Abstract
VAF347 is a low-molecular-weight compound, which activates the aryl hydrocarbon receptor (AhR). Herein, we report that oral administration of a water-soluble derivative of VAF347 (VAG539) promotes long-term graft acceptance and active tolerance in Balb/c mice that receive a transplant of MHC-mismatched pancreatic islet allografts. In vivo VAG539 treatment results in increased frequency of splenic CD4+ T cells expressing CD25 and Foxp3, markers associated with regulatory T (Tr) cells, and in vitro VAF347 treatment of splenic CD4+ T cells improved CD4+CD25+Foxp3+ T-cell survival. Interestingly, transfer of CD11c+ dendritic cells (DCs), but not of CD4+ T or CD19+ B cells, from VAG539-treated long-term tolerant hosts into mice that recently underwent transplantation resulted in donor (C57Bl/6)–specific graft acceptance and in a significantly higher frequency of splenic CD4+CD25+Foxp3+ Tr cells. Furthermore, the transfer of CD4+CD25+ T cells from these mice into mice that recently underwent transplantation promoted graft acceptance. Similarly, cell therapy with in vitro VAF347-treated bone marrow–derived mature DCs prevented islet graft rejection, and reduced OVA-specific T-cell responses in OVA-immunized mice. Collectively, our data indicate that AhR activation induces islet allograft–specific tolerance through direct as well as DC-mediated effects on Tr-cell survival and function.
Introduction
Dendritic cells (DCs) induce and regulate adaptive immune responses by presenting antigens in an immunogenic or tolerogenic context.1-3 The capacity of DCs to induce specific effector functions in naive T cells designates them as attractive means to stimulate beneficial and suppress detrimental immune responses.4-6 However, specific in vivo modulation of DC function is a complex task because DCs encounter and respond to numerous stimuli, and may modify their function accordingly.7 Various factors have been suggested to render DCs tolerogenic by enhancing their capacity to promote the induction and/or activation of regulatory T (Tr) cells.3,6,8,9
Antigen presentation in the absence of costimulation can result in clonal deletion, or in the induction of an anergic and suppressive T-cell phenotype.10 Indeed, both in human tissue culture and in mice, DCs have been shown to expand IL-10–producing type 1 (Tr1) regulatory T (Tr) cells.5,11,12 Furthermore, specific DC subsets, such as plasmacytoid DCs and CD8+ splenic DCs, have been shown to selectively expand CD4+CD25+ Tr cells.13-16 Recent evidence also suggests that LPS-activated mature DCs are capable of expanding CD4+CD25+ Tr cells as well.3,17 Conversely, in inflammatory conditions, DCs present antigens to effector T cells in the context of proinflammatory cytokines and costimulatory surface molecules, and initiate adaptive immune responses by promoting activation and proliferation of antigen-specific naive T cells.1,4 In the context of organ transplantation, it has been demonstrated that activated DCs indeed initiate immune responses toward allogeneic antigens, which result in allograft rejection.18 However, the nature of the maturation signal that activates DCs to initiate an allogeneic immune response remains unclear.19,20 Several preclinical strategies for the prevention of allograft rejection target DCs, in an attempt to abrogate costimulation and T-cell activation, and to promote the expansion of alloantigen-specific Tr cells.21-23
Islet transplantation offers patients with type 1 diabetes mellitus freedom from long-term insulin therapy and a degree of metabolic control that is far superior to injected insulin.24 However, the therapeutic efficacy of this strategy is compromised by the necessity to use robust immunosuppressive regimens to prevent islet allograft rejection.25,26 Therefore, a significant amount of research effort is presently dedicated to the development of novel strategies for the induction of islet donor–specific tolerance.27
VAF347 is a low-molecular-weight compound that has been shown to modify immune responses through a dual mode of action, namely inhibition of DC-mediated T-cell proliferation and cytokine production and isotype-specific inhibition of IgE class switching by human B lymphocytes.28 In vitro, this compound has been demonstrated to act as DC modulator by blocking the capacity of human DCs to stimulate T cells, possibly by inhibiting the expression of CD86, HLA-DR, and IL-6 by human DCs. In a mouse model of allergic lung inflammation, VAF347 inhibited tissue eosinophilia, mucus hyperplasia, and serum IgE production, likely through its effects on DCs.28 Recently, Lawrence et al reported the identification of AhR as the molecular target of VAF347 as well as its critical involvement in the immunomodulatory mode of action of the compound in myeloid cells.29 Interestingly, AhR activation has been previously shown to promote the generation of Tr cells.30 Furthermore, different AhR agonists had been shown to modulate the balance between Tr cells and IL-17–producing Th17 cells.31
Here we investigated the tolerogenic potential of VAF347 and VAG539 (a close derivative that is efficiently converted into VAF347 in vivo). Using a murine model of pancreatic islets allotransplantation, we demonstrate that VAF347/VAG539 can induce long-term active tolerance by a direct effect on Tr-cell survival and function, as well as through DC-mediated induction of Tr cells.
Methods
Low-molecular-weight compound
VAF347 ([4-(3-chloro-phenyl)-pyrimidin-2-yl]-(4-trifluoromethyl-phenyl)-amine) was dissolved in DMSO and added to the cell culture medium. The close derivative VAG539 ([4-(3-chloro-phenyl)-pyrimidin-2-yl]-(4-trifluoromethyl-phenyl)-carbamic acid 2-[(2-hydroxy-ethyl)-methyl-amino]-ethyl ester), which is effectively converted into VAF347 in vivo, was dissolved in saline prior to oral administration.
