Abstract
We analyzed the outcomes of 74 patients diagnosed with BCR-ABL− myeloproliferative neoplasms in blast phase receiving induction chemotherapy (55%), low-intensity therapy (16%), stem cell transplantation (SCT; 3%), or supportive care (26%). Median survival from the date of blastic transformation was 5 months. Patients receiving supportive therapy had a median survival of 6 weeks. Complete remission with or without blood recovery was achieved in 46% of patients receiving induction chemotherapy, but remissions were not durable with a median progression-free survival of only 5 months. Eight patients received SCT either as first therapy or after responding to antileukemia therapy. These patients had a markedly superior survival, with 73% alive at a median follow-up of 31 months. JAK2V617F kinetics were assessed in 16 patients: 0 of 4 negative patients became positive at transformation, and among 12 positive patients, 1 had an increase in JAK2V617F% at transformation, 7 had a substantial decrease, and 4 had stable levels. Myeloproliferative neoplasm blast phase is associated with a dismal prognosis. Responses to chemotherapy can be achieved but are not durable. Long-term survivors had all received SCT either as first therapy or in first remission.
Introduction
The term myeloproliferative neoplasm (MPN) encompasses a number of entities characterized by uncontrolled marrow proliferation in the presence of intact cellular differentiation. The natural history of chronic myelogenous leukemia (CML) serves as a useful template for the understanding of MPN evolution, with a long phase of benign cellular proliferation, followed by a short phase of blastic transformation as the underlying neoplastic clone loses its capacity to differentiate.1 CML is now considered a separate entity because of its unique association with the BCR-ABL translocation, which accounts for its proliferative advantage.1 Recent discoveries relating to the JAK2V617F2 and variant3 mutations, as well as mutations involving the MPL gene,4 have started to shed light on possible mechanisms of excessive proliferation in polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis. However, the pathogenesis of blastic transformation in CML remains poorly understood,5 and there is even less literature regarding blastic transformation in the classic, BCR-ABL-negative MPNs (PV, ET, and primary myelofibrosis).6 It is known that blastic transformation may be related to the use of alkylators, radiation, or other DNA-damaging agents during the chronic phase in some patients.7 Such agents are now rarely used in MPN therapy. To gain insight into the evolution and treatment outcome of patients developing blastic transformation from classic MPN managed in the modern era, we undertook a 13-year retrospective review of this condition at our institution.
Methods
The University of Texas M. D. Anderson Institutional Review Board approved this study. Informed consent was obtained in accordance with the Declaration of Helsinki. Separate searches for patients with the diagnosis of “MPN blast phase,” “MPN leukemic phase,” or dual diagnoses of “MPN” and “acute leukemia” were conducted in databases maintained by the leukemia and hematopathology departments. The charts of identified patients were manually reviewed. Patients were confirmed to have PV, ET, primary myelofibrosis, secondary myelofibrosis, or MPN-unclassifiable (MPN-U) in their chronic phase according to the World Health Organization criteria.8 Blast phase was defined as persistent elevation in peripheral blood or bone marrow blasts of 20% or more.9
Therapy was classified as acute myelogenous leukemia (AML) induction if the intended regimen has an expected toxicity equal to or greater than that of an anthracycline and standard-dose cytosine arabinoside (Ara-C) chemotherapy given in a “7+3” fashion. Low-intensity therapy included investigational agents and single-agent therapy with the anti-CD33/calicheamicin conjugate gemtuzumab ozogamicin. Two patients received stem cell transplantation (SCT) as their first blast phase therapy. All other patients, including those receiving hydroxyurea for suppression of peripheral blood counts, were considered to have received supportive therapy only. Blast phase response was classified using the established criteria for AML,10 with major response categories being complete remission (CR) and complete remission with incomplete blood recovery (CRi). Patients in CR/CRi were allowed to have persistent marrow features of MPN, provided the blast percentage and peripheral blood parameters satisfied the AML criteria. Where available, bone marrow or peripheral blood specimens were tested for the JAK2V617F mutation by pyrosequencing or real-time quantitative polymerase chain reaction, using previously published techniques.11,12 Briefly, pyrosequencing for the 1849G>T JAK2 mutation in genomic DNA was performed using PSQ HS 96 Gold SNP reagents and the PSQ HS 96 pyrosequencing machine (Biotage, Uppsala, Sweden).12 The real-time polymerase chain reaction assay had been modified to enhance allelic discrimination through the synergistic effect of a mismatch in the −1 position and a locked nucleic acid at the −2 position.11 A blinded comparison of the 2 assays had demonstrated excellent correlation of results in a linear regression model (R2 = 0.99, P < .001).11 Both assays have a validated coefficient of variance of less than 10%.11,12
Overall survival (OS) was defined as the time between blast phase diagnosis and patient death. Progression-free survival was censored at the date of SCT for patients undergoing SCT in first response. Categorical data were compared using the Fisher exact or χ2 tests, and continuous data using the Mann-Whitney test, as appropriate. Survival was evaluated using the Kaplan-Meier method, and impact of baseline factors on survival was evaluated using the log-rank and Cox proportional hazards analyses, as appropriate. All P values were 2-sided.
