Abstract
We previously reported the feasibility of clofarabine and cytarabine combinations in AML. Questions remain as to (1) the therapeutic advantage of this combination and (2) the role of lower doses of clofarabine and cytarabine in older patients. We have conducted an adaptively randomized study of lower-dose clofarabine with or without low-dose cytarabine in previously untreated patients with AML aged 60 years and older. Patients received 30 mg/m2 clofarabine intravenously daily for 5 days with or without 20 mg/m2 cytarabine subcutaneously daily for 14 days as induction. Consolidation consisted of 3 days of clofarabine with or without 7 days of cytarabine. Seventy patients were enrolled. The median age was 71 years (range, 60-83 years). Sixteen patients received clofarabine and 54 the combination. Overall, 56% achieved complete remission (CR). CR rate was significantly higher with the combination (63% vs 31%; P = .025). Induction mortality was 19% with the combination versus 31% with clofarabine alone (P = .276). The combination showed better event-free survival (7.1 months vs 1.7 months; P = .04), but not overall survival (11.4 months vs 5.8 months; P = .1). Clofarabine plus low-dose cytarabine has a higher response rate than clofarabine alone with comparable toxicity. This trial is registered at www.clinicaltrials.gov as no. NCT00088218.
Introduction
Over more than 30 years and using standard cytarabine-based therapy, outcome has improved little for patients with acute myeloid leukemia (AML) older than 60 years of age; there is a continuous need to explore new therapies.1-3 Clofarabine (2-chloro-2′-fluoro-deoxy-9-β-D-arabinofuranosyladenine) is a new-generation deoxyadenosine nucleoside analog.4 Compared with its precursors, fludarabine and cladribine, it has several advantages: (1) increased resistance to deamination and phosphorolysis and hence better stability; (2) higher affinity to deoxycytidine kinase (dCyd); (3) prolonged retention of the triphosphate compound in leukemic blasts; and (4) potent inhibition of DNA synthesis and of ribonucleotide reductase (RNR).5-8 Clofarabine has received approval from the US Food and Drug Administration (FDA) for children with relapsed/refractory acute lymphoblastic leukemia (ALL) after at least 2 prior regimens.9
The focus of its development in adults has been in AML.10 We have previously explored the combination of clofarabine with cytarabine in AML salvage and front-line therapy.11,12 Using 40 mg/m2 clofarabine plus 1 g/m2 cytarabine daily for 5 days in untreated patients with AML (median age, 61 years), we reported a response rate of 60%, comparable survival with other induction regimens, and acceptable toxicity.12 Another study has since suggested that lower doses of clofarabine (eg, 30 mg/m2 per dose) can achieve response rates comparable with higher doses.13 Low-dose cytarabine (20 mg/m2 subcutaneously twice daily) was superior to hydroxyurea in a randomized study targeting elderly patients with AML.14 As our interest lay in the development of a clofarabine-based induction program, and because we did not consider low-dose cytarabine alone a very effective nor widely accepted standard in the United States, we designed a study comparing 30 mg/m2 clofarabine daily for 5 days with or without 20 mg/m2 cytarabine daily for 14 days in previously untreated patients with AML and high-risk myelodysplastic syndrome (MDS; > 10% blasts or International Prognostic Scoring System [IPSS] ≥ intermediate-2 risk group) aged 60 years or older. Patients with high-risk MDS were included, as the distinction between AML and MDS therapy is frequently blurred in advanced-stage MDS and because both groups may derive benefit.
Methods
Study group
A total of 70 patients were enrolled. The diagnosis of AML was based on World Health Organization (WHO) criteria whereby for some patients a diagnosis was rendered both in terms of their WHO and French-American-British (FAB) classification. All patients provided informed consent according to institutional guidelines. Prior therapy with hydroxyurea, hematopoietic growth factors, and biologic or targeted therapies (eg, methyltransferase inhibitors, fms-like tyrosine kinase [flt] 3-inhibitors) were allowed. Patients were required to have Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, and adequate hepatorenal function (serum creatinine ≤ 176.8 μM [2 mg/dL]; total bilirubin ≤ 34.2 μM [2 mg/dL]; serum glutamic pyruvic transaminase [SGPT] or serum glutamic oxaloacetic transaminase [SGOT] ≤ ×4 upper limit of normal). Patients were excluded for New York Heart Association Classification grade 3 or higher heart disease. The study was approved by the Institutional Review Board (IRB) of the University of Texas M. D. Anderson Cancer Center (MDACC) and was conducted in accordance with the basic principles of the Declaration of Helsinki.