In vivo experiments
Balb/c and C57Bl/6 mice were purchased from Charles River (Calco, Italy). All mice were kept under specific pathogen-free conditions. Glucose levels in the tail venous blood were quantified using the Glucometer Elite system (Bayer, Wuppertal, Germany) and were always measured in the morning. Diabetes was induced in Balb/c female mice by intravenous injection of streptozotocin (Sigma-Aldrich, St Louis, MO) at 170 mg/kg. A diagnosis of diabetes was made after 2 sequential glucose measurements of less than 13.8 mM (250 mg/dL). All animal care procedures were performed according to protocols approved by the San Raffaele Hospital Institutional Animal Care and Use Committee (IACUC no. 255). After being cultured overnight at 37°C, handpicked C57Bl/6 pancreatic islets were transplanted (300 islets/mouse) under the kidney capsule of recipient diabetic mice. The treatment of Balb/c mice that underwent transplantation began the day after transplantation. VAG539 was diluted in saline and administered orally once daily for 30 consecutive days at a dose of 30 mg/kg or 7.5 mg/kg by gavage. Recipient mice that did not reject the allograft 100 days after transplantation were boosted with donor splenocytes (3 × 107 intraperitoneally).
Immunohistochemical analysis
Tissues were collected 10 days after transplantation. Formalin-fixed, paraffin-embedded samples were cut at 3-μm– to 5-μm–thick sections and stained with hematoxylin and eosin (H&E), or with the indicated monoclonal antibody (BD Biosciences, San Jose, CA). Slides were analyzed by bright-field Axioskop 2 plus microscope, and images were automatically captured using the AxioCam HRc system with Axiovision 3.1 version 4.4 (all products were products from Zeiss, Oberkochen, Germany). Images shown in Figure 1B were taken with a 5×/0.12 NA objective.
Cell purification
Total spleen cells were incubated with collagenase D for 30 minutes at 37°C to obtain a unicellular solution. Magnetic microbeads (for CD11c+, CD4+, CD25+, and CD90+ cells in different experiments) and Macs columns were used according to the manufacturer's instructions (Miltenyi Biotech, Bergisch Gladbach, Germany). Purity was confirmed by flow cytometry using conjugated monoclonal antibodies and was typically 90% to 95%.
Bone marrow–derived DC differentiation
Balb/c bone marrow cells were differentiated into bone marrow–derived DCs (BMDCs) by 6-day culture in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF, 25 ng/mL; R&D Systems, Minneapolis, MN) with or without VAF347 (20 nM). On day 6, some of the cells were activated with LPS (1 μg/mL; Sigma-Aldrich) for 8 hours. BMDCs were injected intravenously (3 × 105) into diabetic Balb/c female 1 day prior to islet allograft transplantation. To evaluate their stimulatory capacity, irradiated BMDCs were used as stimulators for responder C57Bl/6 splenocytes (SPCs) (R/S = 10:1). 3H-thymidine was added after 96 hours of culture for an additional 18 hours.
Tr-cell survival
CD4+ T cells were purified from Balb/c spleen by positive selection using anti-CD4 beads (Miltenyi Biotech), according to the manufacture's instructions. CD4+ T cells were then cultured with or without the presence of VAF347 (20 nM or 100 nM). After 5 days of culture, cells were harvested, washed, and stained with anti-CD4, anti-CD25, directly coupled with PE-conjugated anti-CD4 mAb and PerCP-conjugated anti-CD25 mAb (BD Biosciences). Expression of Foxp3 was determined by intracellular staining with FITC-conjugated anti-Foxp3 mAb (eBioscience, San Diego, CA), following the manufacturer's instructions.
Immunization
Mice were immunized with ovalbumin (Sigma-Aldrich) emulsified in mycobacterium butiricum (final concentration, 1 mg/mL) in the footpads. Seven days later, draining lymph nodes were excised, and cells were analyzed as indicated.
Flow cytometry
Cells were stained with the indicated mAbs (all from BD Biosciences) and analyzed with a fluorescence-activated cell-sorting (FACS) scan equipped with CellQuest software (BD Biosciences). For Foxp3 fluorocytometric analysis, staining of surface markers (CD4-FITC and CD25-PE) was followed by fixation, permeabilization, and an intracellular staining with allophycocyanin (APC)-conjugated anti-Fox3 mAb according to the manufacturer's instructions (eBioscience).
Response to donor cells
Spleen cells from VAG-DC–treated and control mice were stimulated in vitro with irradiated purified C57Bl/6 CD90− APCs. Cytokines released in culture media (96 hours) were quantified by a flow cytometry–based assay according to the manufacturer's instructions (BD Biosciences).
Statistical analysis
Groups of mice were compared according to the day of transplant rejection by a 2-tailed Student t test.
