Abstract
The effects of B-cell depletion with rituximab on regulatory T cells (Tregs) have not been determined. We investigated Tregs in patients receiving rituximab for chronic idiopathic thrombocytopenic purpura (ITP). The peripheral blood Tregs, identified as CD4+FOXP3+ T cells, were measured by flow cytometry prior to and after the immunotherapy. In addition, Tregs were analyzed for their usage of the T-cell receptor (TCR) β-variable (VB) region gene as well as their regulatory function as assessed by cell proliferation assays. Pretreatment data revealed a reduced number and a defective suppressive capacity of Tregs in ITP patients compared with control individuals. In addition, Tregs showed a polyclonal spectratype. Patients, particularly responders, showed restored numbers of Tregs as well as a restored regulatory function upon treatment with rituximab. These results indicate that patients with active ITP have a defective T regulatory cell compartment that can be modulated by a B cell–targeted therapy.
Introduction
The pathophysiology of idiopathic thrombocytopenic purpura (ITP) is heterogeneous and complex.1 While the presence of antibodies against platelet glycoproteins has traditionally been considered to play a central role, several abnormalities involving the cellular mechanisms of immune modulation have been described.1,2
Recently, successful treatment with the B cell–depleting agent rituximab has been associated with regression of some T-cell abnormalities in patients with chronic ITP.3 However, the effects of rituximab treatment on regulatory T cells (Treg) have not been determined. The forkhead family transcription factor FOXP3 can effectively distinguish Tregs from activated CD4+ T cells.4 Although some FOXP3− Tregs can be generated in peripheral lymphoid organs by particular modes of antigen stimulation,5,6 the expression of FOXP3 is a unique marker of Tregs.7
These considerations prompted us to investigate the number and the nature of CD4+FOXP3+ Tregs in patients with ITP. To further define these Tregs and because of recent suggestion of changes in function of Tregs in autoimmunity,8 we assessed the clonality of Tregs and their suppressive function both before and after treatment with rituximab.
Methods
Patients and healthy controls
The study included 26 adults with chronic ITP who received rituximab therapy as part of their treatment. This cohort of patients was different from the one investigated in our earlier report on T-cell changes after rituximab.3 There were 8 men and 18 women, with a mean age of 44.4 years (median 45.5 years, range 24-62 years), and a median ITP duration of 27 months (range 9-87 months). The median platelet count was 19 × 109/L (range 5 to 28 × 109/L). All patients had already received corticosteroids and intravenous immunoglobulin therapy, and the majority had also received other treatments. The median number of treatments received by patients was 3 (range 2-6), including splenectomy in 6 patients. No patient had been challenged previously with rituximab.
Peripheral blood samples were obtained from all patients prior to and 3 months after rituximab therapy. We chose 3 months as the main evaluation point because patients were not supposed to respond after that time, and nonresponders had not yet received other treatments. Additional samples were obtained from responders at 6 months (12 cases) and at 12 months (9 cases). The control group consisted of 26 adult healthy volunteers matched for sex and age (± 3 years) with the study population. The mean age of controls was 44.1 years (median 46 years, range 21-61 years).
Ethical approval for this study was granted by the participating institutional review boards, and informed consent was obtained in accordance with the Declaration of Helsinki.
Phenotypic analysis
The following monoclonal antibodies from BD Biosciences (Milan, Italy) were used for surface antigen staining: anti-CD4 conjugated to fluorescein isothiocyanate (FITC), anti-CD3 conjugated to peridin chlorophyll protein (PerCP), anti-CD25 (p55 IL-2 receptor) conjugated to phycoerythrin (PE). The PE-conjugated FOXP3 antibody reagent kit and IgG1 isotype control from eBioscience (San Diego, CA) were used for intracellular FOXP3 staining according to the manufacturer's instructions, using a fixation/permeabilization solution and permeabilization buffer supplied by the same company. The percentages and absolute numbers of FOXP3+ cells within the CD4+ T-cell population were determined using a forward light scatter/side light scatter (FSC/SSC) gate on lymphocytes and a gate on CD3+CD4+cells. Background fluorescence was assessed using appropriate isotype- and fluorochrome-matched control monoclonal antibodies. The samples were analyzed on a FACScan flow cytometer (BD Biosciences).