Results
Patient characteristics
Seventy-four patients were diagnosed with MPN blast phase between April 1994 and October 2007. Baseline characteristics are summarized in Table 1. With the exception of one patient who had an undifferentiated phenotype, all patients had myeloid leukemic phenotype. Fifty-one study patients were drawn from a total of 476 patients in the MPN database; the remaining 23 study patients were drawn from the acute leukemia and/or the hematopathology databases, and composed of patients with well-defined MPN who presented for management of their blast phase. Median age was 58 years (range, 19-81 years) at the time of MPN diagnosis and 64 years (range, 21-81 years) at the time of blast phase development. Thirty-six (49%) patients had primary myelofibrosis, 18 (24%) had PV, 7 (9%) had ET, 6 (8%) had MPN-U, and 7 (9%) had secondary myelofibrosis evolving from prior PV (n = 5) or ET (n = 2). Patients were a median of 58 months (range, 6-376 months) from initial MPN diagnosis and had received a median of 2 (range, 0-5) prior MPN therapies. Six patients developed blast phase within the first year of MPN diagnosis (4 primary myelofibrosis, 1 secondary myelofibrosis, and 1 MPN-U); these patients had no evidence of excess blasts in their initial MPN workup. Fourteen patients (20%) had less than 20% blasts in their bone marrow but qualified for blast phase based on persistent elevation of peripheral blood blast percentage more than or equal to 20%.
A history of alkylating agent exposure was present in 11 (15%) patients, and a history of radioactive phosphorus exposure was present in 3 (4%) patients. The remaining patients did not have exposure to agents commonly accepted as being leukemogenic. Fifteen (21%) patients had a splenectomy during the MPN phase, with splenectomy being more common in patients with primary or secondary myelofibrosis (12 of 43) than in other histologies (3 of 31, P = .08). Cytogenetics was abnormal at transformation in 42 (72%) patients, with 17 (29%) patients having abnormalities of chromosomes 5, 7, and/or complex (≥ 3) abnormalities, and a further 11 (19%) having rearrangements of chromosome 17. In our preliminary comparison of characteristics of patients in the MPN database who did or did not develop blast phase, the proportion of abnormal chronic phase cytogenetics was similar (P = .86), but the rate of clonal evolution was significantly higher in those patients who eventually transformed to blast phase disease (P < .001). The only other characteristic to be significantly different was the proportion that had elevated peripheral blood blasts more than or equal to 2% at presentation (P = .02).
Response to therapy
There was no treatment protocol specific for MPN blast phase during the study period, and patients were treated at the discretion of the individual physician (Table 2). Forty-one (55%) patients received induction chemotherapy. Two patients were not evaluable for response because of inadequate restaging. In the remaining 39 patients, the rate of CR or CRi was 46% and was not significantly different between the 32 patients receiving high-dose Ara-C-based regimens (CR/Cri, 41%) and the 9 patients receiving standard dose Ara-C-based regimens (CR/Cri, 71%, P = .21). Six patients (15%) died within 4 weeks of AML induction. The median duration of hospitalization was 45 days (range, 21-120 days).
Two (3%) patients received SCT as initial blast phase treatment, without intervening chemotherapy. One patient had a low but persistently elevated blast percentage (21% blood, 13% marrow), and the second patient evolved into blast phase from a SCT performed in the chronic phase. Both patients achieved CR (n = 1) or CRi (n = 1).
