Abstract
The platelet receptor for von Willebrand factor (VWF), glycoprotein (GP) Ib-IX, mediates initial platelet adhesion and transmits signals leading to platelet activation. Src family tyrosine kinases (SFKs) play an important role in VWF-induced GPIb-IX signaling. However, the SFK-dependent downstream signaling pathway is unclear but is thought to involve thromboxane A2 (TXA2) synthesis. Here we show that, although platelets deficient in SFK members, Lyn or Fyn, were defective in the TXA2-dependent second wave of platelet aggregation induced by botrocetin/VWF, only Lyn-knockout platelets were also defective in stable platelet adhesion to VWF under shear stress that is independent of the TXA2 pathway. Lyn-knockout platelets also spread poorly on VWF but spread normally on fibrinogen, indicating an important role for Lyn in VWF-mediated GPIb signaling but not in integrin outside-in signaling. Importantly, Lyn knockout abrogated VWF-induced cGMP elevation. Addition of low concentrations of 8-bromo-cGMP, however, corrected the defective stable adhesion of Lyn-knockout platelets or PP2-treated platelets on VWF. These results demonstrate an important role for Lyn in VWF/GPIb-IX–induced integrin activation mediated via the cGMP signaling pathway independently of TXA2 synthesis and also indicate that Lyn is critically important in GPIb-IX–mediated activation of the cGMP pathway.
Introduction
Under high shear rate flow conditions, the initial platelet adhesion to the disrupted vessel wall is mediated by the interaction between subendothelial-bound von Willebrand factor (VWF) and its platelet receptor, the glycoprotein Ib-IX (GPIb-IX) complex.1,2 In vitro, VWF binding to GPIb-IX can be induced by botrocetin and ristocetin, which mimic the binding of subendothelial matrix to collagen.2-4 The interaction between VWF and GPIb-IX not only mediates transient platelet adhesion but also initiates a signaling cascade leading to platelet integrin αIIbβ3 activation and consequent stable platelet adhesion, spreading, and aggregation.5-8
The exact mechanism of GPIb-IX–mediated platelet activation signaling remains unclear but is known to involve several intracellular signaling molecules and pathways, such as the PI3K-Akt pathway, the cGMP, and mitogen-activated protein kinase (MAPK) pathways, the Syk/phospholipase C pathway, and the thromboxane A2 (TXA2) and adenosine diphosphate (ADP) pathways.8-19 Members of the Src family kinases (SFKs) are known to be important because SFK inhibitors, which do not differentiate different SFK members, have been shown to inhibit GPIb-IX–induced integrin αIIbβ3 activation and multiple signaling events, such as calcium mobilization, PI3K activation, and TXA2 synthesis.12,13,20,21 Six members of SFK are present in platelets, including c-Src, Lyn, Fgr, Fyn, Lck, and Yes.22 Various SFK members have been shown to play distinct roles in various aspects of platelet activation. For example, c-Src binds to the C-terminal region of integrin β3 and is thought to be important in integrin outside-in signaling.23,24 Lyn and Fyn have been implicated in the GPVI-ITAM-Syk signaling pathway.25,26 Two SFK members, c-Src and Lyn, have been reported to form a complex with GPIb-IX and PI3K on VWF binding,21 suggesting potential roles in GPIb-IX signaling. Another SFK, Fyn, although controversial, is also reported by one group to interact with GPIb-IX.27 A recent study by Liu et al showed that Lyn and, to a lesser degree, c-Src are important for the TXA2 and secretion-dependent second wave of platelet aggregation induced by VWF.28 Because Lyn knockout abrogates TXA2 synthesis,28 the TXA2 pathway is thought to be a major downstream pathway of Lyn. In this study, we show that Lyn is not only required for the TXA2 and secretion-dependent second wave of platelet aggregation, but also for GPIb-IX–mediated integrin-dependent platelet stable adhesion that is independent of TXA2, ADP, and the FcRγ-Syk pathway. Another SFK member, Fyn, is important for the amplification of the second wave of platelet aggregation induced by botrocetin/VWF but is not required for GPIb-IX–mediated integrin-dependent platelet stable adhesion to VWF. Importantly, we demonstrate that Lyn is required for GPIb-IX–induced cGMP elevation, and GPIb-IX signaling defect in Lyn-knockout platelets can be corrected by supplementing cGMP. Therefore, an important role of Lyn in GPIb signaling leading to integrin activation is mediated via the cGMP signaling pathway, and Lyn is an upstream mediator required for GPIb-IX–mediated activation of the cGMP signaling pathway.
Methods
Platelet aggregation
Lyn knockout mice were generated by deleting exons 3 to 7 of the Lyn gene29 and were backcrossed to a C57BL/6J background. Fyn knockout mice from The Jackson Laboratory (Bar Harbor, ME) were also backcrossed to C57BL/6J background. Lyn+/+ and Fyn+/+ littermates from heterozygous breeding were used as controls. Blood collection from the abdominal aorta of isofluorane-anesthetized mice and anticoagulation were as described previously.28,30 The animal usage and protocol were approved by the institutional animal care committee of the University of Illinois at Chicago; 0.1 μg/mL prostaglandin E1 and 1 U/mL apyrase were added to the blood before platelet isolation by differential centrifugation. Platelets were isolated from platelet-rich plasma (PRP) and washed in modified Tyrode buffer (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4) containing 5 mM ethylenediaminetetraacetic acid as previously described.14 Washed platelets (3 × 108/mL) were resuspended in the modified Tyrode buffer14 and placed in a turbidometric platelet aggregometer (Chronolog, Havertown, PA) at 37°C with stirring (1000 rpm). Human VWF and botrocetin were purified as described previously.31,32 They were added to the washed platelets to induce platelet aggregation.