Results
Oral administration of VAG539 promotes long-term islet allograft acceptance
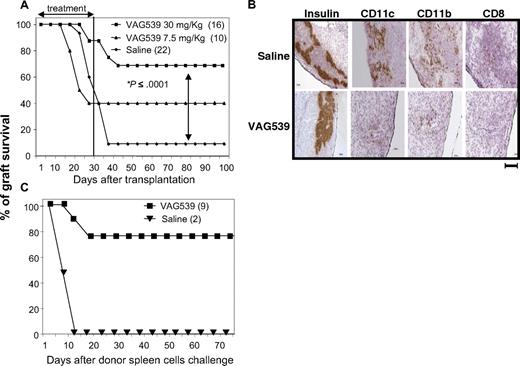
Recipient Balb/c mice rendered diabetic by streptozotocin received a transplant under the kidney capsule with pancreatic islets isolated from C57Bl/6 mice. Daily oral administration of VAG539 at a dose of 30 mg/kg from day 1 to day 30 after transplantation resulted in long-term islet allograft survival in 69% of the recipients (Figure 1A). A lower dose of VAG539 (7.5 mg/kg) was less effective in preventing graft rejection (40%), suggesting a dose-response effect of the compound (Figure 1A). To confirm that normoglycemia was indeed due to the transplanted islets, rather than due to recovery of native pancreas function, islet-bearing kidneys were removed from 3 long-term recipients. Nephrectomized mice became hyperglycemic within 24 hours (data not shown). To determine the effect of VAG539 on the infiltration of immune cells into the transplant, we performed a histologic analysis of the graft 10 days after transplantation. VAG539 treatment reduced graft infiltration as demonstrated by immunohistochemical analysis of CD11c+, CD11b+, and CD8+ cells in the transplant area, which is outlined by staining for insulin-producing cells (Figure 1B).
Inhibition of immune cell infiltration and induction of active tolerance by the low-molecular-weight compound VAG539. (A) Diabetic Balb/c female mice received a transplant of pancreatic islets isolated from C57Bl/6 allogeneic donors and were treated orally with VAG539 from day 1 to day 30 after transplantation (at 30 or 7.5 mg/kg per day). Graft survival was monitored by weekly measurement of blood glucose level. (B) Balb/c recipient mice received a transplant of C57Bl/6 islets and were treated orally with saline or VAG539 (30 mg/kg per day). Ten days after transplantation, kidneys were extracted, cryosectioned at the transplant area, and stained for the indicated markers. Sections from treated mice display reduced infiltration of DCs and CD8+ T cells into the transplant area (bar represents 100 μm). (C) VAG539 (30 mg/kg)–treated and control mice with long-term graft survival, shown in panel A, were challenged with fresh donor-type splenocytes (3 × 107 intraperitoneally). Graft survival was monitored by measurement of blood glucose level (see also Table 1).
Inhibition of immune cell infiltration and induction of active tolerance by the low-molecular-weight compound VAG539. (A) Diabetic Balb/c female mice received a transplant of pancreatic islets isolated from C57Bl/6 allogeneic donors and were treated orally with VAG539 from day 1 to day 30 after transplantation (at 30 or 7.5 mg/kg per day). Graft survival was monitored by weekly measurement of blood glucose level. (B) Balb/c recipient mice received a transplant of C57Bl/6 islets and were treated orally with saline or VAG539 (30 mg/kg per day). Ten days after transplantation, kidneys were extracted, cryosectioned at the transplant area, and stained for the indicated markers. Sections from treated mice display reduced infiltration of DCs and CD8+ T cells into the transplant area (bar represents 100 μm). (C) VAG539 (30 mg/kg)–treated and control mice with long-term graft survival, shown in panel A, were challenged with fresh donor-type splenocytes (3 × 107 intraperitoneally). Graft survival was monitored by measurement of blood glucose level (see also Table 1).
To test whether the compound has a direct effect on the capacity of the islets to withstand immune-mediated damage, we cultured islets for 24 hours in the presence of VAF347 (20 nM) prior to transplantation into mice. This treatment had no effect on graft survival (data not shown). To determine whether long-term graft survival was associated with active tolerance, mice were injected more than 100 days after transplantation (more than 70 days after VAG539 treatment was stopped) with 3 × 107 donor-type splenocytes. Seven (78%) of 9 treated recipients displayed resistance to this challenge by maintaining normal blood glucose levels (Figure 1C; Table 1), indicating that VAG539-treated mice developed immune tolerance.
Oral administration of VAG539 results in induction of active tolerance
| Treatment, days 0-30 . | No rejection (%) . | No rejection after challenge (%) . |
|---|---|---|
| VAG539, 30 mg/kg per d | 11/16 (69) | 7/9 (78) |
| Saline | 2/22 (9) | 0/2 (0) |
| Treatment, days 0-30 . | No rejection (%) . | No rejection after challenge (%) . |
|---|---|---|
| VAG539, 30 mg/kg per d | 11/16 (69) | 7/9 (78) |
| Saline | 2/22 (9) | 0/2 (0) |
Diabetic Balb/c mice received a transplant of C57B1/6 pancreatic islets and were treated orally with VAG539 or with saline. Mice that remained normoglycemic for more than 100 days were injected intraperitoneally with 3 × 107 C57B1/6 splenocytes.
VAG539 treatment induces antigen-specific unresponsiveness
We next investigated whether treatment with VAG539 imposes a nonspecific immunosuppressive effect. To this end, Balb/c mice were immunized with ovalbumin (OVA) and treated daily with VAG539 (30 mg/kg by mouth) or with saline. Analysis of VAG539-treated mice 7 days after immunization demonstrated a significantly lower number of cells in draining lymph nodes compared with saline-treated mice (16 × 106 ± 3 × 106 vs 27 × 106 ± 4 × 106; 2-tailed t test: P < .003). In addition, although the frequency of DCs, B cells, and T cells in VAG539-treated and saline-treated mice was comparable, the expression of CD80 by CD11c+ DCs was significantly lower in VAG539-treated mice compared with saline-treated mice (Figure 2A). Importantly, draining lymph node cells isolated from VAG539-treated mice elicited a significantly lower proliferation in response to OVA compared with cells isolated from saline-treated mice, whereas their ability to proliferate in response to allogeneic stimulators or to Con A was comparable (Figure 2B). These findings suggest that the treatment with VAG539 results in specific inhibition of the ongoing immune response by modulation of APCs, and does not impose an overall effect on the capacity of T cells to proliferate.
