubation with the competitor peptide (red line, n = 3 per genotype).
An inhibitor of Jak2 prevented proliferation of thalassemic erythroid cells
Based on the BrdU results achieved in vivo, we sought further evidence that the expansion of the immature erythroid compartment was sustained by a large number of cycling cells in vitro. Purified erythroid cells isolated from the spleens of healthy and thalassemic mice were cultured in the presence of Epo, with and without colcemid, an antimitotic agent. To visualize cell division, we stained the cultured erythroid cells with CFSE. Once the dye is inside the cell, it binds to cytoskeletal proteins and is divided equally between daughter cells. Thus, it is possible to determine whether cells are dividing by monitoring the reduction of CFSE fluorescence (Figure 3E). After 48 hours in culture, wt cells exhibited some differences, depending upon whether they were cultured with or without colcemid, indicating absent or limited cell proliferation (46% ± 9% and 61% ± 12% of the initial cell numbers, respectively, with and without colcemid; n = 4). In contrast, a large proportion of th3/+ and th3/th3 cells were able to proliferate over the same time period (Figure 3E), leading to an increase in the total cell number (th3/+, 88% ± 18% with colcemid and 132% ± 19% without; th3/th3, 72% ± 25% with and 170% ± 22% without; n = 4 each genotype).
We then investigated the phosphorylation of Jak2 in normal and thalassemic erythroid cells. This analysis showed that a larger percentage of erythroid cells was positive for phospho-Jak2 (pJak2) in thalassemic compared with healthy mice (Figure 3F, n = 3). Based on these observations, we investigated the effect of Jak2 inhibitors on the erythroid cultures. AG490 and TG101209, inhibitors of Jak2,53,54 had the same effect as colcemid, blocking cell proliferation (Figure 3E, only the results for AG490 are shown). The FACS profile and the total number of cells were also similar with colcemid and the Jak2 inhibitors (data not shown). Altogether, these data indicate that the increased number of proliferating cells in β-thalassemia are associated with Jak2-mediated signaling.
Thalassemic erythroid cells differentiated less than similar immature normal erythroid cells in vitro
To determine whether the differences observed in vitro corresponded solely to the relative number of erythroid cells in dissimilar stages of erythroid differentiation in healthy and thalassemic mice, we induced anemia in healthy mice by repeated phlebotomies, decreasing their hemoglobin (Hb) level to less than 40 g/L (4 g/dL). At this point, the mice exhibited splenomegaly similar to that seen in th3/th3 mice with almost exclusive production of CD71+/Ter119+ cells (Figure 4A,B). Cytospin analysis of these cells showed morphology similar to that observed in th3/th3 mice, with the majority of cells showing a predominant primitive progenitor morphology and no hemoglobin content, as assayed with tolidine staining (Figure 4C). A larger number of wt cells, however, were enucleated compared with the th3/+ ones, showing indeed higher Hb content, revealed by the tolidine staining. No enucleated erythroid cells were detectable in the th3/th3 specimens, revealing no Hb content and a proerythroblast morphology (Figure 4D). The CFSE profile of wt cells cultured under colcemid conditions was similar to that of cells from th3/+ mice, corroborating the fact that phlebotomy expanded the immature fraction of erythroid cells (Figure 4E). However, the CFSE profile of erythroid cells derived from phlebotomized wt and th3/+ mice did not completely overlap that of the th3/th3 mice, indicating that the rate of erythroid cell differentiation in thalassemia is proportional to the degree of anemia. Although a larger number of normal cells proliferated compared with those from nonphlebotomized wt animals (data not shown), they rapidly differentiated in culture. This observation suggests that thalassemic erythroid cells have an intrinsic ability to limit their differentiation. Therefore, the differences observed in vivo are likely to have resulted from an expansion of the immature fraction of the erythroid cells limited in their capacity to differentiate.