Platelet adhesion under shear stress
Glass slides were coated with VWF (30 μg/mL) in 0.1 M NaHCO3 (pH 8.3) at 4°C overnight. In some samples, washed wild-type platelets (3 × 108/mL) suspended in modified Tyrode buffer (containing 1 mM CaCl2 and 1 mM MgCl2) were pretreated with different inhibitors, Arg-Gly-Asp-Ser (RGDS) peptide (Bachem California, Torrance, CA), PP2, piceatannol (Calbiochem, San Diego, CA), A3P5P, 2MeSAMP, or aspirin (Sigma-Aldrich, St Louis, MO) for 5 minutes, and then loaded onto the slides. A fluorescence dye, mepacrine (10 μM; Sigma-Aldrich) was added to the platelets before the application of constant shear rate (800 s−1) to the platelets for 5 minutes using a cone-plate rheometer (Thermo Scientific Haake, Waltham, MA). For the cGMP reversal experiment, 10 μM 8-bromo-guanosine 3′, 5′-cyclic monophosphate (8-bromo-cGMP; Calbiochem) was added immediately before the application of constant shear rate (800 s−1). Slides were washed as previously described19 and viewed with a Leica DMI RB inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany) using an N PLAN L 40×/0.55 NA [infinity]/0-2/C objective lens. Images were acquired using a Cool SNAP HQ CCD Camera (Photometrics, Tucson, AZ) and processed with RS Image software, version 1.4 (Photometrics). Stable adherent platelets were counted in 10 randomly selected microscope fields to obtain the average numbers (± SD) of adherent platelets per field. Statistical significance of the difference between groups was analyzed using Student t test.
Platelet spreading on immobilized VWF or fibrinogen
Chamber slides with microtiter wells (Nalge Nunc, Rochester, NY) were coated with 30 μg/mL purified VWF or fibrinogen from human plasma (Calbiochem) in 0.1 M NaHCO3 (pH 8.3) at 4°C overnight. Washed platelets (2 × 107/mL) in modified Tyrode buffer containing 1 mM CaCl2 and 1 mM MgCl233 were preincubated with PP2 for 5 minutes and allowed to adhere and spread on VWF or fibrinogen-coated wells at 37°C for 90 minutes. Botrocetin (1 μg/mL) was also added in VWF-coated wells. In control experiments, platelets were stimulated with ADP (10 μM; Sigma-Aldrich) and allowed spread on VWF and fibrinogen. After 3 washes with phosphate-buffered saline (0.01 M NaH2PO4, 0.15 M NaCl, pH 7.4), the cells were fixed, permeabilized, and stained with fluorescein-labeled phalloidin (Invitrogen, Carlsbad, CA) as previously described.34 Adherent platelets were viewed with a Leica DMI RB fluorescence microscope (Leica) using a HCX PL FLUOTAR 100 × 1.30 NA PH3 oil objective lens with 1.5× magnification. Images were acquired using a CoolSNAP HQ CCD camera (Photometrics) and processed with RS Image version 1.4 software (Photometrics). The spreading area of single platelets was measured using ImageJ software (National Institutes of Health [NIH], Bethesda, MD) with pixel numbers as unit of size. Three randomly selected fields from different tests were used for statistical analysis. Statistical significance of the difference between groups was analyzed using Student t test.
Immunoblot detection of MAPK phosphorylation
Washed platelets in the modified Tyrode's buffer (3 × 108/mL) were stirred (1000 rpm) in a platelet aggregometer with botrocetin (1.2 μg/mL), or botrocetin and VWF (10 μg/mL). The reaction was stopped by addition of an equal volume of 2× SDS sample buffer, containing 0.2 mM E64, 2 mM phenylmethylsulfonyl fluoride, and 34 μg/mL aprotinin (Sigma-Aldrich). Proteins were separated by SDS-PAGE on polyacrylamide gels, transferred to polyvinylidene difluoride membranes, and immunoblotted with either antiphospho-Erk1/2 (Thr202/Tyr204) antibody or antiphospho-p38 (Thr180/Tyr182) antibody (Cell Signaling Technology, Danvers, MA). Corresponding anti-Erk2 and anti-p38 polyclonal antibodies (Cell Signaling Technology) were used to detect the total level of Erk1/2 and p38 MAP kinases.
VWF-induced platelet cGMP elevation
Washed mouse platelets (300 μL, 3 × 108/mL) were incubated with VWF (10 μg/mL) and botrocetin (1.2 μg/mL) in a platelet aggregometer with stirring (1000 rpm) at 37°C for 5 minutes. The reaction was stopped by adding the same volume of ice-cold 12% (w/v) trichloroacetic acid (300 μL). Samples were mixed and centrifuged at 2000 g for 15 minutes at 4°C, and the supernatant was extracted 4 times with 5 volumes of water-saturated diethyl ether followed by lyophilization. The cGMP enzyme immunoassay kit (GE Healthcare, Little Chalfont, United Kingdom) was used to measure cGMP concentration. Bars represent the average (± SD) cGMP concentration derived from 3 experiments.
Results
The roles of Lyn and Fyn in secretion- and integrin-dependent second-wave platelet aggregation induced by VWF and botrocetin
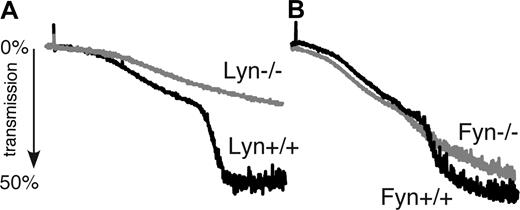
To determine the role of Lyn and Fyn in GPIb-IX–mediated platelet activation, we investigated the effect of Lyn or Fyn knockout on platelet aggregation stimulated with botrocetin and VWF. As expected, botrocetin induced a 2-wave aggregation of wild-type mouse platelets. The first wave mainly comprises GPIb-dependent platelet agglutination; it also contains a minor component of GPIb-IX–induced integrin-dependent platelet aggregation.16,35 The second wave comprises secretion- and integrin-dependent platelet aggregation. It has been previously shown that the second wave of platelet aggregation induced by VWF requires activation of granule secretion and TXA2 synthesis, which greatly amplify integrin activation. Lyn-knockout platelets mainly exhibited impaired second-wave aggregation in response to botrocetin and VWF (Figure 1A), a result consistent with a previous report by Liu et al,28 but also showed a mild decrease in the first wave of platelet aggregation, suggesting the possibility of inhibition of integrin activation in response to VWF and botrocetin. Fyn-knockout platelets, on the other hand, showed only partial inhibition of VWF/botrocetin-induced second-wave platelet aggregation and no decrease in first-wave platelet aggregation (Figure 1B). Thus, it appears that Lyn is required for GPIb-IX–mediated, secretion- and integrin-dependent platelet aggregation, but Fyn is important in the amplification of the second wave of platelet aggregation.