Treatment and monitoring
Patients were randomized to clofarabine alone or clofarabine plus low-dose cytarabine. During the induction, clofarabine was administered as a 1-hour intravenous infusion at 30 mg/m2 daily for 5 days on days 1 through 5. In the combination arm, cytarabine was added at 20 mg/m2 by subcutaneous injection daily for 14 days on days 1 through 14. On days 1 to 5, administration of clofarabine preceded the injection of cytarabine by approximately 4 hours. The rationale for sequencing was based on studies in acute leukemia cell lines demonstrating optimal intracellular ara-CTP accumulation when following clofarabine administration by 3 to 4 hours.15 A second induction cycle was permitted for stable disease, partial response, or hematologic improvement after the first induction cycle. Cycles were repeated every 4 to 7 weeks depending on leukemia response, recovery of normal hematopoiesis, and resolution of toxicities. Patients with at least a complete remission with incomplete count recovery (CRi) could receive up to 12 consolidation cycles. During the consolidation, clofarabine was administered at 30 mg/m2 daily for 3 consecutive days (days 1 through 3). In the combination arm, 20 mg/m2 cytarabine was added by subcutaneous injection daily for 7 consecutive days (days 1 through 7), which was given 4 hours after clofarabine on days 1 through 3. Recovery of the absolute neutrophil count (ANC) to 109/L or greater and platelet count to 60 × 109/L or greater was required before each consolidation course, except for patients who had achieved CRi where further courses could be given with platelet counts of 60 × 109/L or less. In addition, any nonhematologic toxicity experienced by the patient had to return to lower than grade 2 or baseline before the patient continued treatment with the study drugs. Subsequent doses of clofarabine and cytarabine were reduced by 25% for grade 3 and by 50% for grade 4 drug-related nonhematologic toxicities. All patients were admitted to a laminar air flow room, in which they spent the whole duration of the induction (on average 30 days). Supportive measures included further use of prophylactic antibiotics (eg, levaquin, valacyclovir, and itraconazole or voriconazole). Antifungals were withheld on days when clofarabine was given to avoid exacerbation of liver function abnormalities. Hematopoietic growth factors were used at the discretion of the treating physician. Steroids were not routinely used as premedication.
Patients were monitored with complete blood count (CBC)/platelet count, and chemistry profile at least once weekly until remission and then at least every 2 to 4 weeks as long as on therapy. Marrow aspiration was performed starting on day 21 and then every 2 weeks until confirmation of remission or nonresponse. For most patients this was possible with up to 2 more marrow evaluations.
Response criteria
Response was determined according to the revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia.16 Complete remission (CR) required recovery of normal hematopoiesis with ANC of 109/L or greater and platelet count of 100 × 109/L or greater, and normalization of the marrow blasts (≤ 5%). CRi was defined after a period of observation (on average 28 to 35 days), during which neutrophil counts recovered, marrow blasts were 5% or less, but no further platelet recovery occurred. Partial response (PR) required blood count recovery as for CR, but with both a decrease in marrow blasts of at least 50% and not more than 25% blasts in the marrow.
Statistical considerations
The primary study objective was to assess and compare the efficacy of clofarabine alone versus clofarabine plus low-dose cytarabine. Patients were adaptively randomized without stratification factors. Assignment probabilities were based on the observed results in the preceding patients, according to the method of Berry (Bayesian randomization).17 For the first 20 patients, the randomization was balanced with a 50% probability of being randomized to either arm. As data accrued concerning efficacy, the assignment probabilities shifted in favor of the arm with a higher CR rate. Information on each patient's response was entered using a web-based data entry screen that was provided by the Department of Biostatistics at the MDACC. Treatment assignment for each patient was determined and reported back via a customized trial data screen. If at any time the probability of one treatment arm showing superior efficacy than the other one was greater than 0.95, then the trial was to be terminated. In addition, if a CR rate of 35% or higher was unlikely to be true for one of the treatment arms, assignment to that arm was to cease. Should no arm be selected as superior at the conclusion of the trial, the study would be regarded as inconclusive.
Guidelines were also developed for early termination in the event of excessive early mortality (within 14 days of therapy start). The treatment arm was to be discontinued if early death occurred in 2 of the first 4 patients, 3 or more of 9 patients, 4 or more of 14 patients, 5 or more of 19 patients, or 6 or more of 24 patients. Simulations using these stopping boundaries indicated that the probability of early termination for a true early mortality rate of 5%, 15%, or 30% was 7%, 51%, and 96%, respectively.
Chi-square tests were used for analysis of comparisons between the treatment arms with respect to the rates of CR, overall response (OR), resistant disease, induction mortality, and other outcomes.
However, when the sample size was small (< 5 for any entry in the 2 × 2 table), Fisher exact tests were used. Time-to-event distributions were estimated by the Kaplan-Meier method, and the differences between the 2 arms were tested by the log-rank test. Event-free survival was defined as the time from randomization until the date of first objective documentation of disease relapse or death due to any cause, whichever occurred first.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. All patients on the single-agent arm had a diagnosis of AML based on WHO criteria. A total of 4 (8%) of the patients on the combination had a diagnosis of either refractory anemia with excess of blasts (RAEB)–2 (n = 2) or chronic myelomonocytic leukemia (CMML; CMML-1 and CMML-2, one patient each). Two of these patients received prior therapy with DNA methyltransferase inhibitors. The median age was 71 years (range, 60-83 years). A total of 7 (10%) patients were 80 years of age or older (3 [19%] patients on clofarabine and 4 [7%] patients on clofarabine plus low-dose cytarabine).