Twelve (16%) patients received low-intensity therapy. Specific regimens are summarized in Table 2. No patient achieved a CR or CRi, and there were no deaths within 4 weeks of therapy. The duration of hospitalization was significantly shorter than patients receiving induction chemotherapy (median, 33 days; range, 10-64 days, P = .04 compared with induction chemotherapy).
One patient with an undifferentiated blast phenotype had a partial response after vincristine and prednisone, with a decrease in marrow blasts (from 70% to 10%) and improvement in peripheral blood parameters.
Red cell transfusion independence of more than or equal to 4 weeks duration was present in 46% of patients after induction chemotherapy, in both patients after SCT, and in 33% of patients after low-intensity therapy (P = not significant for all comparisons).
Survival
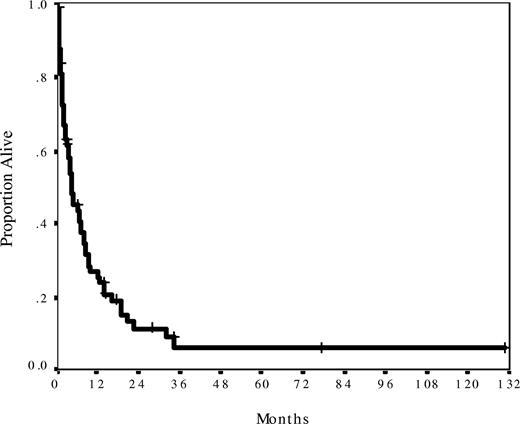
The median follow-up was 14 months (range, 2-131 months) from the time of blast phase development. Median survival was only 5 months, with 27% patients surviving more than or equal to 1 year (Figure 1). Survival was similarly dismal regardless of baseline MPN histology, time from initial MPN diagnosis, number and type of prior therapies, degree of cytopenia, or spleen size (Table 1). Baseline features significantly associated with better survival were age less than 60 years (median OS, 10 months) and peripheral blasts less than 20% (median OS, 13 months), and those significantly associated with inferior survival were performance status more than or equal to 2 (median OS, 1.5 months), prior splenectomy (median OS, 2 months), and chromosome 17 abnormalities (median OS, 3.5 months; Table 1). Compared with nonsplenectomized patients, splenectomized patients were of similar age, had similar cytogenetics, performance status, and blast percentages, and had similar outcomes after induction chemotherapy (P > .20 for all comparisons). However, patients with a history of splenectomy had been exposed to more lines of chronic phase therapy (median, 4 systemic therapies) than patients without a history of splenectomy (median, 2 therapies, P = .002). Cytogenetics categories other than chromosome 17 abnormalities had no effect on survival. This was mainly because patients with intermediate cytogenetics (diploid karyotype, trisomy 8, and miscellaneous noncomplex abnormalities) fared equally poorly as those patients with abnormalities of chromosome 5, 7, and/or complex (≥ 3) abnormalities. A multivariate analysis of all significant baseline factors for survival showed that age more than or equal to 60 years, peripheral blasts more than or equal to 20%, and prior splenectomy were independently associated with survival (Table 3).
Survival for 74 patients from the date of blast phase diagnosis. Median survival was 5 months and actuarial 1-year survival was 27%. Median follow-up was 14 months.
Survival for 74 patients from the date of blast phase diagnosis. Median survival was 5 months and actuarial 1-year survival was 27%. Median follow-up was 14 months.
Whether patients actually received treatment or not had a major impact on survival, with patients receiving supportive therapy only experiencing a short median survival of 6 weeks, compared with 7 months for treated patients (P < .001; Table 1). Recorded reasons for not administering active therapy were poor performance and/or health status (47%), patient preference (37%), active infection (5%), and reason not stated (11%; Table 2). When treatment status (treatment vs supportive care only) was added to a multivariate model considering significant baseline characteristics, including age, performance status, blast percentage, cytogenetics, and splenectomy status, it emerged as the strongest independent associate of survival (Table 3), suggesting that active treatment may improve outcome independent from baseline characteristics associated with therapy tolerance.