Thalassemic erythroid cells differentiated less than similar immature normal erythroid cells in vitro. (A) FACS analysis of wt, th3/+, and th3/th3 splenic erythroid cells before erythroid cell selection. Wt and th3/+ mice were phlebotomized as described in Document S1. (B) FACS analysis was repeated after selection. Numbers on plots are percentages of total cells in the respective gates. (C) Cytospin analysis at time 0 and (D) after culturing the cells for 48 hours in the presence of Epo. Wt cells are all tolidine positive, with the presence of extruded nuclei (arrowhead), and bright tolidine-positive reticulocytes (arrow). The th3/+ sample is characterized by the presence of hemoglobinized polychromatic-orthochromatic erythroblasts (arrowhead), and some rare proerythroblasts (arrow). In the th3/th3 sample, only proerythroblasts/early basophilic erythroblasts (arrow) were detectable, with no presence of tolidine-positive or enucleated cells. For panels C and D, a Plan Fluor 100×/0.75 numeric aperture oil objective was used, along with the same microscope, camera, and software as in Figure 1. (E) CFSE analysis of the erythroid populations. Erythroid cells cultured in the presence of colcemid plus Epo (purple line) or Epo alone (blue line).
Thalassemic erythroid cells differentiated less than similar immature normal erythroid cells in vitro. (A) FACS analysis of wt, th3/+, and th3/th3 splenic erythroid cells before erythroid cell selection. Wt and th3/+ mice were phlebotomized as described in Document S1. (B) FACS analysis was repeated after selection. Numbers on plots are percentages of total cells in the respective gates. (C) Cytospin analysis at time 0 and (D) after culturing the cells for 48 hours in the presence of Epo. Wt cells are all tolidine positive, with the presence of extruded nuclei (arrowhead), and bright tolidine-positive reticulocytes (arrow). The th3/+ sample is characterized by the presence of hemoglobinized polychromatic-orthochromatic erythroblasts (arrowhead), and some rare proerythroblasts (arrow). In the th3/th3 sample, only proerythroblasts/early basophilic erythroblasts (arrow) were detectable, with no presence of tolidine-positive or enucleated cells. For panels C and D, a Plan Fluor 100×/0.75 numeric aperture oil objective was used, along with the same microscope, camera, and software as in Figure 1. (E) CFSE analysis of the erythroid populations. Erythroid cells cultured in the presence of colcemid plus Epo (purple line) or Epo alone (blue line).
In vivo administration of a Jak2 inhibitor reversed splenomegaly
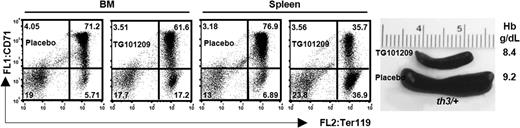
Based on our accumulated data, we postulated 2 hypotheses. In the first one, relentless phosphorylation of the Jak2 protein by high Epo levels might be sufficient to maintain the erythroid cells in active proliferation, limiting their differentiation. However, this is unlikely to be the right scenario, because constitutively active mutant Jak2 tyrosine kinases, such as Jak2V617F, lead to polycythemia vera rather that IE.55,56 Alternatively, we suggest that constitutive activation of the Epo-Jak2 pathway is necessary but not sufficient to cause IE. To discriminate between these 2 alternative hypotheses, we administered TG101209 to cohorts of healthy and thalassemic mice of different ages. We found that 10 and 18 days of treatment were sufficient to dramatically reduce the spleen size in 6- and 12-week-old thalassemic mice (Figure 5; Figures S5, S6). This treatment was associated with a reduced ratio of CD71+/Ter119+ and CD71−/Ter119+ cells in mice treated with TG101209 compared with placebo (Figure 5; Figure S7). However, these changes were associated with decreasing Hb levels (Figure 5; Figure S5). These observations indicate that the main role of pJak2 is to propel the erythropoietic drive. It might also be a factor in IE, but further experiments are required to completely elucidate its potential role in limiting erythroid cell differentiation in thalassemia. Administration of the Jak2 inhibitor also affected both erythropoiesis and the size of the spleen in young healthy animals, at a time when the erythron is still expanding. On one hand, these observations support the notion that pJak2 has a physiologic role in normal erythropoiesis. On the other, the use of Jak2 inhibitors may have limited effects on normal erythropoiesis of healthy adults while still limiting splenomegaly as in thalassemia patients.