Patient characteristics
| Characteristic . | Clofarabine . | Clofarabine plus LD cytarabine . | Total . |
|---|---|---|---|
| No. patients | 16 | 54 | 70 |
| Median age, y (range) | 71 (60-83) | 70 (60-82) | 71 (60-83) |
| Diagnosis, no. patients (%) | |||
| AML | 16 (100) | 50 (92) | 66 (94) |
| RAEB-2 | 2 (4) | 2 (3) | |
| CMML | 2 (4) | 2 (3) | |
| Secondary AML/MDS, no. patients (%) | 7 (41) | 28 (52) | 35 (50) |
| MDS | 6 (38) | 18 (33) | 24 (34) |
| Other AHD | 1 (3)* | 6 (11)† | 7 (10) |
| Solid tumor history | 1 (3)* | 7 (13)‡ | 8 (11) |
| Prior therapy with DNMTI, no. patients (%) | 2 (12) | 9 (17) | 11 (16) |
| FAB classification (AML), no. patients (%) | |||
| M0 | 1 (6) | 1 (2) | 2 (3) |
| M1 | 3 (6) | 3 (5) | |
| M2 | 2 (12) | 5 (10) | 7 (11) |
| M4/M5 | 5 (32) | 8 (16) | 13 (20) |
| M6 | 1 (6) | 3 (6) | 4 (6) |
| Not assigned by FAB | 7 (44) | 30 (59)§ | 37 (55) |
| WHO classification, no. patients (%) | |||
| Recurrent cytogenetic abnormality | 1 (2) | 1 (1) | |
| AML with multilineage dysplasia | 7 (44) | 17 (31) | 24 (34) |
| Therapy-related AML | 1 (6) | 3 (6) | 4 (6) |
| Not otherwise categorized | 7 (44) | 25 (46) | 32 (46) |
| Not available or diagnosis of MDS | 1 (6) | 8 (15) | 9 (13) |
| Karyotype, no. patients (%) | |||
| Diploid | 6 (38) | 27 (50) | 33 (47) |
| Unfavorable | 5 (31) | 20 (37) | 25 (36) |
| −5/−7 | 2 (13) | 14 (26) | 16 (23) |
| del(11)(q23) | 1 (2) | 1 (1) | |
| Complex | 5 (31) | 9 (17) | 14 (20) |
| Flt3, no. patients (%) | |||
| Available | 13 (81) | 50 (93) | 63 (90) |
| Abnormal | 2 (15) | 6 (12) | 8 (11) |
| ITD | 2 (15) | 5 (10)‖ | 7 (14) |
| D835 | 3 (6)‖ | 3 (4) |
| Characteristic . | Clofarabine . | Clofarabine plus LD cytarabine . | Total . |
|---|---|---|---|
| No. patients | 16 | 54 | 70 |
| Median age, y (range) | 71 (60-83) | 70 (60-82) | 71 (60-83) |
| Diagnosis, no. patients (%) | |||
| AML | 16 (100) | 50 (92) | 66 (94) |
| RAEB-2 | 2 (4) | 2 (3) | |
| CMML | 2 (4) | 2 (3) | |
| Secondary AML/MDS, no. patients (%) | 7 (41) | 28 (52) | 35 (50) |
| MDS | 6 (38) | 18 (33) | 24 (34) |
| Other AHD | 1 (3)* | 6 (11)† | 7 (10) |
| Solid tumor history | 1 (3)* | 7 (13)‡ | 8 (11) |
| Prior therapy with DNMTI, no. patients (%) | 2 (12) | 9 (17) | 11 (16) |
| FAB classification (AML), no. patients (%) | |||
| M0 | 1 (6) | 1 (2) | 2 (3) |
| M1 | 3 (6) | 3 (5) | |
| M2 | 2 (12) | 5 (10) | 7 (11) |
| M4/M5 | 5 (32) | 8 (16) | 13 (20) |
| M6 | 1 (6) | 3 (6) | 4 (6) |
| Not assigned by FAB | 7 (44) | 30 (59)§ | 37 (55) |
| WHO classification, no. patients (%) | |||
| Recurrent cytogenetic abnormality | 1 (2) | 1 (1) | |
| AML with multilineage dysplasia | 7 (44) | 17 (31) | 24 (34) |
| Therapy-related AML | 1 (6) | 3 (6) | 4 (6) |
| Not otherwise categorized | 7 (44) | 25 (46) | 32 (46) |
| Not available or diagnosis of MDS | 1 (6) | 8 (15) | 9 (13) |
| Karyotype, no. patients (%) | |||
| Diploid | 6 (38) | 27 (50) | 33 (47) |
| Unfavorable | 5 (31) | 20 (37) | 25 (36) |
| −5/−7 | 2 (13) | 14 (26) | 16 (23) |
| del(11)(q23) | 1 (2) | 1 (1) | |
| Complex | 5 (31) | 9 (17) | 14 (20) |
| Flt3, no. patients (%) | |||
| Available | 13 (81) | 50 (93) | 63 (90) |
| Abnormal | 2 (15) | 6 (12) | 8 (11) |
| ITD | 2 (15) | 5 (10)‖ | 7 (14) |
| D835 | 3 (6)‖ | 3 (4) |
LD indicates low-dose; AHD, antecedent hematologic disorder; IM, insufficient metaphases; ITD, internal tandem duplication; D835, amino acid mutation at codon aspartate 835; and DNMTI, DNA methyltransferase inhibitor (decitabine or azacitidine).
One patient with history of both non-Hodgkin lymphoma (NHL) and carcinoma of the prostate (received radiation therapy [XRT] and chemotherapy for NHL and XRT for carcinoma of the prostate).
One patient with history of both mantle cell lymphoma and MDS.
One patient with carcinoma of the prostate and MDS, and 1 patient with history of renal cell carcinoma and CMML (of the 7 solid tumor patients, 3 received XRT only, 1 each chemotherapy or XRT, respectively, and 2 patients neither XRT nor chemotherapy).