Impact of treatment strategy
We examined whether the choice and sequence of active therapy influenced survival, addressing in particular the question of induction chemotherapy versus low-intensity therapy, and the role of early SCT. An analysis of the treatment arms showed that SCT had a major impact on survival, with the 11 patients receiving SCT at some point in their treatment course experiencing significantly superior survival than non-SCT patients (P = .001). Further analysis in this group showed that the survival benefit was isolated to the 8 patients who were transplanted early in the disease course (SCT as first therapy or after responding to initial therapy). The characteristics of these patients are summarized in Table 4.
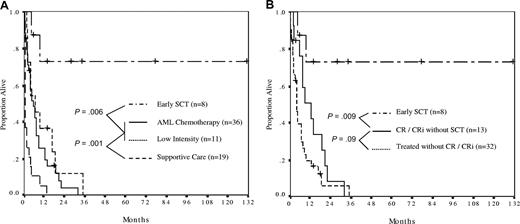
The comparison between treatment arms was therefore between patients receiving early SCT (n = 8), induction chemotherapy without early SCT (n = 36), low-intensity therapy without early SCT (n = 11), and supportive care (Figure 2). In this analysis, patients receiving either induction chemotherapy or low-intensity therapy without early SCT had similarly poor outcomes (median survival, 6 and 7 months, respectively, P = .34). All such patients were projected to have died by 3 years. In contrast, patients receiving early SCT had markedly superior survival, with 73% being alive at a median follow-up of 31 months and one patient being alive in ongoing remission at 131 months (Figure 2A).
The effect of treatment strategy on survival. (A) Patients who received stem cell transplantation (SCT) as first therapy (n = 2) or in first response (n = 6) had the most favorable outcome, with actuarial survival of 73% at a median survivor follow-up of 31 months (range, 6-131 months). Survival was similar between patients receiving AML induction (median, 6 months) and low-intensity therapy (median, 7 months). Patients receiving supportive care only had a median survival of 6 weeks. (B) Survival analysis comparing SCT as first therapy or in first response (n = 8) with patients achieving complete response (CR) plus or minus blood recovery (CRi) who did not proceed to early SCT (n = 13), and patients who received antileukemia therapy but who did not achieve a CR/CRi (n = 32). Median survival was not reached, 13 months and 4 months, respectively.
The effect of treatment strategy on survival. (A) Patients who received stem cell transplantation (SCT) as first therapy (n = 2) or in first response (n = 6) had the most favorable outcome, with actuarial survival of 73% at a median survivor follow-up of 31 months (range, 6-131 months). Survival was similar between patients receiving AML induction (median, 6 months) and low-intensity therapy (median, 7 months). Patients receiving supportive care only had a median survival of 6 weeks. (B) Survival analysis comparing SCT as first therapy or in first response (n = 8) with patients achieving complete response (CR) plus or minus blood recovery (CRi) who did not proceed to early SCT (n = 13), and patients who received antileukemia therapy but who did not achieve a CR/CRi (n = 32). Median survival was not reached, 13 months and 4 months, respectively.
Next, we sought to determine whether SCT was a surrogate marker for therapy sensitive disease by restricting the survival analysis only to patients in CR/CRi (Figure 2B). In this analysis, not only was the beneficial effect of early SCT maintained, the disappointing outcome of CR/CRi patients who did not receive SCT was also apparent; such patients had a median survival of only 13 months, with all patients projected to have died by 3 years. This was the result of inevitable relapse of disease, with median progression-free survival being only 5 months for CR/CRi patients.
The importance of the graft-versus-leukemia effect in leukemia control was underscored by the disease course of 3 patients who showed failure of SCT to control their blastic disease (Table 4). Two patients transplanted in the chronic phase developed blast phase after SCT, and one patient transplanted in blast phase had a relapse. All 3 patients had incomplete T-cell chimerism before blast phase development/relapse. Two patients received repeat SCT with (n = 1) or without (n = 1) induction chemotherapy, with both patients achieving second CR/CRi; one patient subsequently died at 3 months from treatment-related complications, and the second patient remained alive in remission at 4 months. The third patient had a single course of reinduction chemotherapy and withdrawal of his immunosuppression; this patient remained alive in second CRi 19 months later.
Kinetics of the JAK2V617F mutation at transformation and with response to therapy
JAK2V617F mutation status was tested in the MPN phase for 20 patients and in the blast phase for 37 patients (Table 1). Within the small number of patients with known JAK2V617F status, no significant impact of JAK2V617F positivity (at either chronic or blast phase) on survival was detected. The levels of JAK2V617F percentage and marrow blast percentage at the chronic and blast phases are listed in Table 5.