Cell sorting and CDR3 size analysis of TCR VB transcripts
Tregs were sorted using a multistep isolation kit (Miltenyi Biotec, Auburn, CA). The kit contains a cocktail of biotinylated antibodies and antibiotin microbeads for the depletion of non-CD4+ and CD127high cells, and CD25 microbeads for the subsequent positive selection of CD4+CD25+CD127dim/− cells. The purity of the cells after sorting was 92% to 99%.
CDR3 size analysis of TCR VB transcripts (spectratyping) of Tregs was performed as previously described.3 Total RNA was extracted from purified Tregs using Trizol reagent and then subjected to reverse-transcription. The cDNA was amplified with primers specific to VB subfamilies. The CDR3 lengths were analyzed using an ABI 3130xl capillary sequencer (Applied Biosystems, Foster City, CA). Data were collected and analyzed by Genemapper Software version 3.7 (Applied Biosystems).
Functional analysis of Tregs
Purified CD4+CD25− responder T cells (105) were cultured alone or with autologous CD4+CD25+ regulatory T cells (105) in X-VIVO 15 serum-free medium (Lonza Group; Basel, Switzerland) in round-bottom 96-well microtitre plates. Cells were stimulated with 20 000 T cell–activation/expansion beads per well (Miltenyi Biotec). Beads were precoated with biotinylated anti-CD3 plus anti-CD28 monoclonal antibodies, each at the concentration of 5 μg/mL, according to the instructions of the manufacturer. On day 5, each well was pulsed with 1 μCi of 3H-thymidine (3H-TdR; GE Healthcare, Little Chalfont, United Kingdom). After 16 hour of additional incubation, plates were harvested and processed for counting of β-emission in a Wallac Microbeta Trilux counter (Perkin Elmer, Waltham, MA). All these experiments were run in triplicate.
Statistical analysis
Statistics were carried out using NCSS for Windows (NCSS, Kaysville, UT). Continuous variables were summarized by descriptive statistics. Categorical variables were summarized by frequency statistics. Student t test for independent samples was used for comparisons between different groups and the paired sample t test for within-group comparisons. Linear regression was used to determine the correlation between different study parameters. Values of P less than .05 were accepted as statistically significant. All P values are 2-tailed.
Results and discussion
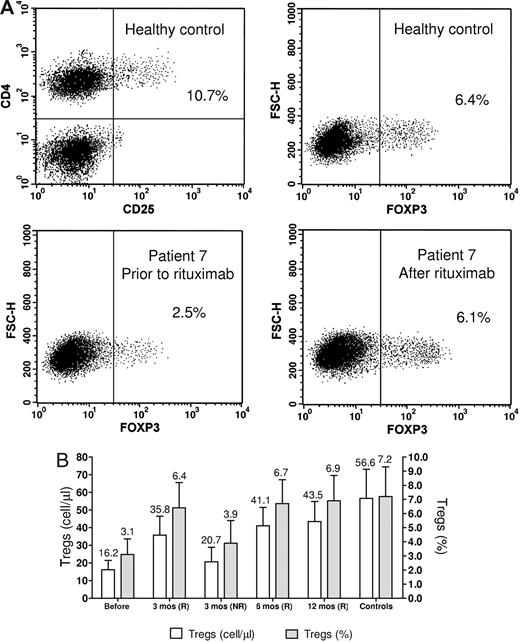
Representative dot-plots of flow cytometry analysis of Tregs are shown in Figure 1A, and results are shown in Figure 1B. The number and the percentage of Tregs in ITP patients at pretreatment (16.2 ± 5.3 cells/μL; 3.1% ± 1.1%) were lower than in controls (56.6 ± 16.5 cells/μL; 7.2% ± 2.1%) and increased significantly in responders at all subsequent controls (P < .01). The percentage of Tregs was not significantly different between patients in remission and controls.