The role of Lyn and Fyn in botrocetin/VWF-induced integrin-dependent platelet aggregation. (A) Washed wild-type and Lyn−/− mouse platelets and (B) washed wild-type and Fyn−/− mouse platelets were stimulated with botrocetin (1.2 μg/mL) and VWF (10 μg/mL) at 37°C. Platelet aggregation was monitored using a turbidometric aggregometer.
The role of Lyn and Fyn in botrocetin/VWF-induced integrin-dependent platelet aggregation. (A) Washed wild-type and Lyn−/− mouse platelets and (B) washed wild-type and Fyn−/− mouse platelets were stimulated with botrocetin (1.2 μg/mL) and VWF (10 μg/mL) at 37°C. Platelet aggregation was monitored using a turbidometric aggregometer.
An important role for Lyn but not Fyn in integrin-dependent stable platelet adhesion to VWF under shear stress that is independent of TXA2, ADP, and Syk
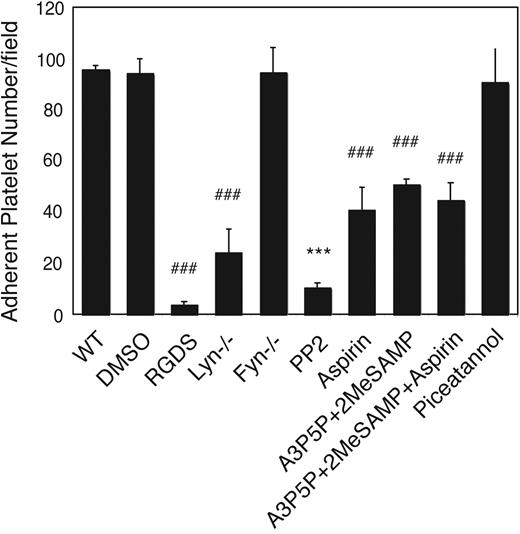
To examine whether Lyn and Fyn are important in shear-induced, stable platelet adhesion to VWF, the cone-plate rheometer was used to apply a constant shear force to platelets. Wild-type and dimethyl sulfoxide (DMSO)–treated mouse platelets stably adhered to the VWF-coated surface at 800 s−1 shear rate. The integrin antagonist, RGDS, inhibited the stable platelet adhesion, indicating that the adhesion is dependent on integrin αIIbβ3, and thus reflects GPIb-IX–mediated integrin activation (Figure 2). Stable platelet adhesion was dramatically inhibited in Lyn knockout mouse platelets, indicating an important role of Lyn in GPIb-IX–mediated integrin-dependent platelet adhesion under shear stress (Figure 2). The SFK inhibitor, PP2, almost completely abolished stable platelet adhesion to VWF (Figure 2). The greater inhibitory effect of PP2 compared with Lyn knockout suggests that other SFK members may also play roles in shear-induced and GPIb-mediated stable platelet adhesion. However, Fyn does not seem to be important because Fyn knockout had no significant effect on stable platelet adhesion to VWF (Figure 2). ADP and TXA2 are important secondary platelet agonists released during VWF-induced, GPIb-IX–dependent platelet activation. Both ADP and TXA2 greatly amplify activation signals and promote maximal platelet response.13,14,16 To understand whether ADP and TXA2 mediate SFK-dependent GPIb-IX signaling pathway, we compared the inhibitory effect of Lyn knockout with that of ADP receptor antagonists and the cyclooxygenase inhibitor, aspirin. The P2Y1 antagonist, A3P5P, together with P2Y12 antagonist, 2MeSAMP, only partially inhibited platelet adhesion to VWF. Aspirin also partially inhibited stable platelet adhesion to VWF. Aspirin and ADP antagonists together showed a similar effect as aspirin alone (Figure 2). These inhibitors showed significantly less inhibitory effect compared with Lyn knockout or PP2-treated platelets. The more dramatic inhibitory effects of Lyn knockout and PP2 indicate that the role of Lyn in GPIb signaling is at least partially independent of the ADP and TXA2 pathways. On the other hand, platelets treated with the Syk inhibitor piceatannol exhibited normal adhesion under shear stress (Figure 2), suggesting that the integrin-dependent stable platelet adhesion to VWF under flow is independent of the ITAM-Syk pathway. Therefore, the ITAM-Syk pathway probably doesn't play a predominant role in Lyn-dependent GPIb signaling leading to integrin activation. Thus, Lyn but not Fyn plays an important role in early GPIb-IX signaling that is independent of the TXA2, ADP, and ITAM-Syk pathways, although TXA2 and ADP play significant amplification roles in the Lyn-mediated platelet activation and stable platelet adhesion.