TG101209, a Jak-2 inhibitor, reduced splenomegaly in thalassemic mice. Representative FACS analysis of 6-week-old th3/+ mice injected for 10 days with TG101209 or placebo, as indicated. The corresponding spleens and the Hb levels are shown. Numbers on plots are percentages of total cells in the respective gates. The weight of the spleens of these and other animals treated with TG101209 or placebo is indicated in Figure S5.
TG101209, a Jak-2 inhibitor, reduced splenomegaly in thalassemic mice. Representative FACS analysis of 6-week-old th3/+ mice injected for 10 days with TG101209 or placebo, as indicated. The corresponding spleens and the Hb levels are shown. Numbers on plots are percentages of total cells in the respective gates. The weight of the spleens of these and other animals treated with TG101209 or placebo is indicated in Figure S5.
Erythroid cells from thalassemic patients exhibit some of the features observed in thalassemic mice
We also analyzed erythroid cells from the peripheral blood of healthy and thalassemic subjects. Using a 2-phase liquid culture system, we amplified erythroid progenitor cells isolated from the blood specimens.55 Although β-globin mRNA and Hb production were reduced in thalassemic patients as expected (data not shown), the levels of cell cycle–related mRNAs such as Jak2, Ki-67, CycA, Bcl-XL, and EpoR were significantly higher than normal (Figure 6A). Up-regulation of Ki-67 was confirmed by immunohistochemical analysis of the cells after various times during the second phase (erythroid expansion and differentiation) of the culture system (Figure 6B). In addition, spleen sections derived from control and thalassemic patients undergoing splenectomy were stained with glycophorin C, alpha-1-spectrin, and Ki-67 antibodies to qualitatively evaluate the number of replicating (Ki-67+) erythroid cells. Both the alpha-1-spectrin and glycophorin C markers recognize early erythroid progenitors, whereas glycophorin C also tags late erythroid cells. Figure 6C shows that thalassemic splenic tissue has more red pulp and a greater number of Ki-67+ erythroid cells than that from a healthy patient (traumatic splenic rupture). These last observations confirm the data from the 2-phase liquid culture system and, for the first time, show that a considerable number of erythroid cells in an enlarged thalassemic spleen were actively proliferating despite the patient having received a transfusion.
Increased number of proliferating erythroid cells in human thalassemic specimens. (A) At the end of the 2-phase liquid culture, the absolute expression of Bcl-XL, EpoR, Ki-67, CycA, and Jak2 mRNA relative to 14S ribosomal control RNA was quantified in 11 patients (black) and 6 healthy controls (white). An unpaired t test was used for statistical analysis. Benzidine staining was used to evaluate the level of erythroid differentiation. Both the amounts of β-globin mRNAs and those of cell cycle–related genes were quantified by quantitative polymerase chain reaction assay. We also performed highperformance liquid chromatography (HPLC) to determine variations in the percentages and absolute amounts of adult Hb in treated and nontreated samples (not shown). Error bars represent SD. (B) Time dependence of proliferating erythroid cells from 3 thalassemic patients (light gray, gray, and black) and one control subject (white). Aliquots of cells after various days of culture (second phase), corresponding to different stages of erythroid differentiation, were collected, cytospun, and stained for the proliferative marker Ki-67. Thalassemic patients showed an increased number of proliferating cells. More than 300 cells were counted to obtain the percentage of Ki-67–positive cells in each aliquot. (C) Spleen sections from a healthy subject (traumatic rupture) and a thalassemic patient (transfused thalassemia intermedia) who underwent splenectomy. Top panels: Ki-67 staining (brown; magnification, 100×). Bottom panels: Ki-67 (brown) and a mixture of glycophorin C and alpha-1-spectrin (red; magnification, 400×). A Plan Fluor 10×/0.30 numeric aperture objective and a Plan Fluor 40×/0.75 numeric aperture objective were used, along with the same microscope, camera, and software as in Figure 1.