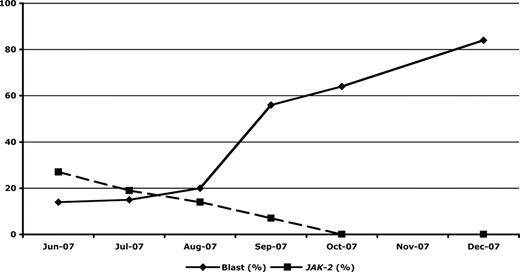
Twelve patients with JAK2V617F+ chronic phase disease had repeat JAK2V617F testing in the blast phase, on unfractionated marrow (n = 11) or peripheral blood (n = 1) samples. As the samples had mixed cell populations and were not enriched for blasts, we chose a conservative cutoff of 100% relative decrease or increase in JAK2V617F percentage to represent definite evidence of change. Using this criterion, 7 (58%) patients had a definite fall in JAK2V617F percentage (with 3 patients becoming undetectable at transformation), 1 (8%) patient had a definite rise in JAK2V617F percentage, and 4 (33%) patients had stable levels. In those patients with a decline in JAK2V617F percentage at transformation, kinetics of blast percentage, and JAK2V617F percentage showed that the JAK2 mutant clone and the leukemia blasts were independent entities (Figure 3).
Kinetics of the JAK2V617F mutation during progression to blast phase in a patient with primary myelofibrosis. Serial analysis of marrow blast percentage and JAK2V617F mutation load showed extinction of the JAK2V617F-bearing clone during blast phase progression. In this patient, the JAK2V617F and the blast phase clones were clearly independent disease processes.
Kinetics of the JAK2V617F mutation during progression to blast phase in a patient with primary myelofibrosis. Serial analysis of marrow blast percentage and JAK2V617F mutation load showed extinction of the JAK2V617F-bearing clone during blast phase progression. In this patient, the JAK2V617F and the blast phase clones were clearly independent disease processes.
Two patients (UPN 32 and 33) had stable JAK2V617F percentage at blastic transformation and were reassessed after achieving complete remission with chemotherapy; in both cases, the JAK2V617F percentage did not substantially change. Both patients then proceeded to allogeneic SCT, which was successful in eliminating the JAK2V617F+ disease. One patient (UPN 71) had a significant fall in JAK2V617F percentage at blastic transformation (from 67% to 11%), which decreased further to 6% after achieving blast phase remission; this patient proceeded to SCT and died at 3 months of complications, and her JAK2 status was not reassessed. One further patient (UPN 9) was JAK2V617F+ (71%) in the blast phase, was confirmed JAK2V617F− after SCT, and then had reappearance of JAK2V617F (65%) when he relapsed with blast phase disease; the JAK2V617F status was not reassessed after he achieved second remission after chemotherapy and withdrawal of immunosuppression.
Four patients had JAK2V617F− chronic phase and were reassessed at blast phase (Table 5); in none of these cases did the JAK2V617F become positive. Table 5 also included the results of patients whose JAK2V617F statuses were uninformative in the chronic phase but who were tested at blast phase. Five patients had PV (> 90% expected JAK2V617F+ in chronic phase), and 4 (80%) of these patients had JAK2V617F+ blast phase. Fifteen patients had primary or secondary myelofibrosis or ET (∼50% expected JAK2V617F+ in chronic phase), of whom only 3 (20%) had JAK2V617F+ blast phase.