The role of Lyn and Fyn in integrin-dependent stable platelet adhesion to VWF under shear stress. Washed wild-type, Lyn−/−, or Fyn−/− mouse platelets were loaded onto VWF-coated glass slides with mepacrine (10 μM). Wild-type platelets were also preincubated with DMSO, RGDS (1 mM), SFK inhibitor PP2 (10 μM), P2Y1 antagonist, A3P5P (0.5 mM), and P2Y12 antagonist, 2MeSAMP (10 μM), aspirin (1 mM), or the Syk inhibitor piceatannol (10 μM) for 2 minutes, before loading onto the VWF-coated glass slides. Platelets were then subjected to constant shear rate (800 s−1) for 5 minutes. After washing, adherent platelets were viewed and photographed as described in “Platelet adhesion under shear stress.” The number of adherent platelets/field were quantitated. Data (mean ± SD) were obtained from 10 randomly selected fields from each of 4 experiments. ###P < .001, compared with wild-type platelet. ***P < .001, compared with DMSO-treated platelet; P < .001, difference between ADP receptor antagonists and PP2; P < .01, difference between ADP receptor antagonists and Lyn knockout or between aspirin and PP2; P < .05, difference between aspirin and Lyn knockout.
The role of Lyn and Fyn in integrin-dependent stable platelet adhesion to VWF under shear stress. Washed wild-type, Lyn−/−, or Fyn−/− mouse platelets were loaded onto VWF-coated glass slides with mepacrine (10 μM). Wild-type platelets were also preincubated with DMSO, RGDS (1 mM), SFK inhibitor PP2 (10 μM), P2Y1 antagonist, A3P5P (0.5 mM), and P2Y12 antagonist, 2MeSAMP (10 μM), aspirin (1 mM), or the Syk inhibitor piceatannol (10 μM) for 2 minutes, before loading onto the VWF-coated glass slides. Platelets were then subjected to constant shear rate (800 s−1) for 5 minutes. After washing, adherent platelets were viewed and photographed as described in “Platelet adhesion under shear stress.” The number of adherent platelets/field were quantitated. Data (mean ± SD) were obtained from 10 randomly selected fields from each of 4 experiments. ###P < .001, compared with wild-type platelet. ***P < .001, compared with DMSO-treated platelet; P < .001, difference between ADP receptor antagonists and PP2; P < .01, difference between ADP receptor antagonists and Lyn knockout or between aspirin and PP2; P < .05, difference between aspirin and Lyn knockout.
The role of Lyn in platelet spreading on immobilized VWF and fibrinogen
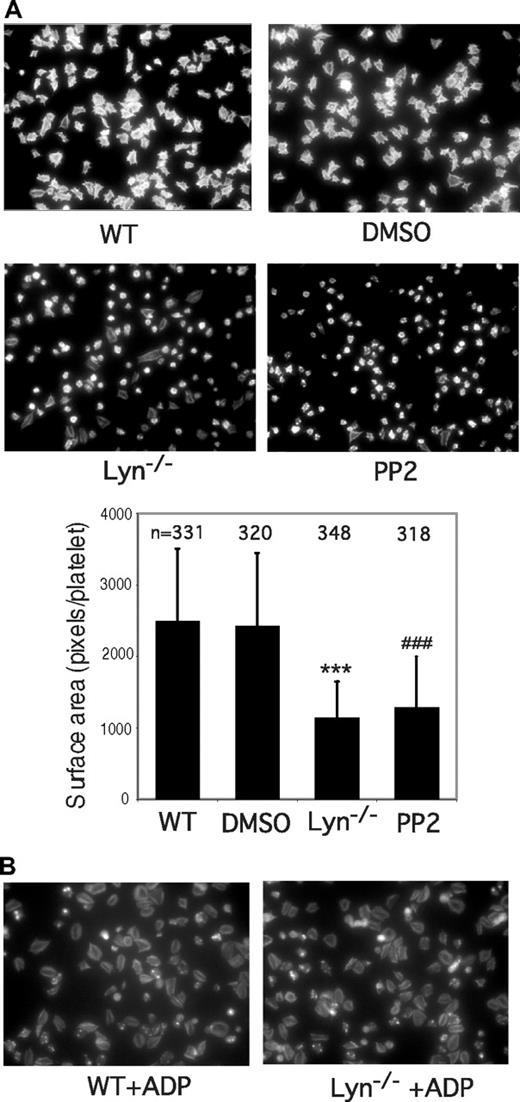
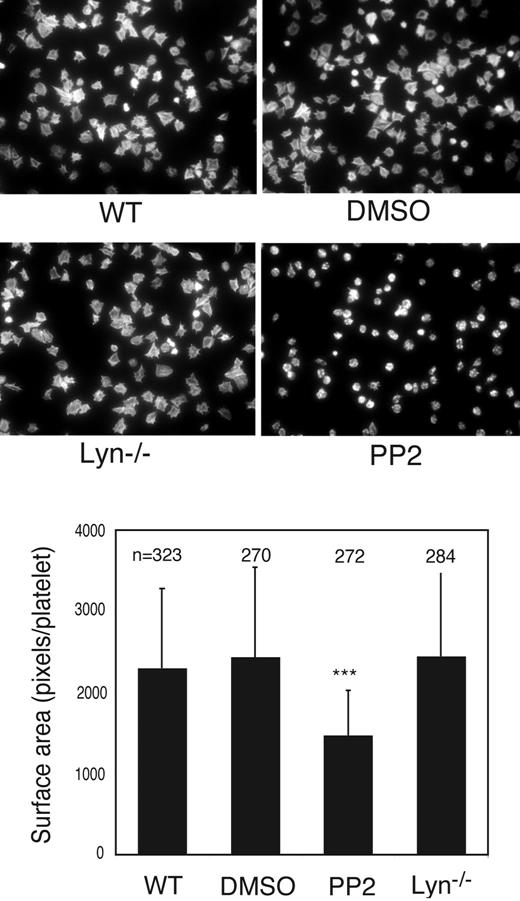
Members of the SFK family have been shown to play a role in integrin outside-in signaling, which is also important in platelet secretion, adhesion, and spreading.23 Thus, it is necessary to differentiate whether Lyn is required for GPIb-IX–mediated inside-out signaling or for integrin outside-in signaling. Platelet spreading on VWF requires GPIb-IX–mediated initial adhesion, GPIb-IX–mediated integrin activation (inside-out signaling), and integrin outside-in signals. In contrast, platelet spreading on immobilized fibrinogen requires the integrin outside-in signaling but does not require inside-out signaling. Wild-type, Lyn knockout, Fyn-knockout, or PP2-treated platelets were allowed to adhere and spread on immobilized VWF and fibrinogen, respectively. As expected, wild-type platelets spread on both VWF and fibrinogen. DMSO did not affect spreading under these conditions. Fyn-knockout platelets also spread normally on VWF (not shown). In contrast, most of Lyn-knockout platelets spread poorly on VWF (Figure 3A). Similarly, the SFK inhibitor PP2 also diminished platelet spreading on VWF (Figure 3A). Interestingly, whereas platelet spreading on immobilized fibrinogen was also inhibited by PP2, indicating an important role for a SFK member (probably c-Src) in integrin outside-in signaling, platelet spreading on fibrinogen was normal for Lyn-knockout platelets (Figure 4). To exclude the possibility that the differential effects of Lyn knockout on platelet spreading on VWF vs fibrinogen result from difference in integrin αIIbβ3 outside-in signaling on different integrin ligands, platelets were stimulated with ADP (that activates integrin αIIbβ3 via GPIb-IX–independent signaling pathways) and allowed to spread on VWF. The spreading of Lyn-deficient platelets was similar to wild-type platelets in the presence of ADP (Figure 3B), suggesting that integrin outside-in signaling on VWF is also independent of Lyn. Therefore, these data demonstrate that Lyn plays an important role in GPIb-IX–mediated signaling leading to integrin activation, but it is not required for integrin outside-in signaling leading to platelet spreading.