Treg analyses. (A) Flow cytometric analysis of Tregs. The representative upper dot-plots are of a healthy control. The suppressive capacity of Tregs in humans seems to be confined to CD4+CD25+ cells with the highest expression of CD25 (CD4+CD25high) whereas CD4+T cell with intermediate expression of CD25 might also contain recently activated T cells without Treg function. However, because of the lack of a defined cut-off for CD25high expression, the use of this technique does not always offer the reproducible results obtained with the determination of FOXP3-positive cells. The lower plots show the increase of the Treg number following rituximab treatment. Dot plots for CD4+Foxp3+ Treg cells were gated on CD4+CD3+ cells. Numbers on plots are percentages of total cells gated within the respective rectangles. (B) Bar histogram showing Treg counts at the various study points. The counts are shown both as absolute concentrations and as percentage of CD4+ cells. The numbers above the bars are the mean values. Error bars represent SD. Pre indicates pretreatment; R, responders; and NR, non responders.
Treg analyses. (A) Flow cytometric analysis of Tregs. The representative upper dot-plots are of a healthy control. The suppressive capacity of Tregs in humans seems to be confined to CD4+CD25+ cells with the highest expression of CD25 (CD4+CD25high) whereas CD4+T cell with intermediate expression of CD25 might also contain recently activated T cells without Treg function. However, because of the lack of a defined cut-off for CD25high expression, the use of this technique does not always offer the reproducible results obtained with the determination of FOXP3-positive cells. The lower plots show the increase of the Treg number following rituximab treatment. Dot plots for CD4+Foxp3+ Treg cells were gated on CD4+CD3+ cells. Numbers on plots are percentages of total cells gated within the respective rectangles. (B) Bar histogram showing Treg counts at the various study points. The counts are shown both as absolute concentrations and as percentage of CD4+ cells. The numbers above the bars are the mean values. Error bars represent SD. Pre indicates pretreatment; R, responders; and NR, non responders.
There was no significant association between clinical parameters (age, sex, duration of disease, pretreatment platelet count, number of treatments, and duration of B-cell depletion after rituximab) and the number or percentage of Tregs at any disease stage.
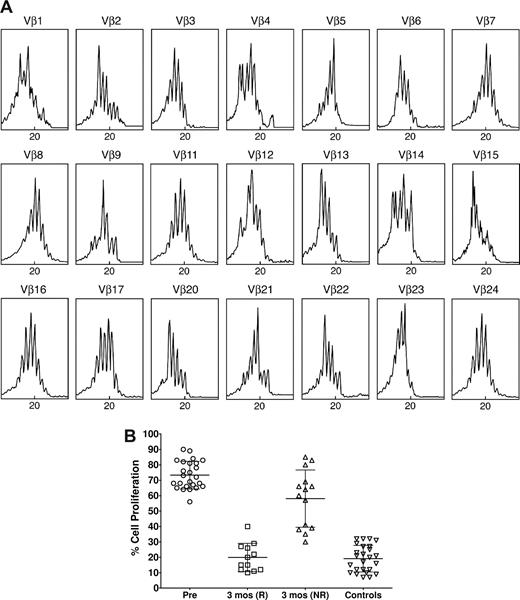
Spectratype analysis in ITP patients revealed a polyclonal pattern in TCR VB families (Figure 2A). In fact, the pretreatment number of abnormal CDR3 size patterns in patients was 2.2 (± 1.4), which was comparable with controls (2.0 ± 1.3). In particular, no patient had a number of oligoclonal/clonal CDR3 size patterns greater than 5. This is different from the changes seen in spectratype analysis of Th1/Th2 and Tc1/Tc2 cells previously observed in patients with ITP.3 On the other hand, the suppressive effect of CD4+CD25+ cells on autologous CD4+CD25− lymphocytes from ITP patients with active disease was significantly diminished compared with controls (P < .01; Figure 2B). Because we did not perform spectratype analysis of other T-cell subsets, we could not establish a correlation between abnormal expression of TCR VB families and Treg function.