Increased number of proliferating erythroid cells in human thalassemic specimens. (A) At the end of the 2-phase liquid culture, the absolute expression of Bcl-XL, EpoR, Ki-67, CycA, and Jak2 mRNA relative to 14S ribosomal control RNA was quantified in 11 patients (black) and 6 healthy controls (white). An unpaired t test was used for statistical analysis. Benzidine staining was used to evaluate the level of erythroid differentiation. Both the amounts of β-globin mRNAs and those of cell cycle–related genes were quantified by quantitative polymerase chain reaction assay. We also performed highperformance liquid chromatography (HPLC) to determine variations in the percentages and absolute amounts of adult Hb in treated and nontreated samples (not shown). Error bars represent SD. (B) Time dependence of proliferating erythroid cells from 3 thalassemic patients (light gray, gray, and black) and one control subject (white). Aliquots of cells after various days of culture (second phase), corresponding to different stages of erythroid differentiation, were collected, cytospun, and stained for the proliferative marker Ki-67. Thalassemic patients showed an increased number of proliferating cells. More than 300 cells were counted to obtain the percentage of Ki-67–positive cells in each aliquot. (C) Spleen sections from a healthy subject (traumatic rupture) and a thalassemic patient (transfused thalassemia intermedia) who underwent splenectomy. Top panels: Ki-67 staining (brown; magnification, 100×). Bottom panels: Ki-67 (brown) and a mixture of glycophorin C and alpha-1-spectrin (red; magnification, 400×). A Plan Fluor 10×/0.30 numeric aperture objective and a Plan Fluor 40×/0.75 numeric aperture objective were used, along with the same microscope, camera, and software as in Figure 1.
Discussion
In β-thalassemia, IE has been attributed to increased expansion of late erythroid progenitor cells in combination with hemolysis and accelerated apoptosis. We have observed that bilirubin and LDH levels in thalassemic mice are only slightly increased compared with those in healthy mice. In addition, the percentage of cells undergoing apoptosis, as measured by others,52,58 including human specimens,16 was relatively low compared with the extreme expansion of medullary and extramedullary erythroid progenitors and predicted by the ferrokinetic studies conducted earlier.10,14 The normal distribution of murine erythroid progenitors is approximately 90% in the bone marrow and 10% in the spleen. In thalassemia, however, the spleen expands up to 20-fold and its erythroid content up to 95% of the total cell population, with mice affected by β-thalassemia major exhibiting even higher levels of IE than animals with the intermedia form of the disease. In addition, th3/th3 mice produce low numbers of reticulocytes. This scenario elicits an “erythroid paradox.” What is the fate of erythroid cells produced in β-thalassemia? Although the percentage of erythroid cells undergoing apoptosis in th3/th3 mice increases 3 to 4 times compared with normal, as evidenced by the CC3 assay, the total number of erythroid progenitors increases dramatically, leading to a large expansion of the erythron just in a few weeks after bone marrow transplantation (BMT). When the fulminant EMH and splenomegaly in these animals reach their peak, the Hb levels are extremely low and the mice die. In mice affected by thalassemia intermedia, erythroid expansion occurs at a slower pace because these animals still have a “relatively efficient” erythropoiesis. They produce up to 10 times more reticulocytes and almost the same number of RBCs as healthy animals. Therefore, in younger mice affected by thalassemia intermedia, hemolytic anemia seems to play a major role, although IE is also a factor as shown by the increased number of CD71+/Ter119+ cells. Nevertheless, as we showed in our previous study,22 the Hb levels in these mice decrease with time, whereas the spleen size, the number of nucleated erythroid cells, and the ratio of liver to spleen iron all increase, resulting in the animals eventually exhibiting some of the features associated with the extreme form of IE observed in th3/th3 mice at 2 months after BMT. At this stage, th3/+ mice start succumbing to the disease. In the th3/th3 mice, which show the greatest number of apoptotic cells, the net expansion of the spleen is 15% to 20% per day. This represents an extremely high rate of cell proliferation. These observations call for alternative explanations for the IE in β-thalassemia.