Effects of SFK inhibitor and Lyn knockout on platelet spreading on immobilized VWF. Microtiter chamber slides were coated with 30 μg/mL of VWF. Washed wild-type mouse platelets were preincubated with DMSO or PP2 (10 μM) for 5 minutes. Wild-type or Lyn−/− platelets were allowed to adhere and spread on VWF for 90 minutes in the presence of (A) botrocetin (1 μg/mL) or (B) ADP (10 μM). Adherent platelets were stained with fluorescein-labeled phalloidin and photographed under a fluorescence microscope. Shown are representative pictures from one of 3 experiments with similar results. The bars in the graph represent the average surface area (± SD) of individual platelets. ###P < .001, compared with WT platelets. ***P < .001, compared with DMSO-treated WT platelets. The number of platelets analyzed for each group is indicated above the bars.
Effects of SFK inhibitor and Lyn knockout on platelet spreading on immobilized VWF. Microtiter chamber slides were coated with 30 μg/mL of VWF. Washed wild-type mouse platelets were preincubated with DMSO or PP2 (10 μM) for 5 minutes. Wild-type or Lyn−/− platelets were allowed to adhere and spread on VWF for 90 minutes in the presence of (A) botrocetin (1 μg/mL) or (B) ADP (10 μM). Adherent platelets were stained with fluorescein-labeled phalloidin and photographed under a fluorescence microscope. Shown are representative pictures from one of 3 experiments with similar results. The bars in the graph represent the average surface area (± SD) of individual platelets. ###P < .001, compared with WT platelets. ***P < .001, compared with DMSO-treated WT platelets. The number of platelets analyzed for each group is indicated above the bars.
Effect of SFK inhibitor and Lyn knockout on platelet spreading on immobilized fibrinogen. Microtiter chamber slides were coated with 30 μg/mL of fibrinogen. Washed wild-type mouse platelets were preincubated with DMSO or PP2 (10 μM) for 5 minutes. Wild-type or Lyn−/− platelets were allowed to adhere and spread on fibrinogen for 90 minutes. Shown in the figure are representative pictures from one of 3 experiments with similar results. The bars in the graph represent the average surface area (± SD) of individual platelets. ***P < .001, compared with DMSO-treated WT platelets. The number of platelets analyzed for each group is indicated above the bars.
Effect of SFK inhibitor and Lyn knockout on platelet spreading on immobilized fibrinogen. Microtiter chamber slides were coated with 30 μg/mL of fibrinogen. Washed wild-type mouse platelets were preincubated with DMSO or PP2 (10 μM) for 5 minutes. Wild-type or Lyn−/− platelets were allowed to adhere and spread on fibrinogen for 90 minutes. Shown in the figure are representative pictures from one of 3 experiments with similar results. The bars in the graph represent the average surface area (± SD) of individual platelets. ***P < .001, compared with DMSO-treated WT platelets. The number of platelets analyzed for each group is indicated above the bars.