Treg clonality and function. (A) Spectratype analysis of a representative ITP patient prior to ritucximab therapy. The normal TCR VB spectratype pattern consists of 5 to 8 bands and shows a Gaussian distribution in which the density of bands is generally higher in the middle part of the spectratype. Contracted spectratypes consisting of 1 to 4 peaks suggest the presence of a monoclonal (1 dominant peak) or oligoclonal (2-4 peaks) T-cell population. In this case almost all patterns appear polyclonal. (B) Suppressive function of Tregs in ITP. The suppressive function of CD4+CD25+ T cells isolated from the peripheral blood of patients with idiopathic thrombocytopenic purpura in active phase was decreased compared with normal controls, but was restored to normal values in those responding to rituximab. The results are expressed as the percentage of cell proliferation, according to the following formula: percent cell proliferation = (counts per minute of CD4+CD25− plus CD4+CD25+ cells/cpm of CD4+CD25− cells alone) × 100. Pre indicates pretreatment; R, responders; and NR, non responders. The central horizontal bar is the median and the top and bottom bars indicate the SD.
Treg clonality and function. (A) Spectratype analysis of a representative ITP patient prior to ritucximab therapy. The normal TCR VB spectratype pattern consists of 5 to 8 bands and shows a Gaussian distribution in which the density of bands is generally higher in the middle part of the spectratype. Contracted spectratypes consisting of 1 to 4 peaks suggest the presence of a monoclonal (1 dominant peak) or oligoclonal (2-4 peaks) T-cell population. In this case almost all patterns appear polyclonal. (B) Suppressive function of Tregs in ITP. The suppressive function of CD4+CD25+ T cells isolated from the peripheral blood of patients with idiopathic thrombocytopenic purpura in active phase was decreased compared with normal controls, but was restored to normal values in those responding to rituximab. The results are expressed as the percentage of cell proliferation, according to the following formula: percent cell proliferation = (counts per minute of CD4+CD25− plus CD4+CD25+ cells/cpm of CD4+CD25− cells alone) × 100. Pre indicates pretreatment; R, responders; and NR, non responders. The central horizontal bar is the median and the top and bottom bars indicate the SD.
The current study confirms previous reports indicating that in ITP patients with active disease Treg cells are both reduced in number9-11 and defective in their suppressive capacity.9 Autoreactive T cells to platelet glycoprotein (GP) IIb-IIIa can be found in the peripheral blood of otherwise normal individuals12 and are involved in the production of GPIIb-IIIa autoantibodies in ITP.13 Because of the impaired function of Tregs, autoreactive Th1 cells of ITP patients are arguably allowed to be activated and induce disease. It is yet unclear why this should be specific to autoimmune destruction of platelets, particularly given the polyclonal nature of the T regulatory cells.13
The lower numbers of Tregs may also be responsible for the proinflammatory Th1 cytokine responses in ITP patients14 and upon remission, the switch to the antiinflammatory Th2 profile that we have described recently.3
In those patients who responded to rituximab, the isolated T regulatory cells were as suppressive as normal controls. The mechanisms leading to the reversal of Treg abnormalities in patients responding to rituximab therapy are also unclear. They probably involve the abrogation of the reciprocal stimulation of B and T cells that is supposed to be abnormally amplified in ITP.1 Interestingly, the TCR VB gene usage pattern was found to be polyclonal. Oligoclonal T cells have been well documented in patients with chronic ITP.3,15 This data suggests that the expansion of pathogenic T cells involves only CD4+ and CD8+ effector cells.
The whole of our findings suggest that autoantibody production in ITP is driven by clonal Th cells that are not suppressed by Tregs. The role of clonal Tc cells remains to be determined, but they are possibly involved in peripheral platelet destruction.16 Because the above abnormalities may be restored upon B-cell depletion, the mechanisms leading to the development of clonal T cells and to defective Treg suppression require further investigation of the B cell–T cell interactions.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.S. designed research, analyzed the data, performed statistical analysis, and drafted the manuscript; N.C. performed research, analyzed and interpreted the data, and contributed to writing the paper; G.D.P., E.S., M.L.E., and E.A. performed research; S.A. designed research and analyzed the data. All authors took part in the revision of the manuscript and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Roberto Stasi, Department of Medical Sciences, Regina Apostolorum Hospital, Via S Francesco, 50, 00041 Albano Laziale, Italy; e-mail: roberto.stasi@uniroma2.it.