One patient with biphenotypic acute leukemia.
Two patients with both ITD and D835.
A total of 35 (50%) patients had secondary AML/MDS, including 24 (34%) patients with a preceding phase of MDS, 7 (10%) with another form of antecedent hematologic disorder (non-Hodgkin lymphoma, mantle cell lymphoma, natural killer cell/T-cell lymphoma, CMML, and plasma cell myeloma), and 8 (11%) with various solid tumors. Prior therapies in cases with preceding MDS included hematopoietic growth factor support, thalidomide, azacitidine, and decitabine.
A total of 35 patients (50%) had abnormal cytogenetics (abnormalities of chromosomes 5 and/or 7 in 16 [23%] patients; del(11)(q23) in one patient). Results for fms-like tyrosine 3 (FLT3) kinase abnormalities were available in 63 (90%) patients. They were abnormal in 8 (11%) patients (2 [15%] with clofarabine; 6 [12%] with the combination). A total of 7 patients had internal tandem duplications (ITDs) and 3 amino acid mutations at the aspartate locus 835 (D835). Two patients had both ITD and D835 mutations.
Outcome
Response.
A total of 39 (56%) patients achieved CR and 2 (3%) patients achieved CRi (OR rate, 59%; Table 2). The response rate was higher with the combination (CR, 63% vs 31%, P = .025; OR, 67% vs 31%, P = .012). Median time to CR was 34 days (range, 25-124 days) with no difference by treatment arm. A total of 9 patients received a second induction course (3 patients with clofarabine, 6 patients with clofarabine plus low-dose cytarabine). A total of 5 (56%) patients achieved CR after 2 induction cycles (4 patients on the combination and 1 patient with clofarabine).
Response
| Response . | Clofarabine, no. (%) . | Clofarabine + LD cytarabine, no. (%) . | Total, no. (%) . | P . |
|---|---|---|---|---|
| Number in group | 16 | 54 | 70 | |
| CR | 5 (31) | 34 (63) | 39 (56) | .025 |
| CRi | 0 | 2 (4) | 2 (3) | |
| OR | 5 (31) | 36 (67) | 41 (59) | .012 |
| Treatment failure | 6 (38) | 8 (19) | 14 (20) | .046 |
| Induction mortality | 5 (31) | 10 (19) | 15 (21) | .276 |
| Response . | Clofarabine, no. (%) . | Clofarabine + LD cytarabine, no. (%) . | Total, no. (%) . | P . |
|---|---|---|---|---|
| Number in group | 16 | 54 | 70 | |
| CR | 5 (31) | 34 (63) | 39 (56) | .025 |
| CRi | 0 | 2 (4) | 2 (3) | |
| OR | 5 (31) | 36 (67) | 41 (59) | .012 |
| Treatment failure | 6 (38) | 8 (19) | 14 (20) | .046 |
| Induction mortality | 5 (31) | 10 (19) | 15 (21) | .276 |
LD indicates low-dose.
The P value of each row indicates comparison between treatment arms within each response category.
Characteristics of response by karyotype, FLT3 status, presence or absence of a diagnosis of secondary AML, and age are shown in Table 3. CR rates were higher in patients with diploid karyotypes (70% vs 47%; P = .046) and those who had no FLT3 abnormalities (65% vs 25%; P = .050). Interestingly, the difference in outcome between diploid and abnormal cytogenetics was most noticeable with the combination treatment (78% vs 48%), but not single-dose clofarabine (33% vs 38%). The low patient numbers in the clofarbine-only arm, however, make interpretation difficult. A continuous decrease of response rates occurred with increasing age from 80% in patients aged between 60 and 64 years to 40% in those older than 70 years (P = .009).
Characteristics of complete responders
| Characteristic . | Clofarabine, ratio (%) . | Clofarabine plus LD cytarabine, ratio (%) . | Total, ratio. (%) . | P . |
|---|---|---|---|---|
| Overall number of patients | 5/16 (31) | 34/54 (63) | 39/70 (56) | .025 |
| Karyotype | ||||
| Diploid | 2/6 (33) | 21/27 (78) | 23/33 (70) | .053 |
| Abnormal | 3/8 (38) | 13/27 (48) | 16/35 (46) | .07 |
| −5/−7 | 1/2 (50) | 6/14 (43) | 7/16 (44) | |
| +8 | 0/1 | 2/4 (50) | 2/5 (40) | |
| del(11)(q23) | 1/1 (100) | 1/1 (100) | ||
| Not available/IM | 0/2 | 0/2 | ||
| Flt3 status | ||||
| No abnormality | 5/11 (45) | 31/44 (70) | 36/55 (65) | .161 |
| Abnormal | 0/2 | 2/6 (33) | 2/8 (25) | |
| ITD | 0/2 | 1/5 (20)* | 1/7 (14) | |
| D835 | 2/3 (67)* | 2/3 (67) | ||
| Secondary AML | ||||
| Yes | 2/7 (29) | 15/28 (54) | 17/35 (49) | .402 |
| Yes with diploid karyotype | 0/1 | 8/12 (67) | 8/13 (62) | |
| No | 3/9 (33) | 19/26 (73) | 22/35 (63) | .051 |