The role of Lyn in GPIb-mediated platelet activation is cGMP-dependent
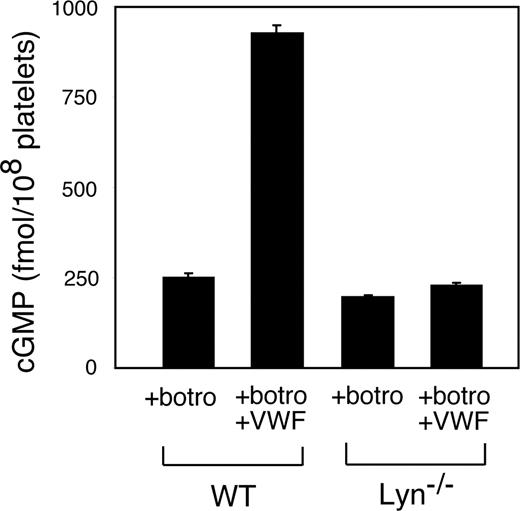
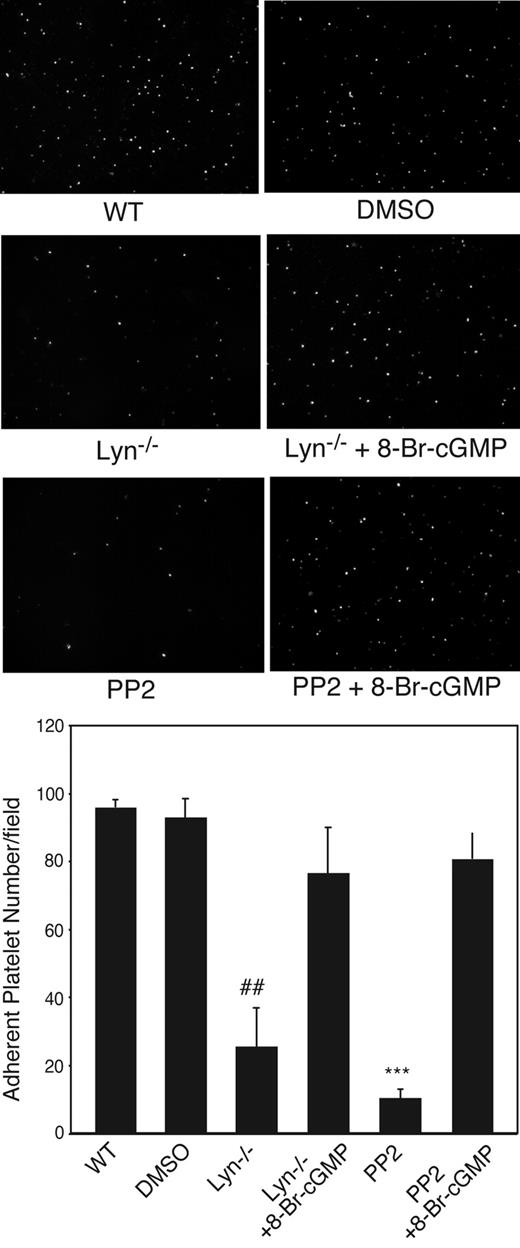
We and others have shown that VWF binding to GPIb-IX induces activation of the nitric oxide (NO)–cGMP signaling pathway in platelets,11,36 which is important in GPIb-IX signaling leading to integrin activation.11,16,19,35 To understand whether the cGMP pathway functions as a downstream mediator of Lyn-dependent GPIb-IX signaling, we investigated whether Lyn knockout affected platelet cGMP elevation stimulated by VWF/botrocetin. VWF and botrocetin induce a significant increase in cGMP level in wild-type platelets as we reported previously.11 Significantly, VWF-induced cGMP elevation was abolished in Lyn-knockout platelets (Figure 5), indicating that Lyn is an important upstream mediator in VWF-induced activation of the cGMP pathway. To determine whether the cGMP pathway is a downstream effector of SFK, particularly Lyn, leading to platelet activation, Lyn knockout or PP2-treated platelets were allowed to adhere to VWF under flow conditions in the presence of low concentrations of a membrane-permeable cGMP analog, 8-bromo-cGMP. Indeed, 8-bromo-cGMP corrected the adhesion defects of Lyn-knockout platelets and PP2-treated platelets on VWF under flow conditions (Figure 6). These results demonstrate that Lyn mediates GPIb-induced integrin activation and stable platelet adhesion via the cGMP pathway.
The role of Lyn in GPIb-IX–mediated elevation of platelet cGMP. Washed wild-type or Lyn−/− platelets were stimulated with botrocetin alone or botrocetin (1.2 μg/mL) and VWF (10 μg/mL) in an aggregometer for 5 minutes. The cGMP levels (mean ± SD) in platelets were measured as described in “VWF-induced platelet cGMP elevation.” Data were obtained from 3 tests.
The role of Lyn in GPIb-IX–mediated elevation of platelet cGMP. Washed wild-type or Lyn−/− platelets were stimulated with botrocetin alone or botrocetin (1.2 μg/mL) and VWF (10 μg/mL) in an aggregometer for 5 minutes. The cGMP levels (mean ± SD) in platelets were measured as described in “VWF-induced platelet cGMP elevation.” Data were obtained from 3 tests.
Correction of impaired stable platelet adhesion to VWF in Lyn-deficient or Src inhibitor-treated platelets by exogenous cGMP. Washed mouse platelets were preincubated with DMSO or PP2 (10 μM). Wild-type (WT), Lyn−/−, or PP2-treated platelets were then treated with 10 μM 8-bromo-cGMP and immediately allowed to adhere to VWF under constant shear rate (800 seconds) for 5 minutes. After washing, stably adherent platelets were counted under a fluorescence microscope as described for Figure 2. Shown in the figure are representative pictures and quantitative data (mean ± SD) from 3 different experiments. ##P < .01, compared with WT platelets. ***P < .001, compared with DMSO-treated WT platelets.
Correction of impaired stable platelet adhesion to VWF in Lyn-deficient or Src inhibitor-treated platelets by exogenous cGMP. Washed mouse platelets were preincubated with DMSO or PP2 (10 μM). Wild-type (WT), Lyn−/−, or PP2-treated platelets were then treated with 10 μM 8-bromo-cGMP and immediately allowed to adhere to VWF under constant shear rate (800 seconds) for 5 minutes. After washing, stably adherent platelets were counted under a fluorescence microscope as described for Figure 2. Shown in the figure are representative pictures and quantitative data (mean ± SD) from 3 different experiments. ##P < .01, compared with WT platelets. ***P < .001, compared with DMSO-treated WT platelets.