VAG539 treatment induces antigen-specific unresponsiveness. VAG539- or saline-treated Balb/c mice (n = 3) were immunized with OVA in CFA. (A) Draining lymph node cells were analyzed 7 days after immunization for expression of CD80 and CD86 by CD11c+ DCs (2-tailed t test: *P < .05). (B) Seven days after immunization, draining lymph node cells were stimulated with irradiated C57Bl/6 CD90− antigen-presenting cells (1:1 ratio; proliferation of stimulator cells alone was < 200 cpm), with Con A (1.25 μg/mL) or with OVA at the indicated concentrations. After 3 days of culture, 1 μCi (0.037 MBq)/well 3H-thymidine was added for additional 16 hours (2-tailed t tests: *P < .05). Error bars represent standard deviation between different mice (n = 3).
VAG539 treatment induces antigen-specific unresponsiveness. VAG539- or saline-treated Balb/c mice (n = 3) were immunized with OVA in CFA. (A) Draining lymph node cells were analyzed 7 days after immunization for expression of CD80 and CD86 by CD11c+ DCs (2-tailed t test: *P < .05). (B) Seven days after immunization, draining lymph node cells were stimulated with irradiated C57Bl/6 CD90− antigen-presenting cells (1:1 ratio; proliferation of stimulator cells alone was < 200 cpm), with Con A (1.25 μg/mL) or with OVA at the indicated concentrations. After 3 days of culture, 1 μCi (0.037 MBq)/well 3H-thymidine was added for additional 16 hours (2-tailed t tests: *P < .05). Error bars represent standard deviation between different mice (n = 3).
VAG539-induced tolerance is transferable by CD11c+ DCs
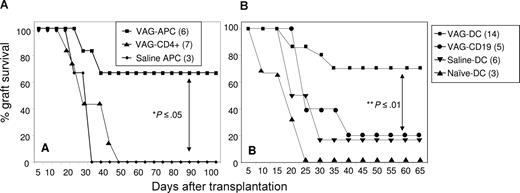
To identify the cell subset that is regulated by orally administered VAG539 and consequently mediates the induction of long-term active tolerance, we purified and transferred different cell subsets from spleens of VAG539-treated mice into Balb/c recipient mice that recently underwent transplantation. Cells isolated from mice that underwent transplantation more than 60 days after transplantation, and therefore more than 30 days after VAG539 withdrawal, were injected intravenously into mice that recently underwent transplantation. As controls, we purified and transferred the same cell subsets from saline-treated littermates, which rejected their transplants, or from age-matched naive Balb/c mice. Transfer of total spleen cells (2 × 107) from VAG539-treated long-term recipients into mice that recently underwent transplantation resulted in a slight prolongation in graft survival. As expected, spleen cells from naive animals had no protective effect (data not shown). Next, we transferred T cell–depleted (CD90−) APCs (5 × 106) or CD4+ T cells (3 × 106) into mice that recently underwent transplantation. Interestingly, 70% of the recipients that received APCs from VAG539-treated hosts (VAG-APC) displayed long-term graft survival, whereas transfer of APCs from control mice (saline APCs) or total CD4+ T cells from VAG539-treated mice did not have any effect on graft survival (Figure 3A). These data indicate that VAG539 induces transferable active tolerance through induction of tolerogenic APCs. CD90− spleen cells contain different APC subsets, among which B cells are of highest abundance (∼ 80%; not shown), whereas CD11c+ DCs represent approximately 3%. B cells have been demonstrated in some cases to promote the induction of active tolerance.32-34 Moreover, VAF347 has been demonstrated to modulate both B cells and DCs.8 Therefore, we investigated whether B cells or DCs could transfer transplantation tolerance. To this end, purified CD19+ B cells (VAG-CD19, 5 × 106) or CD11c+ DCs (VAG-DCs, 3 × 105) from the same VAG539-treated hosts were transferred into Balb/c mice that recently underwent transplantation. As controls, we transferred purified CD11c+ DCs from saline-treated littermates, which rejected their transplants (saline-DCs), and from naive mice (naive-DCs). Interestingly, transfer of VAG-DCs resulted in long-term graft acceptance in 70% of mice that underwent transplantation, whereas transfer of VAG-CD19 cells was not effective (Figure 3B). Together, these data suggest that CD11c+ DCs mediate the tolerogenic effect of VAG539.
Transfer of DCs from VAG539-tolerized mice inhibits allograft rejection. (A) CD90− antigen-presenting cells (VAG-APCs) or CD4+ T cells (VAG-CD4+) were purified from spleens of VAG539-treated graft-accepting mice, and CD90− antigen-presenting cells (saline APCs) were purified from spleens of saline-injected graft-rejecting mice 60 days after transplantation. Cells were injected (intravenously, 5 × 106 APCs or 3 × 106 T cells) into naive mice 1 day prior to islet transplantation. (B) CD11+ (VAG-DCs) or CD19+ (VAG-CD19) cells were purified from spleens of VAG539-treated graft-accepting mice 60 days after transplantation. As control, CD11c+ DCs were purified from spleens of saline-injected graft-rejecting mice 60 days after transplantation (saline-DCs) or of naive BALB/c mice (naive-DCs). Purified CD11c+ DCs (3 × 105) or CD19+ B cells (5 × 106) were injected intravenously 1 day before islet transplantation (2-tailed t test: **P < .01; *P < .05).