| No with diploid karyotype | 1/4 (25) | 11/14 (79) | 12/18 (67) | .083 |
| Age, y | ||||
| 60 to 64 | 1/1 (100) | 7/9 (78) | 8/10 (80) | |
| 65 to 69 | 2/4 (50) | 13/16 (81) | 15/20 (75) | .249 |
| Older than 70 | 2/11 (18) | 14/29 (48) | 16/40 (40) | .148 |
| Characteristic . | Clofarabine, ratio (%) . | Clofarabine plus LD cytarabine, ratio (%) . | Total, ratio. (%) . | P . |
|---|---|---|---|---|
| Overall number of patients | 5/16 (31) | 34/54 (63) | 39/70 (56) | .025 |
| Karyotype | ||||
| Diploid | 2/6 (33) | 21/27 (78) | 23/33 (70) | .053 |
| Abnormal | 3/8 (38) | 13/27 (48) | 16/35 (46) | .07 |
| −5/−7 | 1/2 (50) | 6/14 (43) | 7/16 (44) | |
| +8 | 0/1 | 2/4 (50) | 2/5 (40) | |
| del(11)(q23) | 1/1 (100) | 1/1 (100) | ||
| Not available/IM | 0/2 | 0/2 | ||
| Flt3 status | ||||
| No abnormality | 5/11 (45) | 31/44 (70) | 36/55 (65) | .161 |
| Abnormal | 0/2 | 2/6 (33) | 2/8 (25) | |
| ITD | 0/2 | 1/5 (20)* | 1/7 (14) | |
| D835 | 2/3 (67)* | 2/3 (67) | ||
| Secondary AML | ||||
| Yes | 2/7 (29) | 15/28 (54) | 17/35 (49) | .402 |
| Yes with diploid karyotype | 0/1 | 8/12 (67) | 8/13 (62) | |
| No | 3/9 (33) | 19/26 (73) | 22/35 (63) | .051 |
| No with diploid karyotype | 1/4 (25) | 11/14 (79) | 12/18 (67) | .083 |
| Age, y | ||||
| 60 to 64 | 1/1 (100) | 7/9 (78) | 8/10 (80) | |
| 65 to 69 | 2/4 (50) | 13/16 (81) | 15/20 (75) | .249 |
| Older than 70 | 2/11 (18) | 14/29 (48) | 16/40 (40) | .148 |
Ratios are complete responders per number in group or subgroup.
LD indicates low-dose; IM, insufficient metaphases; ITD, internal tandem duplication; and D835, amino acid mutation at codon aspartate 835.
Two patients with both ITD and D835.
In the clofarabine-only arm, of 8 patients with cytogenetic abnormalities, 3 achieved CR. Of these 3 patients, 2 also achieved a cytogenetic CR (in one patient with complex cytogenetic abnormalities including monosomy 5). In the combination arm, 13 morphologic CRs were observed in 27 patients with cytogenetic abnormalities at diagnosis. Of 12 evaluable patients (in one patient no follow-up cytogenetic material was obtained), 7 (58%) also achieved a cytogenetic CR. This included one patient with 11q23 and 3 (50%) of 6 patients with chromosome 5 and/or chromosome 7 abnormalities.
A total of 15 (21%) patients died during the first course. Of these, 5 (31%) deaths occurred with clofarabine alone at a median of 14 days from start of therapy (range, 6-24 days), and 10 (19%) deaths with the combination at a median of 26 days (range, 9-48 days; P = .276). Early deaths (within 14 days of treatment start) occurred in 3 (19%) patients on clofarabine and 2 (4%) patients on the combination. Mortality was mainly due to infectious complications frequently culminating in multisystem organ failure with ongoing marrow aplasia. One death was due to intracerebral hemorrhage on day 26 of the induction with the combination. The median age of the patients who died during induction was 76 years (range, 67-83 years), with no significant differences by treatment arm.
Consolidation therapy was administered to the 5 (31%) complete responders in the clofarabine arm and a total of 35 (65%) patients (all complete responders and one patient with CRi) of the combination. The median number of consolidation courses was 2 (range, 1-10 courses) in the former and 3 (range, 1-8 courses) in the latter group.
Disease progression and survival.
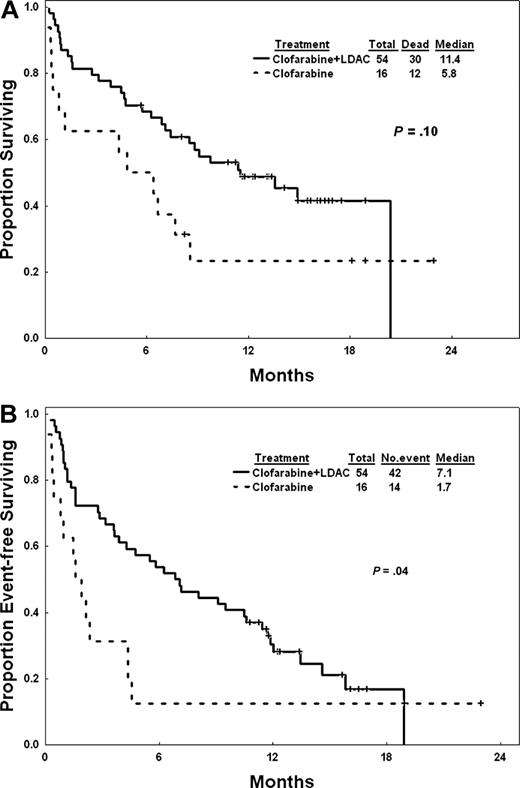
The median follow-up was 14.1 months (range, 5.7-20.4+ months) for clofarabine plus cytarabine and 18.9 months (range, 8.3-23 months) for clofarabine. The median overall survival was 11.4 months for patients on the combination and 5.8 months for those on clofarabine alone (P = .1; Figure 1A). Median event-free survival was 7.1 months for patients on the combination and 1.7 months for those on single-agent clofarabine (P = .04; Figure 1B). The median remission duration (excluding patients with CRi) for patients on the combination was 11.4 months (range, 1.9-18+ months), whereas it was not reached with single-agent clofarabine (range, 3.6-21.7+ months; P = .71; Figure 2A). The median survival of complete responders was not reached for the combination arm and was 8.5 months (range, 3.6-21.7+ months) with single-agent clofarabine (P = .28; Figure 2B).