Lyn mediates the GPIb-induced activation of MAPK
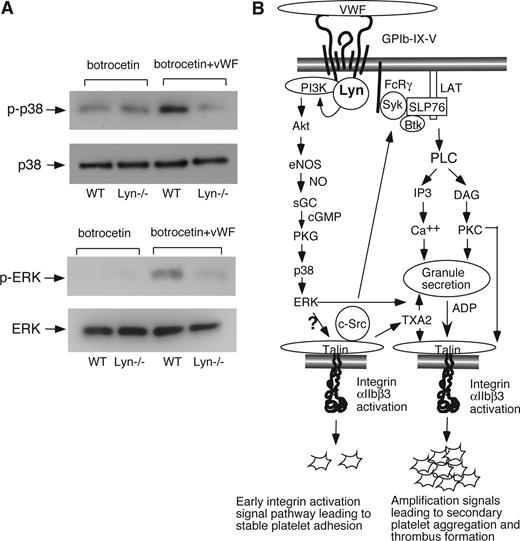
We have previously shown that the cGMP-dependent protein kinase (PKG) activates p38 and ERK MAPK pathways leading to platelet activation.16,35 Thus, if Lyn mediates GPIb-IX signals via the cGMP pathway, Lyn knockout should also show diminished activation of p38 and ERK. Indeed, VWF/botrocetin-induced p38 and ERK phosphorylation were diminished in Lyn-knockout platelets (Figure 7A), providing further support that Lyn is important in GPIb-IX–induced activation of the cGMP-p38-ERK signaling pathway leading to integrin activation and stable platelet adhesion and aggregation.
The role of Lyn in VWF-induced activation of MAPK and a schematic of Lyn-dependent GPIb-IX signaling pathway leading to platelet adhesion and aggregation. (A) Wild-type or Lyn−/− platelets were stimulated with botrocetin (1.2 μg/mL) in the absence or presence of 10 μg/mL of VWF for 8 minutes in a platelet aggregometer. Platelets were solubilized and immunoblotted with anti-phospho-p38 (Thr180/Tyr182) antibody (p-p38) or anti-p38 polyclonal antibody to indicate loading levels (p38; top panels), and anti–phospho-Erk1/2 (Thr202/Tyr204) antibody (p-ERK) or corresponding anti-Erk2 antibody to indicate loading levels (ERK; bottom panels). (B) VWF binding to GPIb–IX activates Lyn, which stimulates the PI3K-Akt-NO-cGMP-PKG-MAPK–dependent early GPIb-IX signaling pathway leading to integrin activation and stable platelet adhesion to VWF under shear stress. This signaling pathway together with integrin outside-in signaling causes TXA2 synthesis and granule secretion of agonists, such as ADP, leading to amplification of integrin activation, increased stable platelet adhesion, and platelet aggregation.
The role of Lyn in VWF-induced activation of MAPK and a schematic of Lyn-dependent GPIb-IX signaling pathway leading to platelet adhesion and aggregation. (A) Wild-type or Lyn−/− platelets were stimulated with botrocetin (1.2 μg/mL) in the absence or presence of 10 μg/mL of VWF for 8 minutes in a platelet aggregometer. Platelets were solubilized and immunoblotted with anti-phospho-p38 (Thr180/Tyr182) antibody (p-p38) or anti-p38 polyclonal antibody to indicate loading levels (p38; top panels), and anti–phospho-Erk1/2 (Thr202/Tyr204) antibody (p-ERK) or corresponding anti-Erk2 antibody to indicate loading levels (ERK; bottom panels). (B) VWF binding to GPIb–IX activates Lyn, which stimulates the PI3K-Akt-NO-cGMP-PKG-MAPK–dependent early GPIb-IX signaling pathway leading to integrin activation and stable platelet adhesion to VWF under shear stress. This signaling pathway together with integrin outside-in signaling causes TXA2 synthesis and granule secretion of agonists, such as ADP, leading to amplification of integrin activation, increased stable platelet adhesion, and platelet aggregation.
Discussion
Although SFK, such as Lyn and c-Src, are known to be important in the botrocetin/VWF-induced second wave of platelet aggregation,28 c-Src is known to be important in integrin outside-in signaling via its interaction with β3.23 It is unclear, however, whether Lyn is important in early GPIb-IX signaling or in integrin outside-in signaling that induces granule secretion leading to the second wave of platelet aggregation. Furthermore, the downstream signaling mechanism of Lyn in GPIb-induced platelet activation remains to be fully elucidated. We show here that Lyn mediates GPIb-IX early signaling leading to integrin activation and integrin-dependent stable platelet adhesion under shear stress independent of TXA2, and, unlike c-Src, is not required for integrin outside-in signaling. Importantly, we show that Lyn mediates GPIb-IX–induced signals leading to elevation of cGMP and that the downstream effect of Lyn in GPIb-IX signaling is mediated via the cGMP signaling pathway.
The role of Lyn in GPIb-IX–mediated platelet activation was previously thought to be mediated through TXA2, the synthesis of which was diminished in Lyn-knockout platelets stimulated by VWF/botrocetin.28 Furthermore, TXA2 is required for the second wave of platelet aggregation.14,16 However, we conclude that Lyn is important in early GPIb-IX signaling that is independent of secondary amplification pathways, including TXA2, ADP, and the ITAM-Syk pathways. This conclusion is supported by data that Lyn-knockout platelets showed significantly decreased adhesion to VWF under shear stress compared not only with wild-type platelets but also with platelets treated with the cyclooxygenase inhibitor, aspirin, ADP receptor antagonists, and a Syk inhibitor. GPIb-IX–mediated platelet activation is often studied by examining the second wave of integrin-dependent platelet aggregation induced by botrocetin or ristocetin in the presence of VWF. This assay, although useful in examining the effects of pharmacologic inhibitors or gene manipulation on GPIb-IX–mediated integrindependent platelet aggregation, does not differentiate between early GPIb-IX signaling, and the secondary amplification signals, such as secreted ADP and TXA2 because the second-wave platelet aggregation requires integrin outside-in signals, TXA2 synthesis, and granule secretion of ADP. In contrast, stable platelet adhesion to VWF under shear stress not only better mimics the physiologic role of GPIb-IX but is also partially independent of secondary amplification signals, such as TXA2 and ADP. Thus, by examining the effect of Lyn knockout and SFK inhibitors on platelet adhesion to VWF under shear stress, our experiments reveal that the role of Lyn (but not Fyn) is independent of TXA2 and ADP, although our results do not exclude that Lyn may also be involved in TXA2 and ADP-dependent signaling.