Transfer of DCs from VAG539-tolerized mice inhibits allograft rejection. (A) CD90− antigen-presenting cells (VAG-APCs) or CD4+ T cells (VAG-CD4+) were purified from spleens of VAG539-treated graft-accepting mice, and CD90− antigen-presenting cells (saline APCs) were purified from spleens of saline-injected graft-rejecting mice 60 days after transplantation. Cells were injected (intravenously, 5 × 106 APCs or 3 × 106 T cells) into naive mice 1 day prior to islet transplantation. (B) CD11+ (VAG-DCs) or CD19+ (VAG-CD19) cells were purified from spleens of VAG539-treated graft-accepting mice 60 days after transplantation. As control, CD11c+ DCs were purified from spleens of saline-injected graft-rejecting mice 60 days after transplantation (saline-DCs) or of naive BALB/c mice (naive-DCs). Purified CD11c+ DCs (3 × 105) or CD19+ B cells (5 × 106) were injected intravenously 1 day before islet transplantation (2-tailed t test: **P < .01; *P < .05).
Induction of donor-specific transplantation tolerance by VAG-DCs
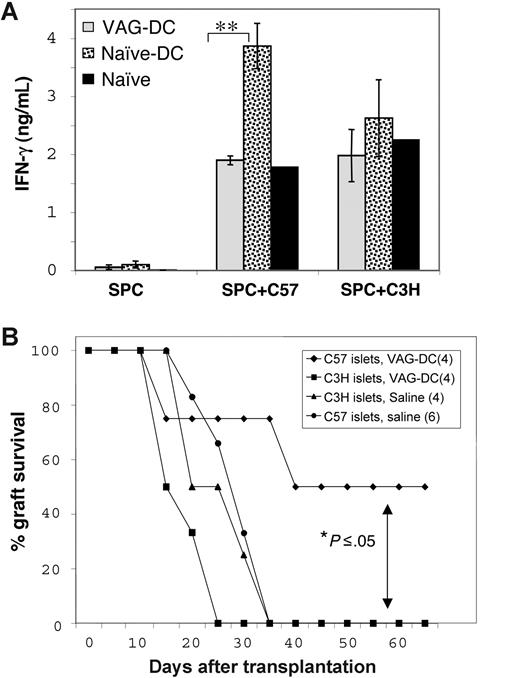
To determine whether VAG-DCs promoted donor-specific tolerance, we characterized proliferation and cytokine production of cells isolated from mice injected with VAG-DCs 100 days after transplantation. As expected, splenocytes (SPCs) from naive-DC–injected mice, which rejected islet allografts, secreted high levels of IFN-γ in response to irradiated CD90− donor-type (C57Bl/6) antigen-presenting cells. However, splenocytes from VAG-DC–injected mice secreted significantly lower IFN-γ levels, similar to those of SPCs from naive mice in response to allostimulation (Figure 4A; 2-tailed t test: P < .01). Notably, SPCs from VAG-DC–injected mice secreted comparable levels of IFN-γ to those observed from SPCs of both naive and naive-DC–injected mice in response to third-party (C3H) stimulator cells (Figure 4A). These results indicate that the transfer of VAG-DCs resulted in reduced IFN-γ production, which may reflect the overall T-cell and NK-cell response toward donor antigens. To support this hypothesis, we transferred VAG-DCs isolated from VAG539-treated tolerant mice into new recipients that received a transplant of either original donor-type (C57Bl/6) or third-party (C3H) islets. Notably, transfer of VAG-DCs prevented rejection of donor-type (C57Bl/6) islets, but not of third-party (C3H) islet grafts (Figure 4B), suggesting that DCs may transfer donor antigens and present them in a tolerogenic context in secondary recipients. Taken together, these results suggest that VAG539 treatment promotes induction of tolerogenic DCs capable of transferring donor-specific transplantation tolerance.
Induction of donor-specific transplantation tolerance by VAG-DCs. (A) SPCs from VAG-DC– and naive-DC–injected mice were isolated 100 days after transplantation, and from naive BALB/c mice (n = 3), and stimulated with irradiated donor-type (C57Bl/6) or third-party (C3H) CD90− APCs. Culture sups were collected after 96 hours and analyzed for the presence of IFN-γ using CBA assay (2-tailed t test: **P < .01). Error bars represent standard error between different mice (n = 3). (B) Balb/c recipient mice received a transplant of C57Bl/6 or C3H islets 1 day after injection of VAG-DCs (3 × 105) isolated from VAG539-treated islet graft–accepting mice. As control, nontreated Balb/c mice received a transplant of C57Bl/6 or C3H islets were used (2-tailed t test: *P < .05).
Induction of donor-specific transplantation tolerance by VAG-DCs. (A) SPCs from VAG-DC– and naive-DC–injected mice were isolated 100 days after transplantation, and from naive BALB/c mice (n = 3), and stimulated with irradiated donor-type (C57Bl/6) or third-party (C3H) CD90− APCs. Culture sups were collected after 96 hours and analyzed for the presence of IFN-γ using CBA assay (2-tailed t test: **P < .01). Error bars represent standard error between different mice (n = 3). (B) Balb/c recipient mice received a transplant of C57Bl/6 or C3H islets 1 day after injection of VAG-DCs (3 × 105) isolated from VAG539-treated islet graft–accepting mice. As control, nontreated Balb/c mice received a transplant of C57Bl/6 or C3H islets were used (2-tailed t test: *P < .05).
