Abstract
Despite the demonstrated clinical efficacy of CD20 monoclonal antibody (mAb) for lymphoma therapy, the in vivo mechanisms of tumor depletion remain controversial and variable. To identify the molecular mechanisms responsible for lymphoma killing by CD20 mAb in a homologous system amenable to mechanistic studies and genetic manipulation, a mouse lymphoma model was developed using primary tumor cells from a C57BL/6 Eμ-cMyc transgenic mouse and mouse antimouse CD20 mAbs. CD20 mAb treatment of syngeneic mice with adoptively transferred lymphomas prevented tumor development or significantly prolonged mouse survival depending on tumor volume, mAb dose, and treatment timing. Cooperative FcγRIV, FcγRIII, and FcγRI interactions mediated optimal lymphoma depletion by CD20 mAb in vivo, whereas clodronate-mediated depletion of macrophages eliminated the therapeutic benefit of CD20 mAb. Although CD20 mAbs activated complement in vitro and in vivo, normal and malignant B-cell depletion was induced through C1q- and C3-independent mechanisms. Thus, the ability of CD20 mAbs to deplete malignant B cells in vivo required FcγR-dependent use of the innate mononuclear cell immune system. These findings allow for mechanism-based predictions of the biologic outcome of CD20 mAb therapy and treatment optimization.
Introduction
Non-Hodgkin lymphoma (NHL) is a heterogeneous group of malignancies that represents approximately 4% of all cancers. More than 90% of NHLs have a B-cell phenotype, and almost all express cell surface CD20, a B cell–specific member of the MS4A gene family.1,2 A chimeric CD20 monoclonal antibody (mAb), rituximab, was the first mAb to be approved for clinical use in cancer therapy.3 Rituximab is currently given along with steroid premedication, either alone or in combination with chemotherapy for the treatment of both indolent and aggressive NHL.4 Despite the demonstrated clinical efficacy of CD20 mAb therapy, the in vivo mechanisms of lymphoma depletion remain controversial
CD20 can serve as a membrane-embedded target for lymphoma destruction in vitro through activation of the innate immune system by initiating complement- and Ab-dependent cytotoxicity.5,6 Furthermore, CD20 mAb treatment alters transmembrane Ca2+ transport and B-cell progression through cell cycle7 and can induce B-cell apoptosis alone6 or following further cross-linking.8 Rituximab and other CD20 mAbs also induce classical pathway complement activation and complement-dependent cytotoxicity (CDC) of fresh B-lymphoma cells and cell lines.5,9-12 Rituximab also activates complement in vivo in both patients13 and primates.14 Furthermore, tumor cell expression of complement regulatory proteins is associated with resistance to CD20 immunotherapy.9,15 Although CD20 mAb depletes human lymphoma cells in vitro through CDC,9-11 tumor susceptibility to CDC and expression of complement inhibitor proteins does not always predict the outcome of CD20 therapy.16 Other Ab-dependent effects also appear important since a chimeric CD20 mAb of an isotype different from that used clinically does not deplete normal B cells in nonhuman primates17 and the antitumor effect of CD20 mAb depends in part on immune activation through Fc receptors for IgG (FcγR).18-21
Mechanistic studies using a panel of mouse anti–mouse CD20 mAbs have shown that B-cell depletion in normal mice requires monocyte FcγR expression.19-21 Although antimouse CD20 mAbs effectively activate complement in vitro, these mAbs deplete endogenous B cells in mice with genetic C3, C4, or C1q deficiencies.19 B-cell depletion in human CD20 transgenic mice by rituximab also requires monocytes and FcγR expression.22 However, rituximab mediates complement-dependent human lymphoma depletion in immunodeficient mouse xenograft models,23,24 and does not cure C1q-deficient mice given syngeneic EL4 lymphoma cells transfected to express human CD20.11 Most recently, rituximab was found to rapidly activate complement in vivo and induce chemokines that activate the innate immune network to eradicate human BJAB lymphoblastoid cell lines in nude mice.25 Thus, there is evidence for both antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated lymphoma depletion following CD20 mAb treatment in vivo.
To identify the molecular mechanisms responsible for lymphoma killing by CD20 mAb in a homologous system amenable to mechanistic studies and genetic manipulation, a preclinical model for mouse lymphoma was developed in C57BL/6 (B6) mice using mouse anti–mouse CD20 mAbs.20,26 This model allowed a comparison between the existing in vitro and in vivo data that shape current models of how CD20 therapies work, and has resulted in mechanism-based predictions of the biologic outcome of mAb therapy.
Methods
Mice
B6.Cg-Tg(IghMyc)22Bri/J (c-MycTG) hemizygous mice were crossed with B6 mice (The Jackson Laboratory, Bar Harbor, ME) to generate cMycTG+/− offspring.27,28 B6 mice from The Jackson Laboratory and National Cancer Institute (NCI)–Frederick Laboratory (Frederick, MD) were used as controls with identical results so all were pooled. FcγRI−/− and FcγRIII−/− mice29 were crossed to generate FcγRI−/−/RIII−/− mice. FcγRIIB−/− (B6;129S4-Fcgr2btm1Ttk/J), BUB/BnJ, and BALB/cJ mice were from The Jackson Laboratory. FcR common γ chain (FcRγ)–deficient mice (FcRγ−/−, B6.129P2-Fcer1gtm1RavN12) were from Taconic Farms (Germantown, NY).
Macrophage-deficient mice were generated by tail vein injections of clodronate-encapsulated liposomes (Sigma-Aldrich, St Louis, MO) with 0.2 mL given on day −1 and 0.1 mL given on days 2, 5, and 9.30,31 To deplete complement in vivo, mice were given cobra venom factor (CVF, 25 U; Quidel, San Diego, CA) intraperitoneally on days 0, 3, 5, and 9. All mice were housed in a specific pathogen-free barrier facility and first used at 6 to 10 weeks of age. All studies were approved by the Animal Care and Use Committee of Duke University.
Tumor immunotherapy
BL3750 spontaneous lymphoma cells were isolated from lymph nodes of a single cMycTG+/− mouse and cultured in complete medium consisting of RPMI 1640 media (Cellgro, Herndon, VA), 20% fetal bovine serum (Sigma-Aldrich), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine (all Cellgro) and 55 μM 2-mercaptoethanol (Invitrogen, Carlsbad, CA) for 7 days before freezing in aliquots. For each experiment, tumor cells were thawed and expanded for 24 to 48 hours in complete medium. Tumor cells in 250 μL PBS were injected through lateral tail veins or subcutaneously into the dorsal skin of recipient mice on day 0. Mice were given purified mAb in 250 μL PBS intravenously except as indicated, and were monitored daily for tumor development and mortality from days 7 to 180. Blood was obtained weekly. Tumor size was measured biweekly using a calibrated micrometer. For tumor measurements, the greatest longitudinal diameter was designated as L and the greatest transverse diameter W. Tumor volume (TV) was calculated as follow: TV = [(W)2 × L]/2. All mice exhibiting distress or tumor volumes exceeding 2.0 cm3 were killed, with the date recorded as death from disease. In some cases, A20 cells (106) were injected subcutaneously into the dorsal skin of BALB/c mice on day 0, followed 1 day later by intravenous mAb injection (250 μg).
MAbs
Sterile mouse anti–mouse CD20 (MB20-11, IgG2c; and MB20-18, IgG2b) mAbs and unreactive mouse control IgG2a were produced in vitro.26 An IgG2a isotype switch variant of the MB20-18 mAb was generated by recombinant DNA technology. IgG2a MB20-18 mAbs containing single amino acid substitutions (Lys322→Ala) were generated by polymerase chain reaction (PCR) techniques using synthetic oligonucleotide mismatch primers as described.32 Recombinant mAb was produced in 293T cells. All mAbs were purified by protein A affinity chromatography (Amersham, Arlington Heights, IL) and were free of endotoxin (Pyrogent Plus test kit, sensitivity of 0.06 EU/mL; Cambrex Bio Science, Walkersville, MD).
Cell isolation and immunofluorescence analysis
CD20 expression was visualized using biotin-conjugated mouse CD20 (MB20-11 and MB20-18) mAbs26 plus phycoerythrin-Cy5 (PE-Cy5) streptavidin (eBioscience, San Diego, CA). CD22 expression was visualized using fluorescein isothiocyanate (FITC)–conjugated CD22 (MB22-10) mAb.26,33 Other mAbs included B220 (RA3-6B2), CD5 (53-7.3), CD16/32 (2.4G2), CD19 (1D3), and CD23 (B3B4) mAbs from BD Biosciences (San Diego, CA). Anti–mouse IgM (1B4B1) and goat anti–mouse IgG2c were from Southern Biotechnology Associates (Birmingham, AL). CD21/CD35 (8D9) mAb was from eBioscience.