Our Western blot results (Figure 2) cannot distinguish whether up-regulation of genes such as CycA and Cdk2 is intrinsic or simply reflects the increased number of proliferating thalassemic erythroid cells compared with normal. In thalassemic animals, high Epo levels support the former hypothesis, whereas the relative increase of CD71+/Ter119+ cells, the latter one. Probably both mechanisms contribute to the results, as would be expected in stress erythropoiesis. In any case, our data in vitro further corroborate the profoundly different behavior of thalassemic versus normal erythroid cells and indicate that additional as-yet-uncharacterized factors, not associated with stress erythropoiesis, serve to limit cell differentiation in thalassemia. These factors might be responsible for the differences between stress and ineffective erythropoiesis. Considered together, these observations challenge the established notion that IE in β-thalassemia is primarily due to cell death and/or hemolysis. In contrast, we propose a novel model of IE in which limited cell differentiation decreases red cell production as well as apoptosis.
High Epo levels might be responsible for preventing apoptosis and enhancing cell proliferation. High levels of Bcl-XL have been shown to protect erythroid progenitor cells from apoptosis, while its disruption leads to severe hemolytic anemia.41,47 Therefore, the relative increase in the amount of EpoR, CycA, and Bcl-XL that we observed supports our hypothesis that Epo stimulates proliferation and simultaneously limits apoptosis in thalassemic erythroid cells. Moreover, the observation by Ghaffari et al53 that Epo stimulates the synthesis of Bcl-XL through the Jak2/Stat-5 signal transduction pathway rather than alternative Jak2-activated pathways such as Akt strongly suggests that the Epo-EpoR-Jak2 axis plays a major role in this process. In fact, our data show that a larger number of thalassemic erythroid cells exhibited phosphorylation of Jak2 than did normal cells. However, based on our data, we propose a model in which the Epo-Jak2 pathway is necessary but not sufficient to cause IE, thus additional factors are required. In this scenario, the relative strength of these hypothetical factors may determine whether high Epo levels lead to effective or ineffective erythropoiesis. For instance, the relative amount of iron overload and related reactive oxygen species (ROSs) might determine the behavior of erythroid cells in the presence of high Epo levels.60 Under these conditions, for instance, high Epo levels might aggravate IE. This would increase the rate of cell proliferation and, concurrently, limit erythrocyte production. In fact, our model predicts that as anemia worsens, Epo levels can increase without leading to higher RBC production. Further studies in th3/+ mice might clarify this point, because higher Epo levels do not correlate with higher Hb levels in a subset of these animals, but rather with the lowest ones (Figure 1I). The most plausible explanation is that a combination of intrinsic and extrinsic factors contributes to this process. One intriguing hypothesis is that the globin chain imbalance or an excess of heme might play a role, providing a signal that prevents cell differentiation, which would otherwise lead to cells with a level of alpha-globin aggregates too toxic for survival. However, under our tissue culture conditions, the cells eventually die even in the presence of Epo. Therefore, diffusible factors such as SCF or cortisol,61,62 hypoxic conditions, or cell-cell and/or cell-stroma interactions, which were not reproduced in our cultures, might be involved.
Turning from the theoretic to the practical, the results achieved with TG101209 in th3/+ mice might have a profound impact on the treatment of splenomegaly and in limiting IE-related damage due to excessive iron absorption, including secondary osteoporosis. The slower progression of the disease in mice affected by thalassemia intermedia closely mimics that in transfusion-independent thalassemic patients who eventually develop splenomegaly with decreasing Hb levels. Although the spleen in these patients likely sequesters sufficient erythrocytes to affect the Hb level, the exact cause of the splenomegaly and characterization of the various splenic populations are unknown. Our data, from both thalassemic mice and patient specimens, shed light on this phenomenon, suggesting that an increased number of proliferating erythroid progenitors accumulate in the spleen under conditions of IE. In such a situation, splenomegaly might arise as the rate of differentiation is further reduced, leading to increased sequestration of erythrocytes progressively exacerbating the process. Transfusion-independent β-thalassemia intermedia patients, if affected by splenomegaly, develop a need for blood transfusion therapy and eventually must undergo splenectomy. The use of Jak2 inhibitors in this situation would not be expected to improve anemia, but rather to limit or reduce splenomegaly. It is possible to envision that patients affected by thalassemia intermedia and splenomegaly could be treated temporarily with these compounds to reduce the spleen size and in the presence of blood transfusion to prevent further anemia. If this treatment were successful, higher Hb levels might be expected in the absence of Jak2 inhibitors, and transfusion support and the need of splenectomy would be obviated. Moreover, even patients affected by β-thalassemia major might benefit from Jak2 inhibitors through reduction of IE and splenomegaly.