Discussion
The current study confirms the poor survival of MPN blast phase reported by other investigators. Three other single institution studies of 13, 23, and 91 patients have all reported a median survival of 3 months or less.6,13,14 The European Collaboration on Low-Dose Aspirin in PV prospectively followed 1638 PV patients over a median of 2.8 years and documented 21 cases of blast phase transformation; none of these patients survived more than 6 months.7 Together, these studies spanned the major categories of classic MPN and support our finding that survival after blast phase development is dismal regardless of the underlying MPN histology. Indeed, the lack of impact of factors normally significant for AML is striking: the length of antecedent marrow disease, the number and type of prior therapies, and the presence of complex cytogenetic abnormalities (with the exception of chromosome 17 abnormalities) all had no impact on survival, mainly because their “less adverse” counterparts also had a poor outcome. The finding of 20% peripheral blasts or more having an independent effect on survival confirms that patients with 20% or more peripheral blasts, but without elevated marrow blasts, should be considered as being in blast phase. This is in keeping with the criteria recently published by the International Working Group on Myelofibrosis Research and Treatment.9 The association between prior splenectomy and poor survival is intriguing and remains incompletely explained. Although more patients with myelofibrosis underwent splenectomy, myelofibrosis itself is not associated with a significantly worse survival. There were no other significant differences in age, cytogenetics, performance status, or blast percentage between splenectomized and nonsplenectomized patients. In addition, treatment outcomes, including early death and CR/CRi rates, were similar between the 2 groups. Splenectomized patients, however, had received more lines of previous MPN therapy, suggesting that the requirement for splenectomy to control chronic phase disease may in itself be a surrogate marker of biologically aggressive and treatment refractory MPN.
A major hurdle to the study of MPN blast phase is the ongoing controversy regarding the relative contribution of disease and therapy to blastic transformation.15 In this regard, it is interesting to observe that, although only a minority of our patients had been exposed to radioactive phosphorus (4%) or alkylating agents (15%), blast phase cytogenetic abnormalities were common (72%) with frequent occurrence of chromosome 5 or 7, and/or complex, abnormalities. In addition, blast phase patients without exposure to DNA-damaging agents did not survive longer than those who were exposed. These observations suggest that it is the underlying disease biology that confers the adverse prognosis, rather than the effects of prior therapy. The only cytogenetic category to have an effect on survival was the presence of chromosome 17 abnormalities. Chromosome 17 abnormalities have been reported in blast phase ET16 and are associated with treatment resistance and poor survival in other hematologic malignancies, including chronic lymphocytic leukemia,17 non-Hodgkin lymphoma,18 and multiple myeloma.19 Its prognostic significance may be related to the deletion of, or mutations involving, the TP53 tumor suppression gene.17 We have recently identified chromosome 17 abnormalities to be a major harbinger of imminent blast phase transformation and/or death in patients with chronic phase myelofibrosis.20 Deletion of 20q is a common finding in MPN that may be associated with a favorable outcome in the chronic phase21,22 ; too few patients were in this cytogenetic category in the current study to draw conclusions regarding whether this abnormality is also favorable in blast phase disease.
The analysis of treatment outcomes showed that the leukemic blasts were responsive to induction chemotherapy, with 46% of patients achieving a CR/CRi by AML response criteria. Within the limited numbers of patients treated, there was no significant advantage or disadvantage to the use of standard-dose Ara-C- or high-dose Ara-C-based regimens. The risk of early death (≤ 4 weeks) was acceptable at 15% and compared favorably to the 33% rate reported by Mesa et al.6 The major reason for treatment failure was the poor durability of CR/CRi after chemotherapy alone, with all patients invariably relapsing at a median of only 5 months. This may be the result of chemoresistance in the leukemic clone and/or failure to eliminate the MPN ancestral cell, which may then recapitulate the blast phase disease. The latter scenario is supported by the finding of stable JAK2V617F levels at the achievement of complete remission in 2 patients, showing that the underlying MPN clone was not eradicated by induction chemotherapy. Both of these patients subsequently underwent SCT and had documented clearance of their JAK2V617F clone by serial molecular studies.
Although the number of patients receiving SCT was small, it is nevertheless striking to note that the only long-term survivors in the current study were patients who had received allogeneic transplantation either as first therapy or in first remission. The activity of SCT in MPN has been reported in several studies demonstrating the potential for long-term remission, and possibly cure, when SCT was administered during chronic phase disease.23-26 Our observation of marrow JAK2V617F elimination after SCT is supported by the results of another recent report27 and provides molecular confirmation of clonal suppression. The potency of the graft-versus leukemia effect against leukemic blasts is underscored by the achievement of a durable (19+ months) second remission after chemotherapy and withdrawal of immunosuppression in a study patient who relapsed after SCT.