Overall and event-free survival of all patients. (A) Overall survival. (B) Event-free survival.
Overall and event-free survival of all patients. (A) Overall survival. (B) Event-free survival.
Remission duration and survival of CR patients. (A) Remission duration. (B) Overall survival of complete responders.
Remission duration and survival of CR patients. (A) Remission duration. (B) Overall survival of complete responders.
Toxicity.
The most common nonhematologic adverse events were gastrointestinal and hepatic (Table 4). With clofarabine alone, bilirubin levels increased at a median of 10 days from start of therapy (range, 11-16 days) and returned to baseline at day 14 (range, 7-26 days). In patients treated with clofarabine plus low-dose cytarabine, hyperbilirubinemia occurred at a median of 6 days (range, 2-23 days) and resolved at a median of 13 days (range, 4-40 days). SGPT and/or SGOT elevations occurred at a median of 6 days (range, 5-9 days) with clofarabine and day 5 (range, 2-22 days) with clofarabine plus low-dose cytarabine, and resolved to baseline at a median of 10 days (range, 7-15 days with clofarabine and 4-38 days with the combination). In all cases, these abnormalities were transient and reversible.
Nonhematologic toxicities (frequency 10% or more of patients)
| Toxicity . | Clofarabine, n = 16 . | Clofarabine plus LD cytarabine, n = 54 . | ||
|---|---|---|---|---|
| Grade 2 or lower, no. (%) . | Grade 3 or higher, no. (%) . | Grade 2 or lower, no. (%) . | Grade 3 or higher, no. (%) . | |
| Diarrhea | 12 (75) | 45 (83) | 3 (6) | |
| Nausea | 12 (81) | 39 (72) | ||
| Hyperbilirubinemia | 4 (25) | 6 (38) | 35 (65) | 14 (26) |
| Mucositis | 2 (13) | 1 (6) | 24 (44) | |
| SGPT ↑ | 6 (38) | 4 (25) | 29 (54) | 6 (11) |
| Skin rashes | 7 (44) | 2 (13) | 27 (50) | 10 (19) |
| Headache | 6 (38) | 27 (50) | ||
| Emesis | 9 (56) | 27 (50) | ||
| Edema | 6 (38) | 29 (35) | ||
| SGOT ↑ | 2 (13) | 3 (19) | 18 (33) | x (13) |
| Anorexia | 3 (19) | 16 (30) | ||
| Alkaline phosphatase ↑ | 3 (19) | 15 (28) | 1 (2) | |
| Creatinine ↑ | 3 (19) | 2 (13) | 11 (20) | 3 (6) |
| Acute renal failure | 3 (19) | 8 (15) | ||
| Fatigue | 3 (19) | 9 (17) | 1 (2) | |
| Atrial fibrillation | 1 (6) | 2 (13) | 6 (11) | 3 (6) |
| Hand-foot syndrome | 2 (13) | 7 (13) | ||
| Facial flushing | 1 (6) | 7 (13) | ||
| Pruritus | 2 (13) | 5 (9) | ||
| Chest tightness | 6 (11) | |||
| Toxicity . | Clofarabine, n = 16 . | Clofarabine plus LD cytarabine, n = 54 . | ||
|---|---|---|---|---|
| Grade 2 or lower, no. (%) . | Grade 3 or higher, no. (%) . | Grade 2 or lower, no. (%) . | Grade 3 or higher, no. (%) . | |
| Diarrhea | 12 (75) | 45 (83) | 3 (6) | |
| Nausea | 12 (81) | 39 (72) | ||
| Hyperbilirubinemia | 4 (25) | 6 (38) | 35 (65) | 14 (26) |
| Mucositis | 2 (13) | 1 (6) | 24 (44) | |
| SGPT ↑ | 6 (38) | 4 (25) | 29 (54) | 6 (11) |
| Skin rashes | 7 (44) | 2 (13) | 27 (50) | 10 (19) |
| Headache | 6 (38) | 27 (50) | ||
| Emesis | 9 (56) | 27 (50) | ||
| Edema | 6 (38) | 29 (35) | ||
| SGOT ↑ | 2 (13) | 3 (19) | 18 (33) | x (13) |
| Anorexia | 3 (19) | 16 (30) | ||
| Alkaline phosphatase ↑ | 3 (19) | 15 (28) | 1 (2) | |
| Creatinine ↑ | 3 (19) | 2 (13) | 11 (20) | 3 (6) |
| Acute renal failure | 3 (19) | 8 (15) | ||
| Fatigue | 3 (19) | 9 (17) | 1 (2) | |
| Atrial fibrillation | 1 (6) | 2 (13) | 6 (11) | 3 (6) |
| Hand-foot syndrome | 2 (13) | 7 (13) | ||
| Facial flushing | 1 (6) | 7 (13) | ||
| Pruritus | 2 (13) | 5 (9) | ||
| Chest tightness | 6 (11) | |||
New onset (n = 8) or exacerbation of preexisting atrial fibrillation (n = 4) occurred in 12 (17%) patients (3 on clofarabine; 9 on clofarabine and low-dose cytarabine) at a median of 5 days (range, 3-24 days) from treatment start. A total of 7 of the 8 patients with new onset atrial fibrillation had a history of hypertension, coronary artery disease, or a previous myocardial infarction; 5 had concurrent infections. Serial assessments of left ventricular ejection fraction (LVEF) were done in 5 patients, with decreases of the LVEF by 8% to 10% in 3 patients.