Transfer of VAG-DCs results in elevated frequency of functional Tr cells
Activation of the molecular target of VAF347, AhR, has been shown to promote in vivo the generation of Tr cells.30 To investigate the cellular mechanism through which AhR activation mediates the induction of tolerance, we investigated whether oral VAG539 administration and VAG-DC transfer modify the frequency of CD4+CD25+Foxp3+ Tr cells. We analyzed the expression of CD25 and Foxp3 in splenic CD4+ T cells of VAG-treated long-term tolerant mice or VAG-DC–injected secondary recipient mice. Interestingly, the frequencies of CD4+CD25+, CD4+Foxp3+, and CD25+Foxp3+ T cells were significantly higher in mice treated orally with VAG539 (30 mg/kg/day by mouth) as well as VAG-DC–injected mice compared with saline-treated mice and with control mice injected with naive-DCs, respectively (Figure 5A,B and data not shown; 2-tailed t test: *P < .05). We next investigated the direct effect of VAF347 on purified CD4+ T cells in vitro. Splenic Balb/C CD4+ T cells were cultured for 5 days with and without the presence of VAF347 (20 nM or 100 nM). As shown in Figure 5C, VAF347 significantly improved the survival of CD4+CD25+FOXP3+ T cells in a dose-dependent manner (n = 4; 2-tailed t test: P < .05 for 20 mM vs medium and 100 mM vs 20 mM; P < .01 for 100 mM vs medium).
Direct and DC-mediated in vivo induction of Foxp3+ CD4+ Tr cells by AhR activation. Mice treated with oral VAG539 (n = 3) and VAG-DC– or naive-DC–injected mice were analyzed 100 days after transplantation for the expression of Foxp3 and CD25 by splenic CD4+ T cells compared with naive Balb/c mice. (A) The percentage of gated CD4+ T cells expressing Foxp3 following oral VAG539 treatment or VAG-DC injection (2-tailed t test: *P < .05). Error bars represent standard error between different mice (n = 3). (B) Top numbers represent percentage of gated CD4+ T cells and bottom numbers represent MFI (Foxp3-PE channel). (C) VAF347 improves survival of CD4+CD25+FOXP3+ T cells in vitro. Splenic CD4+ T cells were cultured for 5 days in the presence or absence of VAF347 at 20 nM or 100 nM. Gated CD4+ T cells were analyzed for the expression of CD25 and FOXP3. Number represents the percentage of CD25+FOXP3+ T cells. (D) Transfer of splenic CD4+CD25+ T cells (5 × 105; purity > 90%) from VAG-DC–injected mice and from naive mice into mice that recently underwent transplantation 1 day prior to allotransplantation (2-tailed t test: *P < .05).
Direct and DC-mediated in vivo induction of Foxp3+ CD4+ Tr cells by AhR activation. Mice treated with oral VAG539 (n = 3) and VAG-DC– or naive-DC–injected mice were analyzed 100 days after transplantation for the expression of Foxp3 and CD25 by splenic CD4+ T cells compared with naive Balb/c mice. (A) The percentage of gated CD4+ T cells expressing Foxp3 following oral VAG539 treatment or VAG-DC injection (2-tailed t test: *P < .05). Error bars represent standard error between different mice (n = 3). (B) Top numbers represent percentage of gated CD4+ T cells and bottom numbers represent MFI (Foxp3-PE channel). (C) VAF347 improves survival of CD4+CD25+FOXP3+ T cells in vitro. Splenic CD4+ T cells were cultured for 5 days in the presence or absence of VAF347 at 20 nM or 100 nM. Gated CD4+ T cells were analyzed for the expression of CD25 and FOXP3. Number represents the percentage of CD25+FOXP3+ T cells. (D) Transfer of splenic CD4+CD25+ T cells (5 × 105; purity > 90%) from VAG-DC–injected mice and from naive mice into mice that recently underwent transplantation 1 day prior to allotransplantation (2-tailed t test: *P < .05).
To demonstrate that Tr cells play a functional role in AhR-mediated graft acceptance, we purified and transferred CD4+CD25+ T cells (5 × 105) from spleens of VAG-DC–injected graft-accepting mice into tertiary recipients that recently underwent transplantation. In accordance with the ex vivo and in vitro findings, CD4+CD25+ T cells purified from VAG-DC–injected mice significantly promoted graft survival, whereas the transfer of CD4+CD25+ T cells from naive mice had a statistically insignificant effect (Figure 5C). These data suggest that the tolerogenic effect of AhR activation is through both a direct effect on Tr-cell survival and modulation of DCs, which can ultimately induce CD4+CD25+Foxp3+ Tr cells.