We also conclude that Lyn plays an important role in early GPIb-IX signaling leading to integrin activation, but, unlike some other SFK members, is not required for integrin outside-in signaling. This conclusion is supported by the data that Lyn-knockout platelets showed defective spreading on VWF, which requires both GPIb-IX signaling leading to integrin activation (inside-out signaling) and integrin outside-in signaling, but spread normally on immobilized fibrinogen, which requires outside-in signaling but not inside-out signals. Similarly, Lyn knockout did not significantly affect platelet spreading on VWF when ADP was used to induce inside-out signaling activation of integrins, which is independent of GPIb-IX signaling. Thus, by combining platelet spreading analysis and in-flow platelet adhesion analysis, we conclude that Lyn plays an important role in early GPIb-IX signaling leading to integrin activation that is independent of secondary amplification signals, including integrin outside-in signaling, TXA2, and ADP.
Unlike Lyn, Fyn does not appear to be important for the VWF-induced early platelet response because GPIb-mediated integrin-dependent stable adhesion to VWF under shear stress was unaffected by Fyn knockout. Nevertheless, botrocetin/VWF-induced second-wave aggregation is partially affected by Fyn knockout, suggesting that Fyn plays a role in the amplification of the second wave of platelet aggregation. Although the mechanism for this remains unclear, a recent study suggested that Fyn is involved in the fine tuning of the β3 integrin outside-in signaling.37 In addition, it has been previously reported that Fyn plays a role in GPVI-mediated ITAM signaling leading to platelet activation.25,26 The GPVI ITAM pathway has been postulated to synergize with GPIb-IX in promoting platelet activation.8,38 Previous studies suggest that c-Src binds to β3 integrin and is important in β3 outside-in signaling.23,24 Consistently, we show here that the nonisoform selective SFK inhibitor, PP2, inhibits platelet spreading on fibrinogen. However, Lyn knockout does not affect integrin outside-in signaling, suggesting that a SFK other than Lyn is important in integrin outside-in signaling. Thus, together with previous studies, our data indicate that Lyn is the major SFK member specifically important in GPIb-IX–mediated integrin inside-out signaling, although we cannot exclude the possibility that other isoforms of SFK may also be involved in early GPIb-IX signaling leading to integrin activation.
We, and recently others, have reported that VWF binding to GPIb–IX induces cGMP elevation.11,36 We have shown that cGMP, by activating p38 and ERK, plays an important role in GPIb-IX–mediated activation of the integrin αIIbβ3.16,35 . Recently, we have further shown that GPIb-IX–mediated cGMP elevation is mediated by the PI3K-Akt pathway, which is also important in early GPIb-IX signaling leading to integrin activation.19 In this study, we conclude that Lyn is a key upstream molecule that is required in GPIb-IX–mediated elevation of cGMP because GPIb-IX–mediated cGMP elevation was abolished in Lyn-knockout platelets. Furthermore, Lyn-knockout platelets show diminished phosphorylation of p38 and ERK during GPIb-IX–dependent platelet activation, both of which are induced via the cGMP-dependent protein kinase pathway.16,35 The role of Lyn in GPIb-IX–mediated cGMP elevation is probably mediated by activating the PI3K-Akt-NO pathway because of VWF/botrocetin induced PI3K-dependent platelet Akt activation,19 which is diminished in Lyn-knockout platelets28 (data not shown). PI3K-Akt pathway is known to activate NO synthesis and thus cGMP elevation.15,39 In addition, although it is still unclear whether Lyn direct activates PI3K, both Lyn and PI3K have been shown to form a complex with the GPIb cytoplasmic domain.21,40,41 More importantly, we have provided novel evidence that not only are Lyn-knockout platelets defective in GPIb-IX–mediated cGMP elevation, but also low concentrations of membrane permeable cGMP analog, 8-bromo-cGMP, reversed the impaired integrin-dependent stable adhesion of Lyn-knockout platelets under shear stress. Although we do not exclude the possibility that Lyn may signal via other mechanisms, our data indicate that cGMP elevation downstream from Lyn is sufficient to mediate GPIb-IX–induced signaling leading to platelet activation. Together with our previous studies,11,19,35 we have thus delineated a novel pathway of GPIb-IX–mediated integrin activation. In this pathway, VWF binding to GPIb-IX activates Lyn, which is required for the activation of PI3K. PI3K sequentially activates Akt, nitric oxide synthase, and guanylate cyclase leading to cGMP elevation. cGMP activates PKG, which activates p38 and ERK leading to integrin activation and stable platelet adhesion to VWF under shear stress (Figure 7B). Thus, our data clearly differentiate the role of Lyn in GPIb-IX signaling from the roles of other SFK members that are mainly important in the amplification signal pathways (Figure 7B).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the NHLB (National Heart, Lung and Blood Institute)/NIH (grants HL062350, HL068819, and HL080264; X.D.) and the National Health and Medical Research Council of Australia (M.C.B.). H.Y. is a recipient of the American Heart Association Midwest Affiliate Predoctoral Fellowship Award. Z.L. is supported by the American Heart Association Scientist Development Grant
National Institutes of Health
Authorship
Contribution: H.Y. performed a major part of experiments, analyzed data, and wrote the paper; J.L. performed important experiments and was involved in data analysis; Z.L. contributed to experiments using Lyn and Fyn knockout mice, data analysis, and discussion; M.C.B. provided key reagents, discussion, and editing; C.A.L. provided the Lyn knockout mice; and X.D. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Xiaoping Du, Department of Pharmacology, University of Illinois College of Medicine, 835 South Wolcott Avenue, Chicago, IL 60612; e-mail: xdu@uic.edu.