Acute renal failure (ARF) requiring temporary hemodialysis occurred in 3 (19%) patients treated with clofarabine alone and 8 (15%) patients treated with the combination. The median time of onset of ARF was 6 days from treatment start in the clofarabine group and 9 days in the combination group. A total of 3 of the patients had baseline renal insufficiency, with a creatinine clearance of less than 0.8335 mL/sec (50 mL/min) before treatment. Tumor lysis syndrome occurred in 2 patients at the time of renal failure. More typically, ARF occurred in the setting of infections resulting in disseminated sepsis and multisystem organ failure in 2 patients.
The possibility of vascular leaking and third spacing of fluids has been raised in other trials with clofarabine. Although in the judgment of the investigators this was not a feature in the current study, it cannot completely be ruled out that some of the episodes of ARF or atrial fibrillation were related to manifestations of capillary leak syndrome.
Grade 3 myelosuppression was ubiquitous during treatment. Prolonged myelosuppression (failure to recover ANC and/or platelet count within 42 days from start of therapy) was infrequent and was only seen in 6 (11%) patients on the combination arm during induction (Table 5).
Myelosuppression and infectious complications
| . | Clofarabine, no. (%) . | Clofarabine plus LD cytarabine, no. (%) . |
|---|---|---|
| Number | 16 | 54 |
| Myelosuppression, prolonged* | 0 | 6 (11) |
| Pneumonia | ||
| Bacterial | 3 (19) | 13 (24) |
| Fungal | 1 (6) | 7 (13) |
| Urinary tract infections | ||
| Bacterial | 7 (13) | |
| Fungal | 1 (2) | |
| Sepsis | ||
| Bacterial | 6 (38) | 13 (24) |
| Fungal | 3 (6) | |
| Fever of unknown origin | 4 (25) | 10 (19) |
| Other | ||
| Sinusitis | 2 (4) | |
| Clostridium difficile toxin+ | 4 (8) | |
| Colitis | 1 (2) | |
| Cellulitis | 4 (25) | 1 (2) |
| Catheter site infections | 1 (2) | |
| Endophthalmitis (fungal) | 1 (2) | |
| Cytomegalovirus antigenemia | 1 (6) | |
| Herpes simplex virus (skin) | 1 (6) |
| . | Clofarabine, no. (%) . | Clofarabine plus LD cytarabine, no. (%) . |
|---|---|---|
| Number | 16 | 54 |
| Myelosuppression, prolonged* | 0 | 6 (11) |
| Pneumonia | ||
| Bacterial | 3 (19) | 13 (24) |
| Fungal | 1 (6) | 7 (13) |
| Urinary tract infections | ||
| Bacterial | 7 (13) | |
| Fungal | 1 (2) | |
| Sepsis | ||
| Bacterial | 6 (38) | 13 (24) |
| Fungal | 3 (6) | |
| Fever of unknown origin | 4 (25) | 10 (19) |
| Other | ||
| Sinusitis | 2 (4) | |
| Clostridium difficile toxin+ | 4 (8) | |
| Colitis | 1 (2) | |
| Cellulitis | 4 (25) | 1 (2) |
| Catheter site infections | 1 (2) | |
| Endophthalmitis (fungal) | 1 (2) | |
| Cytomegalovirus antigenemia | 1 (6) | |
| Herpes simplex virus (skin) | 1 (6) |
LD indicates low-dose.
Lack of blood recovery more than 42 days from treatment start during induction course.
Discussion
Our interest in developing clofarabine combinations had 3 objectives: (1) to address induction therapy of patients aged 60 years and older with AML; (2) to investigate lower doses of clofarabine; and (3) to assess the value of adding low-dose cytarabine to clofarabine. Burnett et al used 30 mg/m2 clofarabine daily for 5 days as induction therapy for patients with AML aged 65 years and older who were considered unsuitable for standard intensive treatment due to age, adverse cytogenetic profile, secondary AML, performance status, or comorbidity.13,14 The primary endpoint was OR rate. Of 19 patients with unfavorable cytogenetics, 53% responded, including a CR/CRi rate of 42%. In patients aged 70 years or older, the OR rate was 56%, with a CR/CRi rate of 49%. A total of 14 (21%) of 66 patients died during induction, of which 6 deaths (9%) were related to clofarabine. Median survival was greater than 6 months. These results confirmed a favorable outcome compared with low-dose cytarabine alone.