Cell therapy with VAF347-DCs prevents allograft rejection
To substantiate our observation that VAG539 promotes tolerance in vivo through the induction of tolerogenic DCs, we evaluated the effect of cell therapy with autologous (BALB/c) bone marrow–derived DCs (BMDCs) differentiated and activated in vitro in the presence of VAF347 (VAF347-BMDCs). BMDCs were injected intravenously (3 × 105) into BALB/c mice one day prior to islet (C57Bl/6) allotransplantation. Injection of LPS-activated VAF347-BMDCs (mDCs-VAG), but not of LPS-activated BMDCs (mDCs) or of nonactivated VAF347-BMDCs, significantly prevented graft rejection (Figure 6A; 2-tailed t test: **P < .01). Interestingly, injection of islet donor-type (C57Bl/6) mDCs-VAF had no effect on graft survival (data not shown). These data demonstrate the capacity of VAF347 to promote in vitro induction of tolerogenic DCs, which could be administered in vivo to promote tolerance.
Cell therapy with VAF347-BMDCs prevents islet allograft rejection. Balb/c bone marrow cells were differentiated into immature BMDCs by 6-day culture in the presence of GM-CSF with or without VAF347 (20 nM). On day 6, an aliquot of cells was activated with LPS (1 μg/mL) for additional 8 hours. (A) LPS-activated VAF347-BMDCs (mDCs-VAF), nontreated BMDCs (mDCs), immature VAF347-BMDCs (iDCs-VAF), and immature nontreated BMDCs (iDCs) were injected intravenously (3 × 105) into diabetic Balb/c females 1 day prior to C57Bl/6 islet allograft transplantation (2-tailed t test: **P < .01). (B) Balb/c mice (n = 3) were immunized with OVA in CFA 1 day after intravenous injection of LPS-activated VAF347-treated (mDCs-VAF) or LPS-activated nontreated BMDCs (mDCs). Seven days later, draining lymph node cells were isolated and stimulated with Con A (1.25 mg/mL), with C57Bl/6 irradiated CD90− APCs (1:1 ratio; proliferation of stimulator cells alone was < 130 cpm), or with OVA at the indicated concentrations (2-tailed t test: *P < .05; ***P < .001). Error bars represent standard deviation between different mice (n = 3).
Cell therapy with VAF347-BMDCs prevents islet allograft rejection. Balb/c bone marrow cells were differentiated into immature BMDCs by 6-day culture in the presence of GM-CSF with or without VAF347 (20 nM). On day 6, an aliquot of cells was activated with LPS (1 μg/mL) for additional 8 hours. (A) LPS-activated VAF347-BMDCs (mDCs-VAF), nontreated BMDCs (mDCs), immature VAF347-BMDCs (iDCs-VAF), and immature nontreated BMDCs (iDCs) were injected intravenously (3 × 105) into diabetic Balb/c females 1 day prior to C57Bl/6 islet allograft transplantation (2-tailed t test: **P < .01). (B) Balb/c mice (n = 3) were immunized with OVA in CFA 1 day after intravenous injection of LPS-activated VAF347-treated (mDCs-VAF) or LPS-activated nontreated BMDCs (mDCs). Seven days later, draining lymph node cells were isolated and stimulated with Con A (1.25 mg/mL), with C57Bl/6 irradiated CD90− APCs (1:1 ratio; proliferation of stimulator cells alone was < 130 cpm), or with OVA at the indicated concentrations (2-tailed t test: *P < .05; ***P < .001). Error bars represent standard deviation between different mice (n = 3).
To investigate the capacity of VAF347-BMDCs to inhibit antigen-specific T-cell activation, we injected mDCs or mDCs-VAF into OVA-immunized BALB/c mice. The proliferative response of draining lymph node cells was analyzed on day 7 after immunization. OVA-specific T-cell response was significantly reduced in draining lymph node cells isolated from mDC-VAF–injected mice compared with control, whereas T cells maintained their ability to respond to alloantigens and to Con A (Figure 6B). Together, these data indicate that cell therapy with mDCs-VAF inhibits islet allograft rejection by specifically suppressing an ongoing immune response, and support the notion that VAF347 induces tolerogenic DCs, which are able to promote antigen-specific transplantation tolerance.
Discussion
The induction of active immune tolerance in the absence of nonspecific immunosuppression is a major requirement for effective treatment of immune pathologies, including autoimmune diseases and graft rejection. VAF347 is a novel low-molecular-weight compound, which activates AhR and has been shown to modulate the function of human DCs.9 The purpose of this work was to define the in vivo effects of VAG539, using a model of allogeneic pancreatic islet transplantation, and to characterize the compound's cellular mechanism of action. We found that VAG539, in the absence of any additional immunosuppressive treatment, prevents the rejection of MHC-mismatched islet allograft and induces long-term active tolerance. The tolerogenic effect of oral VAG539 was accompanied by elevation in the frequency of CD4+CD25+FOXP3+ Tr cells in spleens of treated mice. In addition, VAG539-induced allotolerance could be transferred through CD11c+ DCs into mice that recently underwent transplantation, which consequently displayed elevated frequency of functional CD4+CD25+Foxp3+ Tr cells. DC-induced Tr cells were functional as their transfer into mice that recently underwent transplantation resulted in graft acceptance. Furthermore, we demonstrated that in vitro treatment with VAF347 promotes tolerogenic function that can be maintained in vivo by activated, but not by nonactivated, DCs. These findings highlight the importance of the cross talk between regulatory T and dendritic cells in promoting a specific type of tolerogenic response, and suppressing conflicting immune responses.