Although the proportion of cytogenetic abnormalities at presentation between the patients who did and did not progress to blastic phase was similar, patients who progressed to blast phase were more likely to develop additional karyotypic changes. This observation is analogous to the situation in CML where clonal evolution is a common feature of disease progression to accelerated phase and blast phase.1 Recently proposed models of cancer progression have highlighted the role of oncogenes in promoting the progressive accumulation of genetic abnormalities, including the inactivation of tumor suppressor genes and the amplification and/or activation of key oncogenes.28,29 In CML, it is known that the progression to blast phase is associated with molecular abnormalities not detectable by conventional cytogenetics, including alterations in p53, RB1, c-MYC, p16INK4A, RAS, and other genes.1,5 The search for analogous pathways in MPN blast phase development is a subject of ongoing investigation at our center and other30 centers.
The role of the JAK2V617F in blastic transformation is of particular interest as the examination of its kinetics may give some insight as to whether it is the primeval genetic event in JAK2V617F positive MPN, analogous to the role of BCR-ABL in CML pathogenesis. This determination is of great importance because it allows prediction of whether the novel JAK2 inhibitors may be effective in treating blast phase disease or in preventing its onset if used during the chronic phase. The JAK2V617F data contained in this paper expanded on the previously published results of Jelinek et al.12 At the time of blastic transformation, 0 of 4 assessable JAK2V617F− patients became positive, and only 1 of 12 (8%) assessable JAK2V617F+ patients had a substantial increase in allele burden (from 11% to 69%). In contrast, 7 (58%) JAK2V617F+ patients had a substantial (> 100% relative reduction) fall in allele burden at transformation, with 3 (25%) becoming completely negative. As blasts constituted only 15% to 63% of the marrow cells in these 3 patients (Table 5), the total disappearance of their previous JAK2V617F positivity suggested that the nonblastic elements were also replaced. These observations were similar to that made by Campbell et al31 and Theocharides et al,32 who reported disappearance of JAK2V617F positivity in 3 of 4 (75%) and 7 of 17 (41%) patients, respectively, at the time of blastic transformation. In the Theocharides et al report,32 the authors were able to perform elegant cell separation studies showing that the leukemic blasts were indeed JAK2V617F− in 2 of 6 (33%) positive unfractionated samples and that in 5 patients who transformed from JAK2V617F+ to JAK2V617F− disease, the purified granulocytic fractions were truly JAK2V617F−.32 These observations confirmed that in some patients an entirely new JAK2V617F− clone takes over the disease process within both the myeloproliferative and blastic compartments.
The emergence of JAK2V617F− blast phase from previously positive MPN strongly implies that the JAK2V617F mutation may not be the disease-initiating lesion. Microsatellite studies performed by other authors had excluded the possibility of reversion to wild-type JAK2 by mitotic recombination as the explanation for the JAK2V617F loss.31,32 One of the patients in the Campbell et al report31 lost her chronic phase cytogenetic abnormalities at blastic transformation, raising the possibility that the blast phase may not have arisen from her MPN but rather from a marrow progenitor that was damaged by previous leukemogenic therapy.31 However, in the Theocharides et al report,32 a patient with JAK2V617F-positive unfractionated cells and a negative blast population was found to have a deletion of 11q in 99% of the marrow interphases, indicating that the JAK2V617F-positive and -negative populations were clonally related.32 Collectively, the current study and the 2 previous studies31,32 demonstrate that the JAK2V617F mutation is most commonly decreased or lost at blastic transformation, although patients with stable allele burden were also described in all 3 studies, and one of the patients in the current study actually had a 6-fold increase in JAK2V617F% at transformation. This heterogeneity suggests that the JAK2V617F mutation does not have a consistent role in blastic transformation. It is possible that the normal MPN ancestral pool is composed of a number of different clones with a variety of secondary cytogenetic and molecular abnormalities, and that JAK2V617F positivity (or negativity) in the leukemic phase may simply be a function of which individual MPN clone eventually gives rise to blast phase disease.
Authorship
Contribution: C.S.T., S.M.P., H.M.K., and S.V. designed the research and analyzed the data; C.S.T. and S.V. wrote the paper; C.E.B.-R., T.M., and R.M.N. performed the correlative scientific studies; and U.P., S.E.C., D.A.T., J.A.C., and R.E.C. assisted in the preparation of data, analysis of results, revisions to the paper, and final proofreading.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Srdan Verstovsek, Leukemia Department, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail: sverstov@mdanderson.org.