We intended to combine these results with our experience of the clofarabine combinations from earlier trials and to compare single-agent clofarabine at the same dose and schedule as used by the United Kingdom group with the combination. After 70 patients were randomized, the stopping rule for CR was met, confirming that the combination achieved a significantly higher CR rate compared with clofarabine alone. Time to CR was comparable. However, more patients experienced prolonged myelosuppression with clofarabine plus cytarabine. Use of hematopoietic growth factors (predominantly granulocyte colony-stimulating factor [G-CSF]) was more frequent in the combination arm (63% of patients vs 44% of patients with single-agent clofarabine). Induction mortality was not statistically significant between the 2 arms. Thus, response improved with an only moderately higher rate of toxicities and no clinically significant increase in supportive care requirements. With comparable follow-up time, patients on the combination arm also had better event-free survival, although not better overall survival.
The CR rate of 48% with single-agent clofarabine as reported by Burnett et al was higher than in our single-agent arm.13 This could be explained by differences of pretreatment characteristics and of the size of the 2 study groups. Half of our patients had either secondary AML or abnormal cytogenetics; the respective proportion of these patients was 24% and 31% in the Medical Research Council (MRC) trial. Another problem could have arisen from the Bayesian randomization in our study. Although patients are provided with a higher probability of assignment to the better-performing treatment, there are potential pitfalls with this approach. Adaptive randomization that occurs too soon may be misleading, not reflect an accurate picture of differences between the arms, and therefore skew assignment of patients into one specific treatment arm. Depending on the operating characteristics, study endpoints may be reached with too few patients. This may have happened in the current study, where early in the course of the study, the combination outperformed the single-agent arm, resulting in a significant slowing of the accrual to clofarabine alone. With only a few patient numbers in one arm, it remains difficult to account for biologic differences, and it is possible that patients with unfavorable features were accrued early to the single-agent arm, forcing an inextirpable mark on the treatment assignment that could not be rectified later. The MRC trial also treated a larger number of patients with single-agent clofarabine (n = 66).
The difference in induction mortality between the 2 arms, although not statistically significant, is likely to stem from the same bias. There is no reasonable explanation why single-agent clofarabine should have been any more toxic than the same dose of clofarabine in combination with another myelosuppressive drug. As there was no difference in supportive care (treatment in laminar air flow room) between the arms, neither between this study and any other induction program for older patients with AML, there is no justification to blame differences in supportive care either. It is also conceivable to assume that the combination is faster-acting in controlling manifestations of AML; hence, more complications might have occurred during the early phase with single-agent clofarabine when the disease remained uncontrolled in more patients. This argument, however, could be countered by the experience that clofarabine at 30 mg/m2 is sufficiently myelosuppressive by itself to allow rapid disease control and, in addition, very few of the older patients present with rapidly proliferating white blood cell counts.
The OR rate of the combination arm compares favorably with other investigational agents studied in elderly patients with AML, although an important question remains: how much different it is from standard therapy (eg, the “3+7” schedule of idarubicin plus cytarabine). Baseline expectations for outcome and toxicity with standard front-line chemotherapy for patients with AML older than 60 years have recently been described.18 The response rate in this age group has been 45%, with a few more patients achieving PR or CRi so that the OR rate is approximately 50%. Induction mortality has been as high as 38% depending on which particular front-line combination was used. The induction mortality with specifically idarubicin and cytarabine in patients older than 60 years has been reported to be 23%. Of these patients, 9% required hemodialysis, and additional grade 3/4 renal toxicity was noted in another 4%. A total of 34% required admission to the intensive care unit (ICU), and 20% of the patients required institution of mechanical ventilation.
Single-agent activity of the farnesyltransferase inhibitor tipifarnib in untreated patients older than 65 years with AML and high-risk MDS achieved a CR rate of 18% (OR, 34%).19 In a multicenter phase 2 study of cloretazine in front-line patients with AML (including 43% secondary AML) or high-risk MDS (14%), a CR rate of 27% (OR, 31%) has been reported.20 Luebbert et al treated 52 patients with AML with decitabine and reported a OR rate of 25%.21 In a small series of 11 previously untreated older patients, the combination of decitabine with valproic acid achieved CR in 5 (45%) patients.22
Although patients on the combination arm benefited with respect to better event-free survival, long-term outcome remains unsatisfying. Further efforts should be directed toward postremission consolidation strategies in order to maintain CR for these patients, where continuous repetition of induction-like chemotherapy courses is either of limited benefit or not feasible because of cumulative toxicities.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.F. designed the study, contributed and followed patients, analyzed the data, and wrote the paper; F.R. contributed and followed patients and helped in editing the manuscript; X.H. helped in the design of the study, analysis of data, and editing of the manuscript; G.G.-M., A.F., Z.E., S.V., and D.A.T. contributed and followed patients and helped in editing the manuscript; M.K. assisted in collecting and analyzing the data; and H.K.K. contributed in the design of the study, contributed and followed patients, and helped in editing the manuscript.
Conflict-of-interest disclosure: S.F., F.R., and H.K.K. are members of the Genzyme advisory board and receive research support for clinical studies. The remaining authors declare no competing financial interests.
Correspondence: Stefan Faderl, Department of Leukemia, Unit 428, The University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030; e-mail: sfaderl@mdanderson.org.