Ettmayer et al have identified VAF347 as a low-molecular-weight immunomodulator.28 They demonstrated that VAF347 potently inhibits human monocyte-derived DC-mediated T-cell proliferation and cytokine production in the low nanomolar range. This effect was not due to impaired antigen uptake by DCs, but may be related to a failure of the DCs to deliver appropriate activation signals to T cells, since the expression of HLA-DR and CD86 on the cell surface of human DCs and the secretion of IL-6 were inhibited by VAF347.28 In the present work, we investigated the in vivo effects of VAF347 on murine DC functions and found that it inhibits the expression of CD80 by draining lymph node DCs from immunized mice, and reduces DC infiltration into the graft area in mice that underwent allotransplantation (Figures 1B, 2A). Moreover, DCs exposed to the compound obtained a regulatory function and could transfer allotolerance to untreated recipients.
A large body of data supports the association between tolerogenic DCs and Tr cells.3,16,35 Through the secretion of immunomodulating cytokines, Tr cells can bridge adaptive and innate immunity and promote the induction of regulatory APC subsets, which may in turn induce secondary Tr cells from the naive population.5,12,33 IL-10 and TGF-β produced by Tr1 cells can facilitate the generation of tolerogenic DCs, which consequently promote the induction of Tr cells in vitro and in vivo.36,37 Interestingly, suboptimal DC activation in the presence of TGF-β has been shown to enhance the conversion of naive CD4+ T cells into Foxp3+ suppressor cells.38 The findings of the present work are in line with the notion that tolerance induction is associated with a self-maintaining regulatory loop in which tolerogenic DCs induce/expend Tr cells, and Tr cells program the generation of tolerogenic DCs from DC progenitors.39 This phenomena of infectious tolerance can explain our finding that DCs purified from spleens of treated graft-accepting mice 60 days after VAG539 treatment withdrawal were capable of transferring tolerance.
Our results highlight the importance of the cross talk between APCs with tolerogenic properties and Tr cells in transplantation tolerance.40 However, since DCs constantly respond to their immediate milieu, the question of strength of commitment to a specific phenotype and cytokine profile remains open. It has been suggested that maintenance of tolerogenic phenotype in vivo in variable inflammatory conditions may require prior DC maturation in the presence of immunomodulatory factors.7 Accordingly, DCs differentiated in vitro in the presence of immunomodulatory cytokines such as IL-10 and/or TGF-β, and subsequently activated, resulted in an in vivo induction of Tr cells, which mediated immune tolerance.35,37,41 It has been demonstrated that LPS-activated BMDCs are highly effective at breaking CD4+CD25+ Tr-cell anergy and triggering proliferation and IL-2 production by these Tr cells in vitro.3,17 In addition, mature DCs have been demonstrated to display prolonged presentation of specific MHC-peptide complexes compared with other APC subsets, including immature DCs,42 which can increase their efficiency in differentially mediating induction and activation of either effector or regulatory T-cell subsets. In the present work, we demonstrate that mature, but not immature, VAF347-BMDCs can promote long-term islet allograft survival. It remains to be determined whether VAF347-treated immature DCs were ineffective because they encounter in vivo proinflammatory signals, which reverse their tolerogenic phenotype. Future experiments will have to address whether VAF347/VAG539 treatment of DCs leads to a similar functional profile compared with treatment with immunomodulatory cytokines, especially with regard to AhR activation by VAF347.
In the context of pancreatic islet transplantation, Gregori et al have previously shown that the tolerogenic phenotype of DCs following combined immunosuppressive treatment with the active form of vitamin D3, and mycophenolate mofetil, is maintained in vivo for more than 100 days after drug withdrawal.43 A long-term reduction in mature DCs, associated with transplantation tolerance, could also be induced by peritransplantation administration of other immunosuppressive agents, such as deoxyspergualin combined with anti-CD3 immunotoxin.44 As shown here, the transfer of tolerogenic DCs from treated mice more than 60 days after drug withdrawal resulted in allograft acceptance and in higher frequency of CD4+CD25+Foxp3+ Tr cells in secondary recipients. The capacity of VAG-DCs to transfer allo-specific transplantation tolerance can be related to an ongoing “tolerance response” in mice that underwent transplantation, during which DCs continuously present alloantigens in a tolerogenic manner. This continuous response that is necessary for the maintenance of peripheral tolerance in the absence of thymic central tolerance toward the specific antigen may require the presence of Tr cells. Alternatively, mature tolerogenic DCs may display a prolonged life span in inflammatory conditions and mediate active tolerance toward donor antigens.45
In summary, this study translates the initial in vitro observations, which showed that VAF347 acts as immunomodulatory compound into an in vivo clinically relevant setting of allogeneic islet transplantation. Results provide direct experimental evidence that VAF347 generates tolerogenic DCs, and improves the survival of Tr cells. Thus, VAF437/VAG539-mediated AhR activation promotes tolerance via a direct as well as DC-mediated activation/expansion of Foxp3+ Tr cells. To our knowledge, VAF347 is the first low-molecular-compound with such a biologic profile.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Novartis Pharma AG (Vienna, Austria), and by a grant from the Italian Telethon Foundation (Rome, Italy). E.H. was supported by the International Human Frontier Science Program Organization (Strasbourg, France)
Authorship
Contribution: E.H. designed and performed experiments, analyzed the data, and took a key part in writing the paper; S.G. contributed equally to experimental design and data analysis, and edited the paper; E.D., B.M., and S.O. performed experimental work; M.W. contributed to experimental discussions and paper editing; and M.G.R. supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Maria-Grazia Roncarolo, San Raffaele Telethon Institute for Gene Therapy, Via Olgettina 58, Milan, Italy 20132; e-mail: m.roncarolo@hsr.it.