Single-cell suspensions of bone marrow (bilateral femurs), spleen, and peripheral lymph node (paired axillary and inguinal) were generated by gentle dissection. Blood erythrocytes were lysed after immunofluorescence staining using fluorescence-activated cell sorting (FACS) Lysing Solution (BD Biosciences). In some cases, magnetic cell sorting technology was used to isolate B220+ spleen lymphocytes according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). For immunofluorescence analysis, cells were stained on ice using predetermined optimal concentrations of each Ab for 30 minutes, and fixed as described.34 Cells with the light scatter properties of lymphoma cells or lymphocytes were analyzed by immunofluorescence staining, using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Nonreactive, isotype-matched mAbs (eBioscience/BD Pharmingen, San Diego, CA) were used as staining controls.
CDC assay
Complement-mediated B-cell killing was quantified in vitro as described.35 Briefly, A20 cells were incubated with CD20 mAb (0.01-10 μg/mL) and baby rabbit complement (diluted 5-fold; GIBCO-BRL, Grand Island, NY) in RPMI 1640 supplemented with 0.1% bovine serum albumin (BSA; Sigma-Aldrich), 20 mM HEPES (GIBCO-BRL), 100 IU/mL penicillin, and 100 μg/mL streptomycin. After incubation at 37°C for 5 hours, the cells were stained with propidium iodide (PI; Sigma-Aldrich), with PI exclusion assessed by flow cytometry.
C3 ELISA
Mouse serum C3 levels were measured by enzyme-linked immunosorbent assay (ELISA) as described.36 Blood was allowed to clot on ice for 45 minutes before centrifugation at 2000g for 10 minutes at 4°C. The serum was removed, aliquoted on ice, and stored at −20°C. Microtiter plates were coated overnight at 4°C with 25 μg polyclonal goat IgG anti–mouse C3 (Cappel, Solon, OH) per milliliter diluted in 15 mM Na2CO3/30 mM NaHCO3 buffer, pH 9.6 (wash buffer). The plates were washed and wells incubated for 1 hour with 100 μL 1% BSA in PBS containing 10 mM EDTA, pH 7.5, and then washed with wash buffer containing 0.05% Tween 20. Mouse serum samples (diluted 1:500 in wash buffer containing 0.05% BSA) and mouse C3 (Immunology Consultants Laboratory, Newberg, OR) were added to the wells. Plates were incubated at room temperature for 2 hours and washed, and 100 μL peroxidase-conjugated goat anti–mouse C3 antibody was added to each well. After 1 hour of incubation, the plates were washed twice, and 100 μL ABTS (Sigma-Aldrich) substrate buffer was added. After 30 minutes of substrate conversion, the OD405values of each well were determined.
Statistical analysis
All data are shown as means plus or minus SEM. The Student t test was used to determine the significance of differences between sample means. Comparative statistical analysis of survival using the log-rank test, as well as the generation of Kaplan-Meier cumulative survival plots used Prism software (version 4.0; GraphPad Software, San Diego, CA).
Results
CD20 mAb-induced lymphoma depletion in vivo
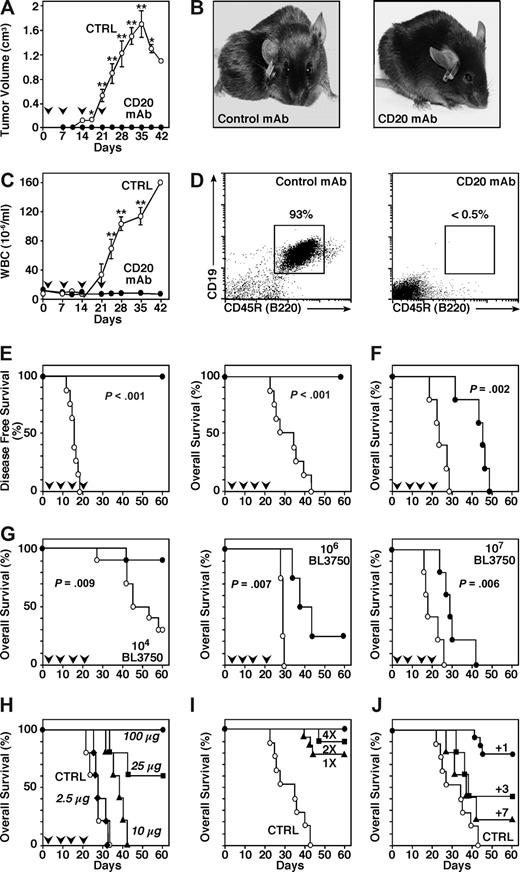
The ability of CD20 mAbs to deplete B-lymphoma cells in a syngeneic mouse model was first assessed using BALB/c mice that received a transplant of the A20 mature B-cell line on day 0 followed by CD20 or control mAb treatment on day 1. Despite high-level CD20 expression by A20 cells, tumors developed similarly in CD20 or control mAb-treated littermates with identical survival rates (Figure 1A). Despite no apparent effect on lymphoma cells, CD20 mAb effectively depleted more than 95% of spleen B cells within 2 days (data not shown), as described.37 Higher CD20 mAb doses, different routes of mAb administration, more frequent mAb administration, reductions in transplanted lymphoma cell numbers, or transfer of lymphoma cells intravenously or intraperitoneally were without benefit (data not shown). The ability of CD20 mAb to deplete primary B-cell malignancies in vivo was therefore assessed using mice given aliquots of primary lymphoma cells (named BL3750) isolated from a syngeneic B6 c-MycTG mouse. The c-Myc proto-oncogene is induced by the Ig heavy-chain enhancer in c-MycTG mice, which primarily develop aggressive, monoclonal B cell–derived lymphomas.27,28 Syngeneic mice were given tumor cells subcutaneously on day 0 and CD20 or control mAb on day 1. CD20 mAb treatment had a significant therapeutic benefit, with most CD20 mAb-treated mice remaining tumor free for up to 60 days (P < .001; Figure 1B).
In vivo model of lymphoma immunotherapy. Survival of mice given (A) A20 cells, or (B) BL3750 tumor cells from a cMycTG+/− mouse. Left panels: CD20 expression by tumor cells (thick line) and the A20 cell line (thin line) assessed by immunofluorescence staining with flow cytometry analysis. Right panels: Mouse survival following subcutaneous transfer of 106 A20 or 105 BL3750 lymphoma cells on day 0. All mice were given CD20 (•) or control (○) mAb intravenously (250 μg/mouse) on day 1 (n = 4-9 mice/group). (C) Cell surface immunophenotype of BL3750 cells evaluated by immunofluorescence staining with gating on live cells as identified by forward/side light scatter as shown. Comparisons of CD19, CD20, and CD22 expression by BL3750 cells (thick line) and spleen B220+ cells (thin line) from cMycTG+/− mice was assessed by 3-color analysis. Cell surface expression of CD45R (B220), IgM, FcγRIIB, IgD, CD21, CD23, and CD5 (thick line) was assessed by 2-color analysis. Results represent those obtained in 3 independent experiments. (A-C) Background staining using a control (CTRL) mAb is shown (dashed line). (D) Hematoxylin and eosin staining of a lymph node tissue section 28 days after mice were given BL3750 cells. Results represent those obtained in 3 mice. Image was acquired with an Olympus BX60 microscope (Olympus, Center Valley, PA; magnification 40×/1.00 oil iris) and Camera SPOT 1.3.0 (Diagnostic Instruments, Sterling Heights, MI). Image was processed with SPOT 4.0.4 (Diagnostic Instruments) and Adobe Photoshop CS2, version 9.0.2 (Adobe Systems, San Jose, CA).
In vivo model of lymphoma immunotherapy. Survival of mice given (A) A20 cells, or (B) BL3750 tumor cells from a cMycTG+/− mouse. Left panels: CD20 expression by tumor cells (thick line) and the A20 cell line (thin line) assessed by immunofluorescence staining with flow cytometry analysis. Right panels: Mouse survival following subcutaneous transfer of 106 A20 or 105 BL3750 lymphoma cells on day 0. All mice were given CD20 (•) or control (○) mAb intravenously (250 μg/mouse) on day 1 (n = 4-9 mice/group). (C) Cell surface immunophenotype of BL3750 cells evaluated by immunofluorescence staining with gating on live cells as identified by forward/side light scatter as shown. Comparisons of CD19, CD20, and CD22 expression by BL3750 cells (thick line) and spleen B220+ cells (thin line) from cMycTG+/− mice was assessed by 3-color analysis. Cell surface expression of CD45R (B220), IgM, FcγRIIB, IgD, CD21, CD23, and CD5 (thick line) was assessed by 2-color analysis. Results represent those obtained in 3 independent experiments. (A-C) Background staining using a control (CTRL) mAb is shown (dashed line). (D) Hematoxylin and eosin staining of a lymph node tissue section 28 days after mice were given BL3750 cells. Results represent those obtained in 3 mice. Image was acquired with an Olympus BX60 microscope (Olympus, Center Valley, PA; magnification 40×/1.00 oil iris) and Camera SPOT 1.3.0 (Diagnostic Instruments, Sterling Heights, MI). Image was processed with SPOT 4.0.4 (Diagnostic Instruments) and Adobe Photoshop CS2, version 9.0.2 (Adobe Systems, San Jose, CA).
Primary BL3750 cells expressed CD20 at lower levels than spleen B cells, but expressed normal levels of CD19 and CD22. BL3750 also expressed B220 (CD45R) and IgM, but not CD5, CD23, IgD, CD90, CD4, or CD8 (Figure 1C; data not shown). BL3750 cells retained their phenotype, even after 40 days of continuous culture (data not shown). Moreover, lymphoma cells isolated from mice that developed tumors after mAb treatment retained parental cell surface CD20 expression levels (data not shown), confirming the stability of this newly established tumor cell line. After BL3750 cell transfer into mice, lymphoid tissues were infiltrated by monotonous appearing intermediate-sized lymphocytes with round to oval nuclei, small indistinct nucleoli, and a rim of basophilic cytoplasm (Figure 1D). Apoptotic bodies of lymphocyte derivation were numerous within tissues. Although there was no “starry sky” appearance within lymph nodes, BL3750 cells exhibited many of the other phenotypic (BCL6+, BCL2−; data not shown) and histologic characteristics of Burkitt lymphoma–like cells. Finally, BL3750 tumor cells had monoclonal VH and VK gene rearrangements (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
CD20 mAb depletion of lymphoma cells in vivo under different conditions was assessed using aliquots of primary BL3750 cells (105) transplanted subcutaneously into syngeneic mice that were randomly given CD20 or control mAb weekly for 4 weeks after tumor transfer. In control mAb-treated mice, tumor growth was detectable at the site of injection with a median of 16 days (range, 12-19; Figure 2A,B). Tumor growth was accompanied by lymph node, spleen, and liver enlargement, with bone marrow infiltration as described.28 After 5 weeks, circulating leukocyte numbers increased 20-fold (Figure 2C), with lymphoblastic B cells representing more than 90% of blood leukocytes (Figure 2D; data not shown). CD20 mAb treatment prevented tumor development in all recipients (P < .001), while the median survival of control mAb-treated mice was 32 days (range, 23-44 days; Figure 2E). BL3750 cells given intravenously led to acute tumor dissemination and death in both CD20 and control mAb-treated mice (Figure 2F). Nonetheless, CD20 mAb treatment significantly (P = .002) prolonged mouse survival. Thus, adoptively transferred BL3750 cells provided an in vivo model for spontaneous B-cell lymphomas responsive to CD20 immunotherapy.
CD20 mAb treatment eliminates BL3750 tumors in vivo. (A-E) Mice were given 105 BL3750 cells subcutaneously on day 0 with CD20 (•, n = 6) or control (○, n = 8) mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward  ). (A) Tumor volumes (± SEM) for CD20 or control mAb-treated mice. (B) Representative control mAb-treated mice exhibited tumors on their backs 5 weeks after BL3750 cell transfer, whereas tumors were not detectable in CD20 mAb-treated mice. (C) Circulating white blood cell numbers (± SEM) for CD20 or control mAb-treated mice. (A,C) Significant differences between sample means are indicated (*P < .05; **P < .001). (D) Circulating CD19+B220+ cells in representative mice 5 weeks after transfer of BL3750 tumor cells and CD20 or control mAb treatment. The percentages indicate relative frequencies of cells within the indicated gates. (E) Mouse tumor-free and overall survival rates following BL3750 cell transfers. (F) Mice were given 105 BL3750 cells intravenously on day 0 with CD20 (•, n = 5) or control (○, n = 5) mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward
). (A) Tumor volumes (± SEM) for CD20 or control mAb-treated mice. (B) Representative control mAb-treated mice exhibited tumors on their backs 5 weeks after BL3750 cell transfer, whereas tumors were not detectable in CD20 mAb-treated mice. (C) Circulating white blood cell numbers (± SEM) for CD20 or control mAb-treated mice. (A,C) Significant differences between sample means are indicated (*P < .05; **P < .001). (D) Circulating CD19+B220+ cells in representative mice 5 weeks after transfer of BL3750 tumor cells and CD20 or control mAb treatment. The percentages indicate relative frequencies of cells within the indicated gates. (E) Mouse tumor-free and overall survival rates following BL3750 cell transfers. (F) Mice were given 105 BL3750 cells intravenously on day 0 with CD20 (•, n = 5) or control (○, n = 5) mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward  ). (G-J) Mouse survival following transfer of BL3750 cells subcutaneously on day 0 with CD20 (•) or control (○) mAb treatments as indicated (downward
). (G-J) Mouse survival following transfer of BL3750 cells subcutaneously on day 0 with CD20 (•) or control (○) mAb treatments as indicated (downward  ). (G) Mouse survival following transfer of 104 (n = 10 mice/group), 106 (n = 4/group), or 107 (n = 5/group) BL3750 cells on day 0, with CD20 or control mAb (250 μg/mouse) given at days 1, 7, 14, and 21. (H) Mouse survival following transfer of 105 BL3750 cells with CD20 (2.5 μg/mouse, ♦; 10 μg, ▴; 25 μg, ■; or 100 μg, •) or control mAb (250 μg, ○) given on days 1, 7, 14, and 21 (n = 4-5/group). (I) Mouse survival (n = 6-8/group) following transfer of 105 BL3750 cells with CD20 or control mAb (250 μg/mouse) given on day 1 (1×, ▴), on days 1 and 7 (2×, ■), or on days 1, 7, 14, and 21 (4×, •). (J) Mouse survival (n = 5-8/group) following transfer of 105 BL3750 cells with CD20 or control mAb (250 μg/mouse) given on days 1 (+1, •), 3 (+3, ■), or 7 (+7, ▴).
). (G) Mouse survival following transfer of 104 (n = 10 mice/group), 106 (n = 4/group), or 107 (n = 5/group) BL3750 cells on day 0, with CD20 or control mAb (250 μg/mouse) given at days 1, 7, 14, and 21. (H) Mouse survival following transfer of 105 BL3750 cells with CD20 (2.5 μg/mouse, ♦; 10 μg, ▴; 25 μg, ■; or 100 μg, •) or control mAb (250 μg, ○) given on days 1, 7, 14, and 21 (n = 4-5/group). (I) Mouse survival (n = 6-8/group) following transfer of 105 BL3750 cells with CD20 or control mAb (250 μg/mouse) given on day 1 (1×, ▴), on days 1 and 7 (2×, ■), or on days 1, 7, 14, and 21 (4×, •). (J) Mouse survival (n = 5-8/group) following transfer of 105 BL3750 cells with CD20 or control mAb (250 μg/mouse) given on days 1 (+1, •), 3 (+3, ■), or 7 (+7, ▴).
CD20 mAb treatment eliminates BL3750 tumors in vivo. (A-E) Mice were given 105 BL3750 cells subcutaneously on day 0 with CD20 (•, n = 6) or control (○, n = 8) mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward  ). (A) Tumor volumes (± SEM) for CD20 or control mAb-treated mice. (B) Representative control mAb-treated mice exhibited tumors on their backs 5 weeks after BL3750 cell transfer, whereas tumors were not detectable in CD20 mAb-treated mice. (C) Circulating white blood cell numbers (± SEM) for CD20 or control mAb-treated mice. (A,C) Significant differences between sample means are indicated (*P < .05; **P < .001). (D) Circulating CD19+B220+ cells in representative mice 5 weeks after transfer of BL3750 tumor cells and CD20 or control mAb treatment. The percentages indicate relative frequencies of cells within the indicated gates. (E) Mouse tumor-free and overall survival rates following BL3750 cell transfers. (F) Mice were given 105 BL3750 cells intravenously on day 0 with CD20 (•, n = 5) or control (○, n = 5) mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward
). (A) Tumor volumes (± SEM) for CD20 or control mAb-treated mice. (B) Representative control mAb-treated mice exhibited tumors on their backs 5 weeks after BL3750 cell transfer, whereas tumors were not detectable in CD20 mAb-treated mice. (C) Circulating white blood cell numbers (± SEM) for CD20 or control mAb-treated mice. (A,C) Significant differences between sample means are indicated (*P < .05; **P < .001). (D) Circulating CD19+B220+ cells in representative mice 5 weeks after transfer of BL3750 tumor cells and CD20 or control mAb treatment. The percentages indicate relative frequencies of cells within the indicated gates. (E) Mouse tumor-free and overall survival rates following BL3750 cell transfers. (F) Mice were given 105 BL3750 cells intravenously on day 0 with CD20 (•, n = 5) or control (○, n = 5) mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward  ). (G-J) Mouse survival following transfer of BL3750 cells subcutaneously on day 0 with CD20 (•) or control (○) mAb treatments as indicated (downward
). (G-J) Mouse survival following transfer of BL3750 cells subcutaneously on day 0 with CD20 (•) or control (○) mAb treatments as indicated (downward  ). (G) Mouse survival following transfer of 104 (n = 10 mice/group), 106 (n = 4/group), or 107 (n = 5/group) BL3750 cells on day 0, with CD20 or control mAb (250 μg/mouse) given at days 1, 7, 14, and 21. (H) Mouse survival following transfer of 105 BL3750 cells with CD20 (2.5 μg/mouse, ♦; 10 μg, ▴; 25 μg, ■; or 100 μg, •) or control mAb (250 μg, ○) given on days 1, 7, 14, and 21 (n = 4-5/group). (I) Mouse survival (n = 6-8/group) following transfer of 105 BL3750 cells with CD20 or control mAb (250 μg/mouse) given on day 1 (1×, ▴), on days 1 and 7 (2×, ■), or on days 1, 7, 14, and 21 (4×, •). (J) Mouse survival (n = 5-8/group) following transfer of 105 BL3750 cells with CD20 or control mAb (250 μg/mouse) given on days 1 (+1, •), 3 (+3, ■), or 7 (+7, ▴).
). (G) Mouse survival following transfer of 104 (n = 10 mice/group), 106 (n = 4/group), or 107 (n = 5/group) BL3750 cells on day 0, with CD20 or control mAb (250 μg/mouse) given at days 1, 7, 14, and 21. (H) Mouse survival following transfer of 105 BL3750 cells with CD20 (2.5 μg/mouse, ♦; 10 μg, ▴; 25 μg, ■; or 100 μg, •) or control mAb (250 μg, ○) given on days 1, 7, 14, and 21 (n = 4-5/group). (I) Mouse survival (n = 6-8/group) following transfer of 105 BL3750 cells with CD20 or control mAb (250 μg/mouse) given on day 1 (1×, ▴), on days 1 and 7 (2×, ■), or on days 1, 7, 14, and 21 (4×, •). (J) Mouse survival (n = 5-8/group) following transfer of 105 BL3750 cells with CD20 or control mAb (250 μg/mouse) given on days 1 (+1, •), 3 (+3, ■), or 7 (+7, ▴).
Tumor burden and CD20 mAb dosing affect BL3750 depletion
CD20 mAb efficiency was assessed over a range of BL3750 cell dosages (104-107/mouse; Figure 2G). Transfer of 104 BL3750 cells caused tumor growth in 70% of syngeneic mice treated with control mAb (median death, 45 days; range, 27-58 days). By contrast, weekly CD20 mAb (250 μg) treatment for 4 weeks prevented leukemia/lymphoma in 90% of recipients (P = .009; Figure 2G). Transplantation of 106 BL3750 cells resulted in death of all control mAb-treated mice (median, 28 days; range, 27-29 days), whereas CD20 mAb treatment delayed tumor growth in 75% of mice (median death, 37 days; range, 33-43 days; P = .007; Figure 2G). Transplantation of 107 BL3750 cells resulted in median death at 18 days (range, 17-26 days) in control mAb-treated mice, whereas CD20 mAb treatment significantly delayed death (median, 29 days; range, 24-42 days; P = .006). Thus, CD20 mAb demonstrated optimal therapeutic benefit with low tumor cell doses.
Lymphoma cell depletion was also assessed over a range of mAb concentrations (2.5-100 μg/mouse). Transplantation of 105 BL3750 tumor cells resulted in death (median, 28 days; range, 22-34 days; Figure 2H) in control mAb-treated mice. Mice that received 2.5 μg CD20 mAb weekly for 4 weeks had a median survival of 28 days (range, 26-33 days). By contrast, 10 μg, 25 μg, and 100 μg CD20 mAb given weekly for 4 weeks significantly delayed or prevented tumor growth (P < .02). Thus, CD20 mAb displayed the greatest efficacy when given at a dose of 100 μg or more per mouse.
CD20 mAb efficiency was assessed using different therapeutic schedules whereby CD20 mAb (250 μg) was given on day 1 (1×), days 1 and 7 (2×), or days 1, 7, 14, and 21 (4×). Transplantation of 105 BL3750 cells resulted in the death of all control mAb-treated mice, whereas CD20 mAb treatment once, twice, or 4 times prevented tumor growth in 75% to 100% of mice (P ≤ .001; Figure 2I). CD20 or control mAb (250 μg/mouse) was also given on days 1, 3, or 7 after transplantation of 105 BL3750 cells. CD20 mAb given at day 1 prevented tumor growth in 75% of mice (P < .001), whereas CD20 mAb efficiency diminished dramatically when given on day 3 or 7 (Figure 2J).
The effect of tumor burden on circulating CD20 mAb levels in vivo was assessed in mice given high-dose BL3750 cells to establish leukemia. One day after CD20 mAb treatment, the majority of circulating tumor cells displayed cell surface CD20 mAb at saturating levels, whereas control mAb-treated mice did not have detectable IgG2a mAb bound to the tumor cell surface (Figure 3A). However, the number of circulating tumor cells with CD20 mAb on their surface declined thereafter, with only a minority of leukemia cells expressing significant amounts of cell surface CD20 mAb by day 7 (Figure 3A,B). Decreased CD20 mAb bound to the surface of tumor cells did not result from CD20 internalization, as the addition of fresh CD20 mAb before staining in vitro revealed normal cell surface CD20 densities (Figure 3A,B; data not shown). In parallel, circulating tumor cell numbers were depleted on day 2 after CD20 mAb treatment, but reappeared and expanded 2 days later when circulating CD20 mAb levels had decreased. Thus, rapid tumor cell expansion along with normal B-cell and BL3750-cell depletion significantly hasted CD20 mAb consumption in vivo compared with what is observed in tumor-free mice.37
BL3750 tumor cell expansion depletes circulating CD20 mAb in vivo. Mice were given 106 BL3750 cells subcutaneously with CD20 (IgG2c, •, n = 6) or control (IgG2a, ○, n = 8) mAb (250 μg/mouse) given intravenously on day 0 after the mice had demonstrable leukemia. Blood cells were isolated before mAb treatment and on the indicated days after mAb treatment. (A) CD20 mAb binding to circulating tumor cells. Numbers indicate the relative frequencies of gated CD19+ lymphoblast cells with IgG2a/c mAb bound in vivo on days 1 and 7. Blood leukocytes were incubated with either control IgG2c or MB20-11 (IgG2c) CD20 mAb in vitro, washed, stained using fluorochrome-conjugated IgG2c-specific secondary antibody, and analyzed by flow cytometry. (B) Percentages of circulating B lymphoblasts with IgG2a/c mAb bound to their cell surface. Values indicate mean (± SEM) percentages of circulating CD19+ lymphoblast cells staining positive as shown in panel A. (C) Mean white blood cell (WBC) numbers (± SEM) in mice given CD20 or control mAb.
BL3750 tumor cell expansion depletes circulating CD20 mAb in vivo. Mice were given 106 BL3750 cells subcutaneously with CD20 (IgG2c, •, n = 6) or control (IgG2a, ○, n = 8) mAb (250 μg/mouse) given intravenously on day 0 after the mice had demonstrable leukemia. Blood cells were isolated before mAb treatment and on the indicated days after mAb treatment. (A) CD20 mAb binding to circulating tumor cells. Numbers indicate the relative frequencies of gated CD19+ lymphoblast cells with IgG2a/c mAb bound in vivo on days 1 and 7. Blood leukocytes were incubated with either control IgG2c or MB20-11 (IgG2c) CD20 mAb in vitro, washed, stained using fluorochrome-conjugated IgG2c-specific secondary antibody, and analyzed by flow cytometry. (B) Percentages of circulating B lymphoblasts with IgG2a/c mAb bound to their cell surface. Values indicate mean (± SEM) percentages of circulating CD19+ lymphoblast cells staining positive as shown in panel A. (C) Mean white blood cell (WBC) numbers (± SEM) in mice given CD20 or control mAb.
FcγR-dependent ADCC mediates lymphoma depletion in vivo
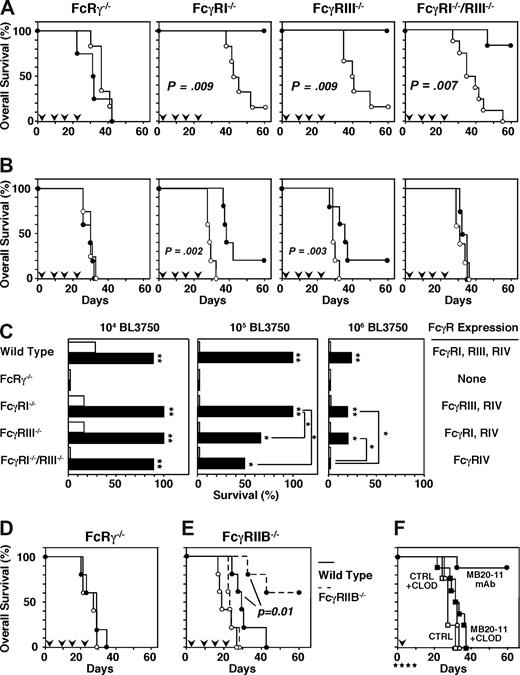
The dependence of lymphoma depletion on FcγR expression and effector cells was assessed using IgG2c CD20 mAb and mice deficient in FcRγ, FcγRI, FcγRIII, or both FcγRI/RIII. FcRg−/− mice lack cell surface FcγRI, FcγRIII, and FcγRIV expression. FcRg−/− mice given 104 BL3750 cells subcutaneously survived similarly after control (median, 41 days; range, 31-42 days) or CD20 (median, 37 days; range, 22-42 days) mAb treatment (Figure 4A,C). The mode of CD20 mAb action is suggested to differ according to the site of tumor injection.23 However, FcRg−/− mouse survival was similar with CD20 or control mAb treatment regardless of whether the tumor cells were transplanted intravenously or subcutaneously (Figure 4A,D). Thus, CD20 mAb-mediated lymphoma depletion required FcRγ chain expression regardless of tumor site.
FcγR-bearing macrophages mediate lymphoma depletion by CD20 mAb in vivo. (A-E) Mouse survival following BL3750 cell transfer on day 0 with CD20 or control mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward  ). (A) Mouse survival following transfer of 104 BL3750 cells subcutaneously into FcRγ−/− (n = 5/group), FcγRI−/− (n = 4-5/group), FcγRIII−/− (n = 5/group), or FcγRI/RIII−/− (n = 7/group) recipient mice with CD20 (•) or control (○) mAb treatment. (B) Mouse survival following transfer of 106 BL3750 cells subcutaneously into FcRγ−/− (n = 5/group), FcγRI−/− (n = 5/group), FcγRIII−/− (n = 5/group), or FcγRI/RIII−/− (n = 4-5/group) recipient mice with CD20 (•) or control (○) mAb treatment. (C) Mouse survival at day 60 following transfer of 104 (left panel), 105 (middle panel), or 106 (right panel) BL3750 cells into wild-type, FcRγ−/−, FcγRI−/−, FcγRIII−/−, or FcγRI/RIII−/− recipient mice with CD20 (▬) or control (▭) mAb treatment (n = 4-7/group) as in panels A,B. Significant differences in mean survival of mice treated with CD20 or control mAbs as well as survival of CD20 mAb-treated mice are indicated (*P < .05; **P < .001). (D) Mouse survival following transfer of 105 BL3750 cells intravenously into FcRγ−/− mice with CD20 (•) or control (○) mAb treatment (n = 5/group). (E) Mouse survival following transfer of 107 BL3750 cells into wild-type (—, n = 5/group) or FcγRIIB−/− (
). (A) Mouse survival following transfer of 104 BL3750 cells subcutaneously into FcRγ−/− (n = 5/group), FcγRI−/− (n = 4-5/group), FcγRIII−/− (n = 5/group), or FcγRI/RIII−/− (n = 7/group) recipient mice with CD20 (•) or control (○) mAb treatment. (B) Mouse survival following transfer of 106 BL3750 cells subcutaneously into FcRγ−/− (n = 5/group), FcγRI−/− (n = 5/group), FcγRIII−/− (n = 5/group), or FcγRI/RIII−/− (n = 4-5/group) recipient mice with CD20 (•) or control (○) mAb treatment. (C) Mouse survival at day 60 following transfer of 104 (left panel), 105 (middle panel), or 106 (right panel) BL3750 cells into wild-type, FcRγ−/−, FcγRI−/−, FcγRIII−/−, or FcγRI/RIII−/− recipient mice with CD20 (▬) or control (▭) mAb treatment (n = 4-7/group) as in panels A,B. Significant differences in mean survival of mice treated with CD20 or control mAbs as well as survival of CD20 mAb-treated mice are indicated (*P < .05; **P < .001). (D) Mouse survival following transfer of 105 BL3750 cells intravenously into FcRγ−/− mice with CD20 (•) or control (○) mAb treatment (n = 5/group). (E) Mouse survival following transfer of 107 BL3750 cells into wild-type (—, n = 5/group) or FcγRIIB−/− ( , n = 5/group) mice with CD20 (•) or control (○) mAb treatment. (F) Mouse survival following transfer of 105 BL3750 tumor cells subcutaneously on day 0 with CD20 (■ and •) or control (□ and ○) mAb (250 μg/mouse) given intraperitoneally on day 1 (downward
, n = 5/group) mice with CD20 (•) or control (○) mAb treatment. (F) Mouse survival following transfer of 105 BL3750 tumor cells subcutaneously on day 0 with CD20 (■ and •) or control (□ and ○) mAb (250 μg/mouse) given intraperitoneally on day 1 (downward  ). Mice (n = 4-8/group) were given clodronate-encapsulated (■ and □) or PBS-encapsulated (• and ○) liposomes intravenously on days − 1, 2, 5, and 9 (downward
). Mice (n = 4-8/group) were given clodronate-encapsulated (■ and □) or PBS-encapsulated (• and ○) liposomes intravenously on days − 1, 2, 5, and 9 (downward  ).
).
FcγR-bearing macrophages mediate lymphoma depletion by CD20 mAb in vivo. (A-E) Mouse survival following BL3750 cell transfer on day 0 with CD20 or control mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward  ). (A) Mouse survival following transfer of 104 BL3750 cells subcutaneously into FcRγ−/− (n = 5/group), FcγRI−/− (n = 4-5/group), FcγRIII−/− (n = 5/group), or FcγRI/RIII−/− (n = 7/group) recipient mice with CD20 (•) or control (○) mAb treatment. (B) Mouse survival following transfer of 106 BL3750 cells subcutaneously into FcRγ−/− (n = 5/group), FcγRI−/− (n = 5/group), FcγRIII−/− (n = 5/group), or FcγRI/RIII−/− (n = 4-5/group) recipient mice with CD20 (•) or control (○) mAb treatment. (C) Mouse survival at day 60 following transfer of 104 (left panel), 105 (middle panel), or 106 (right panel) BL3750 cells into wild-type, FcRγ−/−, FcγRI−/−, FcγRIII−/−, or FcγRI/RIII−/− recipient mice with CD20 (▬) or control (▭) mAb treatment (n = 4-7/group) as in panels A,B. Significant differences in mean survival of mice treated with CD20 or control mAbs as well as survival of CD20 mAb-treated mice are indicated (*P < .05; **P < .001). (D) Mouse survival following transfer of 105 BL3750 cells intravenously into FcRγ−/− mice with CD20 (•) or control (○) mAb treatment (n = 5/group). (E) Mouse survival following transfer of 107 BL3750 cells into wild-type (—, n = 5/group) or FcγRIIB−/− (
). (A) Mouse survival following transfer of 104 BL3750 cells subcutaneously into FcRγ−/− (n = 5/group), FcγRI−/− (n = 4-5/group), FcγRIII−/− (n = 5/group), or FcγRI/RIII−/− (n = 7/group) recipient mice with CD20 (•) or control (○) mAb treatment. (B) Mouse survival following transfer of 106 BL3750 cells subcutaneously into FcRγ−/− (n = 5/group), FcγRI−/− (n = 5/group), FcγRIII−/− (n = 5/group), or FcγRI/RIII−/− (n = 4-5/group) recipient mice with CD20 (•) or control (○) mAb treatment. (C) Mouse survival at day 60 following transfer of 104 (left panel), 105 (middle panel), or 106 (right panel) BL3750 cells into wild-type, FcRγ−/−, FcγRI−/−, FcγRIII−/−, or FcγRI/RIII−/− recipient mice with CD20 (▬) or control (▭) mAb treatment (n = 4-7/group) as in panels A,B. Significant differences in mean survival of mice treated with CD20 or control mAbs as well as survival of CD20 mAb-treated mice are indicated (*P < .05; **P < .001). (D) Mouse survival following transfer of 105 BL3750 cells intravenously into FcRγ−/− mice with CD20 (•) or control (○) mAb treatment (n = 5/group). (E) Mouse survival following transfer of 107 BL3750 cells into wild-type (—, n = 5/group) or FcγRIIB−/− ( , n = 5/group) mice with CD20 (•) or control (○) mAb treatment. (F) Mouse survival following transfer of 105 BL3750 tumor cells subcutaneously on day 0 with CD20 (■ and •) or control (□ and ○) mAb (250 μg/mouse) given intraperitoneally on day 1 (downward
, n = 5/group) mice with CD20 (•) or control (○) mAb treatment. (F) Mouse survival following transfer of 105 BL3750 tumor cells subcutaneously on day 0 with CD20 (■ and •) or control (□ and ○) mAb (250 μg/mouse) given intraperitoneally on day 1 (downward  ). Mice (n = 4-8/group) were given clodronate-encapsulated (■ and □) or PBS-encapsulated (• and ○) liposomes intravenously on days − 1, 2, 5, and 9 (downward
). Mice (n = 4-8/group) were given clodronate-encapsulated (■ and □) or PBS-encapsulated (• and ○) liposomes intravenously on days − 1, 2, 5, and 9 (downward  ).
).
When 104 BL3750 tumor cells were transferred into FcγRI−/−, FcγRIII−/−, or FcγRI−/−/RIII−/− mice, CD20 mAb treatment significantly improved survival (Figure 4A,C). FcγRI−/−/RIII−/− mice survived for a median of 34 days (range, 26-54 days) after control mAb treatment, whereas CD20 mAb treatment prevented leukemia/lymphoma in 86% of mice (P < .001; Figure 4A). CD20 mAb was less efficient at depleting tumor cells when 105 BL3750 cells were injected into FcγRIII−/− and FcγRI−/−/RIII−/− mice, while all FcγRI−/− and wild-type mice survived (Figure 4C; data not shown). Transfer of 105 BL3750 cells into FcγRI−/−/RIII−/− mice resulted in 100% tumor growth in control mAb-treated mice, but in only 50% of CD20 mAb-treated mice (P = .02). Transfer of 106 BL3750 cells into FcγRI−/−/RIII−/− mice resulted in tumor growth in all control and CD20 mAb-treated mice, with no significant difference in survival rates (Figure 4B). However, CD20 mAb-treated FcγRIII−/− mice that express both FcγRI and FcγRIV had a better survival rate than FcγRI−/−/RIII−/− mice that express only FcγRIV (P = .02; Figure 4B,C). Thus, FcγRIV, FcγRIII, and FcγRI cooperate to mediate optimal lymphoma depletion by CD20 mAb in vivo.
FcγRIIB inhibits lymphoma depletion in vivo
In contrast to FcγRs that stimulate effector cells, FcγRIIB contains cytoplasmic ITIM sequences that inhibit effector cell responses when engaged.38 Lymphoma depletion was assessed using FcγRIIB−/− and wild-type mice given 107 BL3750 cells with weekly mAb treatment for 4 weeks. Median survival for wild-type mice given control mAb was 18 days (range, 17-26 days), and the median survival of FcγRIIB−/− mice given control mAb was 22 days (range, 21-28 days; Figure 4E). By contrast, median survival for wild-type mice given CD20 mAb was 29 days (range, 24-42 days), whereas CD20 mAb treatment prevented tumor growth in 60% of FcγRIIB−/− mice (P = .01). Thereby, FcγRIIB deficiency prolonged mouse survival following CD20 mAb treatment.
Monocytes mediate lymphoma depletion in vivo
To determine the contribution of macrophages to lymphoma depletion, mice given BL3750 cells on day 0 were also treated with clodronate- or PBS-encapsulated liposomes on days −1, 2, 5, and 9. Survival rates of mice given control mAb were similar whether or not the mice were given clodronate- or PBS-liposomes (Figure 4F). CD20 mAb treatment prevented lymphoma development in 88% of BL3750 cell recipients given PBS-liposomes. By contrast, all mice given CD20 mAb plus clodronate-liposomes developed lymphomas (median survival, 32 days; range 21-37 days), which was significantly different (P = .001) from mice given CD20 mAb plus PBS-liposomes. Thus, clodronate-mediated depletion of macrophages neutralized the therapeutic benefit of CD20 mAb treatment.
C1q binding does not facilitate B-cell depletion in vivo
The role of complement in B-cell depletion by CD20 mAb was assessed using B6 and BUB mice. BUB mice have exceptionally potent complement hemolytic activity relative to common mouse strains, although serum C3 levels and complement opsonic activity appear similar.39 A mutant CD20 mAb, IgG2a MB20-18K322A, that does not bind C1q efficiently was also generated. Mutation of Lys322 to Ala reduces the affinity of IgG for C1q by at least 30-fold.32 Both MB20-18 and MB20-18K322A mAbs reacted uniformly with B220+ primary B cells from B6 and BUB mice in vitro (Figure 5A). In the presence of complement in vitro, MB20-18K322A mAb induced significantly less lysis of A20 cells compared with parental MB20-18 mAb (Figure 5B). The ability of the MB20-18 and MB20-18K322A mAbs to activate complement in vivo was also assessed by measuring serum C3 levels in B6 and BUB mice after injecting CD20 mAb. Serum C3 levels decreased transiently by 6 hours (B6) or 4 hours (BUB) after MB20-18 (20 μg) mAb injection (Figure 5C). By contrast, there was significantly less C3 consumption in response to MB20-18K322A mAb treatment. However, the MB20-18 and MB20-18K322A mAbs effectively depleted mature B cells in blood, spleen, lymph nodes, and bone marrow of both B6 and BUB mice even at relatively low mAb doses (Figure 5D). Thus, CD20 mAbs may induce complement activation in vivo, but normal B-cell depletion was induced through C1q-independent mechanisms.
CD20 mAb-mediated lymphoma depletion is complement independent in vivo. (A) Reactivity of CD20 mAbs with spleen B cells in B6 (left panel) and BUB (right panel) mice. Mean fluorescence intensity (MFI) of IgG2a MB20-18 (■) and MB20-18K322A (□) staining over a range of mAb concentrations. Staining was visualized using PE-conjugated isotype-specific secondary Abs with flow cytometric analysis. Results represent those obtained in 3 or more experiments. (B) In vitro complement-dependent cytotoxicity of A20 cells by MB20 mAbs. Values represent mean (± SEM) percentages of B220+ cells that were PI+ in 3 or more experiments. (C) Serum C3 levels in B6 and BUB mice determined by ELISA after injection of MB20-18 (■) or MB20-18K322A (□) mAb (downward  ). Results represent 2 different experiments with 4 mice for each group and strain. C3 values in CVF-treated mice are shown (▴, n = 3 mice) in the left panel. Significant differences between sample means are indicated (*P < .05; **P < .001). (D) MB20-18 (■) or MB20-18K322A (□) mAb depletion of B cells in B6 and BUB mice. Bone marrow (mature IgM+B220hi), blood (B220+), spleen (mature CD24+CD21+B220+), and peripheral lymph node (B220+) B-cell numbers were determined 7 days after mAb treatment at the indicated mAb doses. Values (± SEM) represent percentages of B cells present in mAb-treated mice relative to control mAb-treated littermates (≥ 2 mice per value). (E) B6 mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with MB20-18 (■), MB20-18K322A (□), or control (○) mAb (100 μg/mouse; n = 6 mice/group) given intravenously on day 1 (downward
). Results represent 2 different experiments with 4 mice for each group and strain. C3 values in CVF-treated mice are shown (▴, n = 3 mice) in the left panel. Significant differences between sample means are indicated (*P < .05; **P < .001). (D) MB20-18 (■) or MB20-18K322A (□) mAb depletion of B cells in B6 and BUB mice. Bone marrow (mature IgM+B220hi), blood (B220+), spleen (mature CD24+CD21+B220+), and peripheral lymph node (B220+) B-cell numbers were determined 7 days after mAb treatment at the indicated mAb doses. Values (± SEM) represent percentages of B cells present in mAb-treated mice relative to control mAb-treated littermates (≥ 2 mice per value). (E) B6 mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with MB20-18 (■), MB20-18K322A (□), or control (○) mAb (100 μg/mouse; n = 6 mice/group) given intravenously on day 1 (downward  ). (F) Mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with CD20 (MB20-11) or control mAb (250 μg/mouse) given intraperitoneally on day 1 (downward
). (F) Mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with CD20 (MB20-11) or control mAb (250 μg/mouse) given intraperitoneally on day 1 (downward  ). CVF or PBS was given on days 0, 3, 5, and 9 (downward
). CVF or PBS was given on days 0, 3, 5, and 9 (downward  ). Mice were treated with CVF and CD20 (■) or control (□) mAb. As controls, PBS was given along with CD20 (•) or control (○) mAb treatments (n = 5 mice/group).
). Mice were treated with CVF and CD20 (■) or control (□) mAb. As controls, PBS was given along with CD20 (•) or control (○) mAb treatments (n = 5 mice/group).
CD20 mAb-mediated lymphoma depletion is complement independent in vivo. (A) Reactivity of CD20 mAbs with spleen B cells in B6 (left panel) and BUB (right panel) mice. Mean fluorescence intensity (MFI) of IgG2a MB20-18 (■) and MB20-18K322A (□) staining over a range of mAb concentrations. Staining was visualized using PE-conjugated isotype-specific secondary Abs with flow cytometric analysis. Results represent those obtained in 3 or more experiments. (B) In vitro complement-dependent cytotoxicity of A20 cells by MB20 mAbs. Values represent mean (± SEM) percentages of B220+ cells that were PI+ in 3 or more experiments. (C) Serum C3 levels in B6 and BUB mice determined by ELISA after injection of MB20-18 (■) or MB20-18K322A (□) mAb (downward  ). Results represent 2 different experiments with 4 mice for each group and strain. C3 values in CVF-treated mice are shown (▴, n = 3 mice) in the left panel. Significant differences between sample means are indicated (*P < .05; **P < .001). (D) MB20-18 (■) or MB20-18K322A (□) mAb depletion of B cells in B6 and BUB mice. Bone marrow (mature IgM+B220hi), blood (B220+), spleen (mature CD24+CD21+B220+), and peripheral lymph node (B220+) B-cell numbers were determined 7 days after mAb treatment at the indicated mAb doses. Values (± SEM) represent percentages of B cells present in mAb-treated mice relative to control mAb-treated littermates (≥ 2 mice per value). (E) B6 mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with MB20-18 (■), MB20-18K322A (□), or control (○) mAb (100 μg/mouse; n = 6 mice/group) given intravenously on day 1 (downward
). Results represent 2 different experiments with 4 mice for each group and strain. C3 values in CVF-treated mice are shown (▴, n = 3 mice) in the left panel. Significant differences between sample means are indicated (*P < .05; **P < .001). (D) MB20-18 (■) or MB20-18K322A (□) mAb depletion of B cells in B6 and BUB mice. Bone marrow (mature IgM+B220hi), blood (B220+), spleen (mature CD24+CD21+B220+), and peripheral lymph node (B220+) B-cell numbers were determined 7 days after mAb treatment at the indicated mAb doses. Values (± SEM) represent percentages of B cells present in mAb-treated mice relative to control mAb-treated littermates (≥ 2 mice per value). (E) B6 mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with MB20-18 (■), MB20-18K322A (□), or control (○) mAb (100 μg/mouse; n = 6 mice/group) given intravenously on day 1 (downward  ). (F) Mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with CD20 (MB20-11) or control mAb (250 μg/mouse) given intraperitoneally on day 1 (downward
). (F) Mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with CD20 (MB20-11) or control mAb (250 μg/mouse) given intraperitoneally on day 1 (downward  ). CVF or PBS was given on days 0, 3, 5, and 9 (downward
). CVF or PBS was given on days 0, 3, 5, and 9 (downward  ). Mice were treated with CVF and CD20 (■) or control (□) mAb. As controls, PBS was given along with CD20 (•) or control (○) mAb treatments (n = 5 mice/group).
). Mice were treated with CVF and CD20 (■) or control (□) mAb. As controls, PBS was given along with CD20 (•) or control (○) mAb treatments (n = 5 mice/group).
Lymphoma depletion in vivo is complement independent
The ability of the MB20-18 and MB20-18K322A mAbs to deplete lymphomas in vivo was assessed in mice given BL3750 cells on day 0, with each mAb given on day 1 (100 μg). All control mAb-treated mice developed lymphomas and died (median survival, 28 days; range, 17-39 days; Figure 5E). By contrast, the MB20-18 and MB20-18K322A mAbs prevented lymphoma development in 75% and 88% of recipients, respectively. Thus, the MB20-18 and MB20-18K322A mAbs depleted lymphoma cells equally in vivo.
The role of complement in CD20 mAb-mediated lymphoma depletion was also assessed using CVF to deplete C3 in vivo.40 Mice given BL3750 cells on day 0 were treated with CVF or PBS on days 0, 3, 5, and 9. Within 24 hours after CVF injection, C3 levels decreased dramatically in sera of CVF-treated mice for at least 3 days (Figure 5C; data not shown). Mice treated with control mAb either with or without CVF had similar survival rates (Figure 5F). By contrast, CD20 mAb treatment alone or with CVF treatment prevented leukemia/lymphoma in 80% of recipients, with no significant difference in survival (Figure 5F). Thus, the ability of CD20 mAbs to activate complement did not enhance tumor clearance in vivo.
Discussion
Although multiple mechanisms have been proposed for CD20 mAb depletion of tumors in vivo, the present findings using a new mouse lymphoma model (Figures 1,2) demonstrate that FcγR-bearing macrophages are necessary and sufficient to mediate this process (Figure 4). The protective activity of CD20 immunotherapy was abolished in FcγR-deficient or macrophage-depleted lymphoma-bearing mice (Figure 4A-C,F). Consistent with this, the innate immune system also eliminates normal B cells after CD20 mAb therapy in mice.19,22 For lymphoma depletion, high-affinity FcγRI, low-affinity FcγRIII, and intermediate-affinity FcγRIV each contributed to lymphoma depletion when an IgG2c CD20 mAb was used (Figure 4). Likewise, normal B-cell depletion correlates closely with CD20 mAb isotype, with IgG2a/c mAbs exhibiting the greatest potency due to their ability to engage FcγRI, FcγRIII, and FcγRIV.19,20 Thus, macrophage FcγR expression is sufficient for both normal and malignant cell depletion in vivo during CD20 immunotherapy.
Lymphoma studies of CD20 mAbs in vivo have predominantly relied on xenograft models using severe combined immunodeficient (SCID) or irradiated nude mice with altered innate or adaptive immunity.18,23,25 The variability among published results with cell lines may result in part from their heterogeneity, transformed nature, and histories of long-term culture. Similarly, mouse mature CD20+ A20 lymphoma cells were resistant to CD20 mAb treatment in vivo (Figure 1A). Mechanisms of lymphoma resistance to CD20 immunotherapy are unknown.4 Since it is not logistically feasible to systematically study the effects of CD20 mAb treatment on spontaneous lymphomas in controlled experiments, a primary c-Myc–induced lymphoma was isolated, expanded minimally in culture, and used to determine how CD20 mAbs deplete lymphomas in healthy mice. CD20 mAb treatment efficiently prevented tumor growth, leukemia, and death in B6 mice (Figure 1). Lymphoma depletion was highly reproducible, and was dependent on the number of transplanted tumor cells, the CD20 mAb dose, and schedule of tumor and mAb injections (Figure 2).
FcγR expression was required for CD20 mAb-induced BL3750 lymphoma depletion in vivo (Figure 4). CD20 mAb lymphomacidal activity was absent in FcRγ−/− mice (Figure 4A-C) that lack FcγRI, FcγRIII, and FcγRIV. Moreover, FcγRI, FcγRIII, and FcγRIV were each involved, with cooperative interactions mediating optimal CD20 mAb-dependent lymphoma depletion (Figure 4C). FcγRIV is the mouse ortholog of primate FcγRIIIa.41,42 At a low tumor dose, FcγRIV could mediate effective lymphoma depletion in the absence of FcγRI and/or FcγRIII expression (Figure 4A). However, FcγRI, FcγRIII, and FcγRIV expression were all important for lymphoma depletion as the tumor burden increased, with FcγRIII appearing to contribute more than FcγRI when CD20 mAb was given at optimal concentrations and the lymphoma cells were most likely saturated for CD20 mAb binding (Figure 3B). The role for FcγRI is likely to increase under conditions where CD20 mAb is limiting, as demonstrated for normal B-cell depletion by low-dose CD20 mAb in vivo.20 Thereby, high-affinity FcγRI binding to normal serum IgG2a/c does not prevent its participation in lymphoma killing in cooperation with FcγRIII and FcγRIV. Consistent with these mouse studies, the effectiveness of CD20 mAb therapy in lupus and lymphoma patients also correlates with human FcγRIIa and FcγRIIIa polymorphisms.43,44 A human FcγRIIIa polymorphism that confers higher affinity for human IgG1 is associated with increased follicular lymphoma responses when rituximab is used alone.43,44 These improved clinical outcomes may be due to increased CD16 expression, rituximab binding, and rituximab-mediated ADCC.45 In this context, FcγRIIB deficiency significantly enhanced lymphoma killing by CD20 mAb in vivo (Figure 4E), as previously described for normal mouse B cells20 and nude mice given human lymphoma xenografts.18 Circumventing monocyte inhibitory FcγRII function in vivo is likely to result in more effective immunotherapies, particularly when CD20 mAb levels, target CD20 molecules, or localized effector cell numbers are low. Thus, FcγR function is likely to be the most critical factor regulating lymphoma depletion in both humans and mice.
CD20 mAb lymphomacidal activity was absent in macrophage-depleted mice (Figure 4F). Macrophages are also crucial for normal B-cell depletion in wild-type mice,19 for B-cell depletion in human CD20-transgenic mice,22 for human lymphoma xenografts in nude mice,25 and for CD19 mAb-mediated B-cell depletion in human CD19-transgenic mice.46 Macrophage-mediated lymphoma depletion during CD20 immunotherapy has far-reaching clinical implications that may also be applicable to other cell-directed mAb therapies. For example, augmenting monocyte numbers or function may increase CD20 mAb effectiveness in vivo.47,48 Thereby, high rates of failure for CD20 immunotherapy may in some cases result from concomitant myelosuppression or reduced numbers of tissue monocytes. Since monocytes provide a fundamental and essential mechanism for malignant B-cell depletion, there is a critical need for monitoring monocyte numbers and function in patients with lymphoma undergoing CD20 and other mAb-based therapies.
Complement did not appear to contribute to BL3750 lymphomacidal activity in vivo even though the MB20-11 mAb efficiently activates complement in vitro19 and binds C1q (not shown). These findings parallel previous studies in mice with C3, C4, or C1q deficiencies where CD20 mAb depleted normal B cells effectively.19 Furthermore, a mutant CD20 mAb with reduced C1q binding depleted normal blood, spleen and bone marrow B cells (Figure 5D), and lymphoma cells (Figure 5E) as efficiently as the parental mAb, even at low mAb concentrations in vivo. This mutation significantly reduced the ability of MB20-11 mAb to induce A20 cell lysis in vitro in the presence of complement (Figure 5B). Furthermore, only the C1q-binding MB20-18 mAb depleted serum C3 levels in B6 and BUB mice after administration (Figure 5C). Although these are the first studies assessing complement levels in mice after CD20 mAb injection, rituximab injection can lead to rapid complement activation in patients, and may play a key role in the side effects associated with rituximab treatment.13,49 Thereby, CD20 mAbs may activate complement in vivo and induce CDC in vitro, but complement was not necessary for CD20 mAb-mediated BL3750 lymphoma depletion in vivo.
The current studies contrast with studies proposing CDC as the central effector mechanism for rituximab.11,24,25 Indeed, the protective activities of rituximab (human IgG1 chimera) and the 1F5 (mouse IgG2a) antihuman CD20 mAbs are reported to be absent in C1q−/− mice but conserved in a mouse tumor model after natural killer (NK) cell and neutrophil depletion.11 Complement, but not NK cells, neutrophils, or macrophages, was also required for rituximab therapy in mice given human CD20-expressing mouse 38C13 B-lymphoma cells subcutaneously or within lymph nodes.24 Although simple explanations for these different results are not obvious, it remains possible that complement activation facilitates but does not induce lymphoma cell depletion. In fact, a recent study using the BJAB xenograft tumor model proposed that rituximab rapidly activates complement and induces β-chemokines in vivo, thereby activating the innate immune network.25 The fine specificities of different antihuman CD20 mAbs may also influence their in vivo effects.10,23 For example, complement activation by rituximab and the 1F5 mAb is critical for lymphoma therapy in xenotransplantation models, whereas the B1 (IgG2a) CD20 mAb activates complement in vitro50 but does not depend on complement activation for therapeutic benefit in lymphoma xenotransplantation studies.23 The link between complement activation and β-chemokine induction remains to be elucidated, as well as why the innate immune network was required in the current and other studies,25 whereas some studies did not require NK cells, neutrophils, or macrophages for CD20 mAb efficiency.11,24 Regardless, the current study using a completely homologous experimental system in normal mice failed to reveal a role for complement in lymphoma depletion following CD20 mAb treatment.
This study reinforces the importance of monocytes in lymphoma depletion and the importance of maintaining circulating CD20 mAb levels to allow effective lymphoma depletion. Moreover, these results validate the concept that enhancing therapeutic mAb interactions with monocyte FcγRs may enhance ADCC in vivo. The homologous lymphoma model described in the current study also provides a preclinical model for evaluating treatment modalities and testing therapeutic strategies in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Paul Chadwick who performed pathology studies and all members of the Department of Immunology who provided expert technical support and valuable discussions.
This work was supported by grants from the National Institutes of Health (NIH, Bethesda, MD; CA105001, CA96547, and AI56363), La Fondation de France (Paris, France), and La Federation Nationale des Centres de Lutte Contre le Cancer (Paris, France; V.M.-C.).
National Institutes of Health
Authorship
Contribution: V.M.-C. and Y.X. designed and conducted research, analyzed data, and wrote the paper; M.H., C.M.M., and Y.H. conducted research; J.C.P. and K.M.H. contributed to the design of research; and T.F.T. designed research and wrote the paper.
Conflict-of-interest disclosure: T.F.T. is a paid consultant for MedImmune, Inc. and Angelica Therapeutics, Inc. J.C.P. and K.M.H are paid consultants for Angelica Therapeutics, Inc. The remaining authors declare no competing financial interests.
Correspondence: Thomas F. Tedder, Box 3010, Department of Immunology, Rm 353 Jones Bldg, Research Dr, Duke University Medical Center, Durham, NC 27710; e-mail thomas.tedder@duke.edu.
References
Author notes
*V.M.-C. and Y.X. contributed equally to these studies and share first authorship.








 ). (A) Tumor volumes (± SEM) for CD20 or control mAb-treated mice. (B) Representative control mAb-treated mice exhibited tumors on their backs 5 weeks after BL3750 cell transfer, whereas tumors were not detectable in CD20 mAb-treated mice. (C) Circulating white blood cell numbers (± SEM) for CD20 or control mAb-treated mice. (A,C) Significant differences between sample means are indicated (*P < .05; **P < .001). (D) Circulating CD19+B220+ cells in representative mice 5 weeks after transfer of BL3750 tumor cells and CD20 or control mAb treatment. The percentages indicate relative frequencies of cells within the indicated gates. (E) Mouse tumor-free and overall survival rates following BL3750 cell transfers. (F) Mice were given 105 BL3750 cells intravenously on day 0 with CD20 (•, n = 5) or control (○, n = 5) mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward
). (A) Tumor volumes (± SEM) for CD20 or control mAb-treated mice. (B) Representative control mAb-treated mice exhibited tumors on their backs 5 weeks after BL3750 cell transfer, whereas tumors were not detectable in CD20 mAb-treated mice. (C) Circulating white blood cell numbers (± SEM) for CD20 or control mAb-treated mice. (A,C) Significant differences between sample means are indicated (*P < .05; **P < .001). (D) Circulating CD19+B220+ cells in representative mice 5 weeks after transfer of BL3750 tumor cells and CD20 or control mAb treatment. The percentages indicate relative frequencies of cells within the indicated gates. (E) Mouse tumor-free and overall survival rates following BL3750 cell transfers. (F) Mice were given 105 BL3750 cells intravenously on day 0 with CD20 (•, n = 5) or control (○, n = 5) mAb (250 μg/mouse) given intravenously on days 1, 7, 14, and 21 (downward 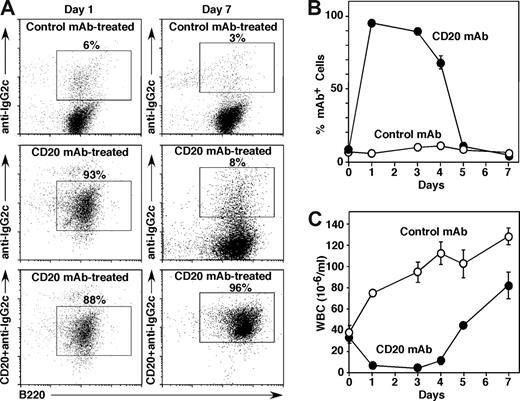
 , n = 5/group) mice with CD20 (•) or control (○) mAb treatment. (F) Mouse survival following transfer of 105 BL3750 tumor cells subcutaneously on day 0 with CD20 (■ and •) or control (□ and ○) mAb (250 μg/mouse) given intraperitoneally on day 1 (downward
, n = 5/group) mice with CD20 (•) or control (○) mAb treatment. (F) Mouse survival following transfer of 105 BL3750 tumor cells subcutaneously on day 0 with CD20 (■ and •) or control (□ and ○) mAb (250 μg/mouse) given intraperitoneally on day 1 (downward 
 ). Results represent 2 different experiments with 4 mice for each group and strain. C3 values in CVF-treated mice are shown (▴, n = 3 mice) in the left panel. Significant differences between sample means are indicated (*P < .05; **P < .001). (D) MB20-18 (■) or MB20-18K322A (□) mAb depletion of B cells in B6 and BUB mice. Bone marrow (mature IgM+B220hi), blood (B220+), spleen (mature CD24+CD21+B220+), and peripheral lymph node (B220+) B-cell numbers were determined 7 days after mAb treatment at the indicated mAb doses. Values (± SEM) represent percentages of B cells present in mAb-treated mice relative to control mAb-treated littermates (≥ 2 mice per value). (E) B6 mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with MB20-18 (■), MB20-18K322A (□), or control (○) mAb (100 μg/mouse; n = 6 mice/group) given intravenously on day 1 (downward
). Results represent 2 different experiments with 4 mice for each group and strain. C3 values in CVF-treated mice are shown (▴, n = 3 mice) in the left panel. Significant differences between sample means are indicated (*P < .05; **P < .001). (D) MB20-18 (■) or MB20-18K322A (□) mAb depletion of B cells in B6 and BUB mice. Bone marrow (mature IgM+B220hi), blood (B220+), spleen (mature CD24+CD21+B220+), and peripheral lymph node (B220+) B-cell numbers were determined 7 days after mAb treatment at the indicated mAb doses. Values (± SEM) represent percentages of B cells present in mAb-treated mice relative to control mAb-treated littermates (≥ 2 mice per value). (E) B6 mouse survival following transfer of 105 BL3750 cells subcutaneously on day 0 with MB20-18 (■), MB20-18K322A (□), or control (○) mAb (100 μg/mouse; n = 6 mice/group) given intravenously on day 1 (downward