Our paper emphasizes the proliferative component of IE and introduces the notion that limited cell differentiation also plays a role in this process. Two main consequences stem from this approach, the quest for alternative factors that control erythroid differentiation in IE and a potential clinical role for erythroid inhibitors such as TG101209 in treating thalassemic patients. In addition, we believe that the use of Epo, which is often suggested for patients affected by IE, should be carefully weighed. Depending on the relative strength of the various factors that control erythroid differentiation in each patient affected by IE, Epo may or may not be beneficial. In particular, in those patients in whom Epo would not be beneficial, treatment might exacerbate anemia and EMH, that is, more erythroid precursors would be made with limited production of differentiated erythroid cells. In conclusion, our data challenge the dogma that increased apoptosis is solely responsible for IE. In addition to moderate apoptosis, we propose that IE in β-thalassemia is driven by a large number of proliferating cells and limited differentiation, mimicking tumorlike behavior. In addition, our study suggests that use of Jak2 inhibitors has the potential to fundamentally transform the management of this disorder.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kristine McKinney, Vanesa Gottifredi, Mirella Misiaszek, Kimberly Young, Prim Singh, Scott A. Kerns, and the members of the Pasta and Red Cells Society of New York for technical support and helpful discussions.
S.R. was supported by grants from The National Institutes of Health–National Institute of Diabetes and Digestive and Kidney Diseases (NIH-NIDDK, Bethesda, MD) R21DK065169, the Carlo and Micol Schejola Foundation (Atlanta, GA), the Roche Foundation for Anemia Research (RoFAR, Meggen, Switzerland), the Cooley's Anemia Foundation (CAF, New York, NY), and the Children's Cancer and Blood Foundation. L.B. was supported by Associazione per la Lotta alla Talassemia (AVLT, Rovigo, Italy) and the Cooley's Anemia Foundation; P.R and M.D.S., by the American Portuguese Biomedical Fund (APBRF, USA)/Inova grant; and P.J.G., by HRSA-MCHB H87MC00281; R.W.G., by NIH-R01 DK055463.
National Institutes of Health
Authorship
Contribution: I.V.L., L.M., E.C.G., and R.S. maintained the mouse colony, collected the mouse organs, performed the bone marrow transplantations, the CBC, and RNA and Western blot assays, and analyzed the data; P.R. performed the FACS analysis on embryonic and adult erythroid cells; L.B. performed the 2-phase liquid culture and gene expression analysis; A.C. and Y.L. performed the pathologic examination of the tissue samples and the immunohistochemistry staining; T.S., M.K., B.B.-L., and J.G. provided vital reagents and performed the Ki-67 and Mcm3 tissue staining; M.P. provided advice and some reagents to perform the Western blot assays; M.S., R.W.G., M.D.C., P.J.G., E.A.R., J.D.H., and J.G. provided vital reagents and analyzed the data; S.R. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: J.D.H. is the Director of Research, TargeGen, Inc., which provided the Jak2 inhibitor TG101209. The remaining authors declare no competing financial interests.
Correspondence: Stefano Rivella, Weill Medical College of Cornell University, 515 E 71st St, S702, New York, NY 10021; e-mail: str2010@med.cornell.edu.
References
Author notes
I.V.L., E.C.G., L.M., and R.S. contributed equally to this work.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal