Abstract
We performed a retrospective comparison of presenting features, planned treatment, complete remission (CR) rate, and outcome of 321 adolescents and young adults (AYAs) ages 16 to 20 years with newly diagnosed acute lymphoblastic leukemia (ALL) who were treated on consecutive trials in either the Children's Cancer Group (CCG) or the Cancer and Leukemia Group B (CALGB) from 1988 to 2001. CR rates were identical, 90% for both CALGB and CCG AYAs. CCG AYAs had a 63% event-free survival (EFS) and 67% overall survival (OS) at 7 years in contrast to the CALGB AYAs, in which 7-year EFS was only 34% (P < .001; relative hazard rate [RHR] = 2.2) and OS was 46% (P < .001; RHR = 1.9). While CALGB AYAs aged 16 to 17 years achieved similar outcomes to all CCG AYAs with a 7-year EFS of 55%, the EFS for 18- to 20-year-old CALGB patients was only 29%. Comparison of the regimens showed that CCG AYAs received earlier and more intensive central nervous system prophylaxis and higher cumulative doses of nonmyelosuppressive agents. There were no differences in outcomes of those who reached maintenance therapy on time compared with those who were delayed. Based on these observations, a prospective study for AYAs with ALL using the more successful approach of the CCG has been initiated.
Introduction
Older adolescents and young adults (AYAs) ages 16 to 20 years with acute lymphoblastic leukemia (ALL) have worse outcomes than younger children, for whom cure now approaches 80% to 85%. Major decrements in survival start above 15 years of age.1,2 Specific outcome data for this age group is relatively sparse for several reasons. ALL is relatively uncommon among AYAs. These patients receive treatment from either pediatric or adult oncologists depending on referral pattern. Overall, the number of patients in this age range who are enrolled onto pediatric or adult clinical trials is small compared with the proportion of younger children or older adults who enter clinical trials.3 Furthermore, because these older adolescents comprise a relatively small percentage of either pediatric or adult ALL trial populations, they are often analyzed together with patients aged 10 to 15 years in pediatric series, or with patients 20 to 30 years and older in adult clinical trials
Biologically, there are important disease characteristics in the AYA ALL population. ALL in this age group is characterized by a higher incidence of precursor T-cell immunophenotype, higher hemoglobin levels at diagnosis, and a lower incidence of lymphomatous features compared with younger children.4 This age group has a significantly lower incidence of favorable cytogenetic features, such as the t(12;21)(p13;q22) cryptic translocation that results in the TEL/AML1 fusion gene and hyperdiploidy (chromosome number ≥ 51), compared with younger children. The incidence of the Philadelphia (Ph+) chromosome increases with age, and is present in only 5% to 7% of these patients, which is higher than the incidence in younger children, but much lower than in older adults.4-6 A number of studies have recently begun to examine the prognostic significance of gene expression patterns in children with ALL.7,8 However, distinct expression patterns in AYA patients have not yet been reported.
The treatment approach for AYAs differs between pediatric and adult ALL trials with variations in induction therapy, the intensity and duration of postremission therapy, the type and intensity of central nervous system (CNS) prophylaxis, and the duration of maintenance chemotherapy. AYAs treated on adult studies often receive chemotherapy agents and doses that are designed, in part, to be tolerable to a broad age group, ranging from 16 to 60 years of age. The Children's Cancer Group (CCG) has reported significant improvements in the outcome of high-risk patients, including adolescents (ages 13-20 years) with ALL, who were treated with more intensive postremission therapy, achieving a 5-year event-free survival (EFS) of 75%.2,8,9 The adult cooperative groups, including the Cancer and Leukemia Group B (CALGB), have also reported more favorable outcomes for younger adults in the last 10 years when intensified postremission strategies modeled after those pioneered in pediatric trials have been used.10-12
We wished to determine whether the outcome for AYA patients differed depending on their enrollment on pediatric compared with adult cooperative group trials. We performed a retrospective comparison of presenting clinical and molecular-cytogenetic features, type and dosage of planned treatment, remission rate, and clinical outcome of AYA patients with newly diagnosed ALL who were treated on consecutive CCG or CALGB cooperative group protocols from 1988 to 2001. Our results have generated hypotheses that are now being tested in prospective trials.
Methods
All patients provided informed consent according to federal and institutional guidelines and in accordance with the Declaration of Helsinki. All CALGB and CCG studies included in this analysis have been performed using Institutional Review Board–approved protocols. Many participating centers contributed to these trials, which were National Cancer Institute (NCI)–approved treatment trials administered by the CCG and the CALGB. These studies were all previously registered at www.ClinicalTrials.gov. The trials included in this analysis are CCG 1882, CCG 1901, CALGB 8811, CALGB 9111, CALGB 9311, CALGB 9511, and CALGB 19802.
Patient characteristics
Patient characteristics are shown in Table 1.
Patient characteristics
| . | CCG, 16 to 20 y, n = 197 . | CALGB, 16 to 20 y, n = 124 . | P . |
|---|---|---|---|
| Median age, y | 16 | 19 | < .001 |
| Sex, no. (%) male | 129 (65) | 87 (70) | .45 |
| Ethnic distribution, no. (%) | .89 | ||
| White | 141 (72) | 90 (73) | |
| Hispanic | 32 (16) | 19 (15) | |
| African-American | 13 (7) | 10 (8) | |
| Other | 11 (5) | 5 (4) | |
| Immunophenotype, no. (%), evaluable* | 143 | 92 | |
| Precursor-T | 23 (16) | 23 (25) | .56 |
| Precursor-B | 93 (65) | 64 (70) | .13 |
| Other† | 27 (19) | 5 (5) | .006 |
| Cytogenetics, no. (%), evaluable* | 67 | 81 | |
| t(9;22) | 2 (3) | 5 (6) | .60 |
| t(4;11) | 2 (3) | 2 (2) | .75 |
| Initial WBCs more than 50 × 109/L, no. (%) | 47 (24) | 26 (21) | .64 |
| . | CCG, 16 to 20 y, n = 197 . | CALGB, 16 to 20 y, n = 124 . | P . |
|---|---|---|---|
| Median age, y | 16 | 19 | < .001 |
| Sex, no. (%) male | 129 (65) | 87 (70) | .45 |
| Ethnic distribution, no. (%) | .89 | ||
| White | 141 (72) | 90 (73) | |
| Hispanic | 32 (16) | 19 (15) | |
| African-American | 13 (7) | 10 (8) | |
| Other | 11 (5) | 5 (4) | |
| Immunophenotype, no. (%), evaluable* | 143 | 92 | |
| Precursor-T | 23 (16) | 23 (25) | .56 |
| Precursor-B | 93 (65) | 64 (70) | .13 |
| Other† | 27 (19) | 5 (5) | .006 |
| Cytogenetics, no. (%), evaluable* | 67 | 81 | |
| t(9;22) | 2 (3) | 5 (6) | .60 |
| t(4;11) | 2 (3) | 2 (2) | .75 |
| Initial WBCs more than 50 × 109/L, no. (%) | 47 (24) | 26 (21) | .64 |
WBC indicates white blood cell.
Patients were reviewed and confirmed by central cooperative group pathology and cytogenetic committees.
Different criteria were used by CCG and CALGB to characterize the immunophenotype and may account for the differences in numbers of patients in the ″other″ category. For CCG, the ″other″ category includes patients with coexpression of both B and T markers on lymphoblasts (14 patients) and 13 patients where the immunophenotype could not be fully resolved. For CALGB, only patients with coexpression of B and T markers were included in the ″other″ category.
CALGB
From 1988 to 2001, 124 patients ages 16 to 20 years were enrolled on 5 sequential CALGB trials for newly diagnosed adult ALL. Patients with mature B-cell ALL (Burkitt type) were excluded from these trials. A total of 87 (70%) patients were male. Of those, 90 (73%) were white, 19 were Hispanic, 10 were African-American, and 5 had other ethnic backgrounds. The median presenting white blood cell (WBC) count was 10.4 × 109/L (range, 0.2-401 × 109/L); 26 (21%) patients had a presenting WBC count greater than 50 × 109/L. Immunophenotype was reviewed centrally in 92 patients; 64 (70%) had precursor B-cell ALL, 23 (25%) had precursor T-cell ALL, and 5 had ALL, otherwise unclassified. Central cytogenetic review was evaluable for 81 patients and included 5 with a t(9;22)(q34;q11) and 2 with a t(4;11)(q21;q23).
CCG
A total of 197 patients aged 16 to 20 years were enrolled on CCG studies from 1989 to 1995. Of these, 129 (65%) were male. A total of 141 (72%) were white; 32 were Hispanic, 13 were African-American, and 11 patients had other ethnic backgrounds. The median presenting WBC count was 12.2 × 109/L (range 0.6-587 × 109/L). A presenting WBC count greater than 50 × 109/L was noted in 47 (24%) patients. Central immunophenotypic review revealed that 93 (65%) were precursor B-cell ALL and 23 (16%) were precursor T-cell ALL. Central cytogenetic review was performed for 67 patients; of these, 2 had a t(9;22)(q34;q11) and 2 had a t(4;11)(q21;q23).
Chemotherapy
A total of 759 adults (aged 16-81 years), including the 124 (16%) patients aged 16 to 20 years, with previously untreated ALL were enrolled on one of 5 sequential CALGB studies from 1988 to 2001 (Table 2): CALGB 8811 (1988-1990),11 CALGB 9111 (1991-1992),12 CALGB 9311 (1993-1994),13 CALGB 9511 (1995-1998),14 and CALGB 19802 (1999-2001).15 The basic regimen for each of these trials, except protocol 19802, was similar, consisting of a 5-drug induction followed by an intensive postremission consolidation modeled after the Berlin-Frankfurt-Munster (BFM) program and included intrathecal chemotherapy and cranial irradiation (see Table 2 for regimen of CALGB 8811). All subsequent trials had slight variations from the 8811 schema. Briefly, CALGB 9111 used the identical treatment but contained a randomization to granulocyte colony-stimulating factor (G-CSF) or placebo during induction and early intensification therapy. CALGB 9311 added an experimental course of treatment with an anti-B4 blocked ricin monoclonal antibody for patients with B-lineage disease, or high-dose cytarabine for patients with T-lineage ALL that was introduced between the 2 early intensification courses. In CALGB 9511, pegylated asparaginase was substituted for L-asparaginase during induction and consolidation therapy. In CALGB 19802, the daunorubicin and cytarabine doses were escalated in separate treatment modules, and high-dose systemic methotrexate with intrathecal methotrexate (IT MTX) replaced prophylactic cranial irradiation (see Tables 4 and 5). In all 5 CALGB studies, the first scheduled lumbar puncture for IT MTX occurred on or shortly after day 29 of induction. One CALGB AYA patient underwent allogeneic stem cell transplantation in first remission.
CALGB 8811 regimen
| . | Method . | Dose . | Day . |
|---|---|---|---|
| Induction (4 wk) | |||
| Cyclophosphamide | IV | 1200 mg/m2 | 1 |
| Daunorubicin* | IV | 45 mg/m2 | 1, 2, 3, |
| Vincristine | IV | 2 mg | 1, 8, 15, 22 |
| Prednisone | PO/IV | 60 mg/m2 per day | 1-21 |
| l-asparaginase | SC | 6000 IU/m2 | 5, 8, 11, 15, 18, 22 |
| Early intensification (4 wk—repeat once) | |||
| Intrathecal methotrexate | 15 mg | 1 | |
| Cyclophosphamide | IV | 1000 mg/m2 | 1 |
| 6-mercaptopurine | PO | 60 mg/m2 per day | 1-14 |
| Cytarabine | SC | 75 mg/m2 per day | 1-4, 8-11 |
| Vincristine | IV | 2 mg | 15, 22 |
| l-asparaginase | SC | 6000 IU/m2 | 15, 18, 22, 25 |
| CNS prophylaxis and interim maintenance | |||
| Cranial irradiation | 2400 cGy | 1-12 | |
| Intrathecal methotrexate | 15 mg | 1, 8, 15, 22, 29 | |
| 6-mercaptopurine | PO | 60 mg/m2 per day | 1-70 |
| Methotrexate | PO | 20 mg/m2 | 36, 43, 50, 57, 64 |
| Late intensification (8 wk) | |||
| Doxorubicin | IV | 30 mg/m2 | 1, 8, 15 |
| Vincristine | IV | 2 mg | 1, 8, 15 |
| Dexamethasone | PO | 10 mg/m2 per day | 1-14 |
| Cyclophosphamide | IV | 1000 mg/m2 | 29 |
| 6-thioguanine | PO | 60 mg/m2 per day | 29-42 |
| Cytarabine | SC | 75 mg/m2 per day | 29-32, 36-39 |
| Long-term maintenance (until 24 mo from diagnosis) | |||
| Vincristine | IV | 2 mg | 1 of every 4 wk |
| Prednisone | PO | 60 mg/m2 per day | 1-5 of every 4 wk |
| Methotrexate | PO | 20 mg/m2 | 1, 8, 15, 22 |
| 6-mercaptopurine | PO | 60 mg/m2 per day | 1-28 |
| . | Method . | Dose . | Day . |
|---|---|---|---|
| Induction (4 wk) | |||
| Cyclophosphamide | IV | 1200 mg/m2 | 1 |
| Daunorubicin* | IV | 45 mg/m2 | 1, 2, 3, |
| Vincristine | IV | 2 mg | 1, 8, 15, 22 |
| Prednisone | PO/IV | 60 mg/m2 per day | 1-21 |
| l-asparaginase | SC | 6000 IU/m2 | 5, 8, 11, 15, 18, 22 |
| Early intensification (4 wk—repeat once) | |||
| Intrathecal methotrexate | 15 mg | 1 | |
| Cyclophosphamide | IV | 1000 mg/m2 | 1 |
| 6-mercaptopurine | PO | 60 mg/m2 per day | 1-14 |
| Cytarabine | SC | 75 mg/m2 per day | 1-4, 8-11 |
| Vincristine | IV | 2 mg | 15, 22 |
| l-asparaginase | SC | 6000 IU/m2 | 15, 18, 22, 25 |
| CNS prophylaxis and interim maintenance | |||
| Cranial irradiation | 2400 cGy | 1-12 | |
| Intrathecal methotrexate | 15 mg | 1, 8, 15, 22, 29 | |
| 6-mercaptopurine | PO | 60 mg/m2 per day | 1-70 |
| Methotrexate | PO | 20 mg/m2 | 36, 43, 50, 57, 64 |
| Late intensification (8 wk) | |||
| Doxorubicin | IV | 30 mg/m2 | 1, 8, 15 |
| Vincristine | IV | 2 mg | 1, 8, 15 |
| Dexamethasone | PO | 10 mg/m2 per day | 1-14 |
| Cyclophosphamide | IV | 1000 mg/m2 | 29 |
| 6-thioguanine | PO | 60 mg/m2 per day | 29-42 |
| Cytarabine | SC | 75 mg/m2 per day | 29-32, 36-39 |
| Long-term maintenance (until 24 mo from diagnosis) | |||
| Vincristine | IV | 2 mg | 1 of every 4 wk |
| Prednisone | PO | 60 mg/m2 per day | 1-5 of every 4 wk |
| Methotrexate | PO | 20 mg/m2 | 1, 8, 15, 22 |
| 6-mercaptopurine | PO | 60 mg/m2 per day | 1-28 |
CCG treatment schema
| Standard therapy (CCG-BFM) . | Augmented BFM therapy . | ||
|---|---|---|---|
| Treatment . | Dose . | Treatment . | Dose . |
| Consolidation phase (5 wk) | Consolidation phase (9 wk) | ||
| Prednisone | 7.5 mg/m2, d 0; 3.75 mg/m2, d 1, 2 | Cyclophosphamide | 1000 mg/m2 IV, d 0, 28 |
| Cyclophosphamide | 1000 mg/m2 IV, d 0, 14 | Cytarabine | 75 mg/m2 SC or IV, d 1-4, 8-11, 29-32, 36-39 |
| Mercaptopurine | 60 mg/m2 PO, d 0-27 | Mercaptopurine | 60 mg/m2 PO, d 0-13, 28-41 |
| Vincristine | 1.5 mg/m2 IV, d 14, 21, 42, 49 | Vincristine | 1.5 mg/m2 IV, d 14, 21, 42, 49 |
| Cytarabine | 75 mg/m2 IV, d 1-4, 8-11, 15-18, 22-25 | Asparaginase | 6000 U/m2 IM, d 14,16, 18, 21, 23, 25, 42, 44, 46, 49, 51, 53 |
| Intrathecal methotrexate* | 12 mg, d 1, 8, 15, 22 | Intrathecal methotrexate* | 12 mg, d 1, 8, 15, 22 |
| Radiotherapy† | Cranial, 1800 cGy; cranial, 2400 cGy, and spinal, 600 cGy | Radiotherapy† | Cranial, 1800 cGy; cranial, 2400 cGy, and spinal, 600 cGy; testicular, 2400 cGy |
| Interim maintenance phase (8 wk) | Interim maintenance phase (8 wk) | ||
| Mercaptopurine | 60 mg/m2 PO, d 0-41 | Vincristine | 1.5 mg/m2 IV, d 0, 10, 20, 30, 40 |
| Methotrexate | 15 mg/m2 PO, d 0, 7, 14, 21, 28, 35 | Methotrexate | 100 mg/mg2 IV, d 0, 10, 20, 30, 40 (escalate by 50 mg/m2 per dose) |
| Asparaginase | 15 000 IU/m2 IM, d 1, 11, 21, 31, 41 | ||
| Delayed intensification phase (7 wk) | Delayed intensification I phase (8 wk) | ||
| Reinduction phase (4 wk) | Reinduction phase (4 wk) | ||
| Dexamethasone | 10 mg/m2 PO, d 0-20, then taper for 7 d | Dexamethasone | 10 mg/m2 PO, d 0-20, then taper for 7 d |
| Vincristine | 1.5 mg/m2 IV, d 0,14, 21 | Vincristine | 1.5 mg/m2 IV, d 0, 14, 21 |
| Doxorubicin | 25 mg/m2 IV, d 0, 7, 14 | Doxorubicin | 25 mg/m2 IV, d 0, 7, 14 |
| Reconsolidation phase (3 wk) | Reconsolidation phase (4 wk) | ||
| l-Asparaginase | 6000 IU/m2 IM, d 3, 5, 7, 10, 12, 14 | l-Asparaginase | 6000 /m2 IM, d 3, 5, 7, 10, 12, 14 |
| Vincristine | 1.5 mg/m2 IV, d 42, 29 | Vincristine | 1.5 mg/m2 IV, d 42, 49 |
| Cyclophosphamide | 1000 mg/m2 IV, d 28 | Cyclophosphamide | 1000 mg/m2 IV, d 28 |
| Thioguanine | 60 mg/m2 PO, d 28-41 | Thioguanine | 60 mg/m2 PO, d 28-41 |
| Cytarabine | 75 mg/m2 SC or IV, d 29-32, 36-39 | Cytarabine | 75 mg/m2/d SC or IV, d 29-32, 36-39 |
| Intrathecal methotrexate* | 12 mg, d 29, 36 | Intrathecal methotrexate* | 12 mg, d 29, 36 |
| l-asparaginase | 6000 IU/m2 IV, d 42, 44, 46, 49, 51,53 | ||
| Interim maintenance II phase (8 wk) | |||
| Vincristine | 1.5 mg/m2 IV, d 0, 10, 20, 30, 40 | ||
| Methotrexate | 100 mg/m2 IV, d 0, 10, 20, 30, 40 (escalate by 50 mg/m2 per dose) | ||
| [SCAP]L[R]-asparaginase | 15 000 IU/m2 IM, d 1, 11, 21, 31, 41 | ||
| Intrathecal methotrexate* | 12 mg, d 0, 20, 40 | ||
| Delayed intensification II phase (8 wk) | Same as for delayed intensification I phase | ||
| Long-term maintenance phase (12 wk)‡ | Long-term maintenance phase (12 wk)‡ | ||
| Vincristine | 1.5 mg/m2 IV, d 0, 28, 56 | Vincristine | 1.5 mg/m2 IV, d 0, 28, 56 |
| Prednisone | 40 mg/m2 PO, d 0-4, 28-32, 56-60 | Prednisone | 60 mg/m2 PO, d 0-14, 28-32, 56-60 |
| Mercaptopurine | 75 mg/m2 PO, d 0-83 | Mercaptopurine | 75 mg/m2 PO, d 0-83 |
| Methotrexate | 20 mg/m2 PO, d 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77 | Methotrexate | 20 mg/m2 PO, d 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77 |
| Intrathecal methotrexate* | 12 mg, d 0 | Intrathecal methotrexate* | 12 mg, d 0 |
| Standard therapy (CCG-BFM) . | Augmented BFM therapy . | ||
|---|---|---|---|
| Treatment . | Dose . | Treatment . | Dose . |
| Consolidation phase (5 wk) | Consolidation phase (9 wk) | ||
| Prednisone | 7.5 mg/m2, d 0; 3.75 mg/m2, d 1, 2 | Cyclophosphamide | 1000 mg/m2 IV, d 0, 28 |
| Cyclophosphamide | 1000 mg/m2 IV, d 0, 14 | Cytarabine | 75 mg/m2 SC or IV, d 1-4, 8-11, 29-32, 36-39 |
| Mercaptopurine | 60 mg/m2 PO, d 0-27 | Mercaptopurine | 60 mg/m2 PO, d 0-13, 28-41 |
| Vincristine | 1.5 mg/m2 IV, d 14, 21, 42, 49 | Vincristine | 1.5 mg/m2 IV, d 14, 21, 42, 49 |
| Cytarabine | 75 mg/m2 IV, d 1-4, 8-11, 15-18, 22-25 | Asparaginase | 6000 U/m2 IM, d 14,16, 18, 21, 23, 25, 42, 44, 46, 49, 51, 53 |
| Intrathecal methotrexate* | 12 mg, d 1, 8, 15, 22 | Intrathecal methotrexate* | 12 mg, d 1, 8, 15, 22 |
| Radiotherapy† | Cranial, 1800 cGy; cranial, 2400 cGy, and spinal, 600 cGy | Radiotherapy† | Cranial, 1800 cGy; cranial, 2400 cGy, and spinal, 600 cGy; testicular, 2400 cGy |
| Interim maintenance phase (8 wk) | Interim maintenance phase (8 wk) | ||
| Mercaptopurine | 60 mg/m2 PO, d 0-41 | Vincristine | 1.5 mg/m2 IV, d 0, 10, 20, 30, 40 |
| Methotrexate | 15 mg/m2 PO, d 0, 7, 14, 21, 28, 35 | Methotrexate | 100 mg/mg2 IV, d 0, 10, 20, 30, 40 (escalate by 50 mg/m2 per dose) |
| Asparaginase | 15 000 IU/m2 IM, d 1, 11, 21, 31, 41 | ||
| Delayed intensification phase (7 wk) | Delayed intensification I phase (8 wk) | ||
| Reinduction phase (4 wk) | Reinduction phase (4 wk) | ||
| Dexamethasone | 10 mg/m2 PO, d 0-20, then taper for 7 d | Dexamethasone | 10 mg/m2 PO, d 0-20, then taper for 7 d |
| Vincristine | 1.5 mg/m2 IV, d 0,14, 21 | Vincristine | 1.5 mg/m2 IV, d 0, 14, 21 |
| Doxorubicin | 25 mg/m2 IV, d 0, 7, 14 | Doxorubicin | 25 mg/m2 IV, d 0, 7, 14 |
| Reconsolidation phase (3 wk) | Reconsolidation phase (4 wk) | ||
| l-Asparaginase | 6000 IU/m2 IM, d 3, 5, 7, 10, 12, 14 | l-Asparaginase | 6000 /m2 IM, d 3, 5, 7, 10, 12, 14 |
| Vincristine | 1.5 mg/m2 IV, d 42, 29 | Vincristine | 1.5 mg/m2 IV, d 42, 49 |
| Cyclophosphamide | 1000 mg/m2 IV, d 28 | Cyclophosphamide | 1000 mg/m2 IV, d 28 |
| Thioguanine | 60 mg/m2 PO, d 28-41 | Thioguanine | 60 mg/m2 PO, d 28-41 |
| Cytarabine | 75 mg/m2 SC or IV, d 29-32, 36-39 | Cytarabine | 75 mg/m2/d SC or IV, d 29-32, 36-39 |
| Intrathecal methotrexate* | 12 mg, d 29, 36 | Intrathecal methotrexate* | 12 mg, d 29, 36 |
| l-asparaginase | 6000 IU/m2 IV, d 42, 44, 46, 49, 51,53 | ||
| Interim maintenance II phase (8 wk) | |||
| Vincristine | 1.5 mg/m2 IV, d 0, 10, 20, 30, 40 | ||
| Methotrexate | 100 mg/m2 IV, d 0, 10, 20, 30, 40 (escalate by 50 mg/m2 per dose) | ||
| [SCAP]L[R]-asparaginase | 15 000 IU/m2 IM, d 1, 11, 21, 31, 41 | ||
| Intrathecal methotrexate* | 12 mg, d 0, 20, 40 | ||
| Delayed intensification II phase (8 wk) | Same as for delayed intensification I phase | ||
| Long-term maintenance phase (12 wk)‡ | Long-term maintenance phase (12 wk)‡ | ||
| Vincristine | 1.5 mg/m2 IV, d 0, 28, 56 | Vincristine | 1.5 mg/m2 IV, d 0, 28, 56 |
| Prednisone | 40 mg/m2 PO, d 0-4, 28-32, 56-60 | Prednisone | 60 mg/m2 PO, d 0-14, 28-32, 56-60 |
| Mercaptopurine | 75 mg/m2 PO, d 0-83 | Mercaptopurine | 75 mg/m2 PO, d 0-83 |
| Methotrexate | 20 mg/m2 PO, d 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77 | Methotrexate | 20 mg/m2 PO, d 7, 14, 21, 28, 35, 42, 49, 56, 63, 70, 77 |
| Intrathecal methotrexate* | 12 mg, d 0 | Intrathecal methotrexate* | 12 mg, d 0 |
Induction was as follows: vincristine, 1.5 mg/m2 per week for 4 weeks; daunomycin, 25 mg/m2 per week for 4 weeks; prednisone, 60 mg/m2 per day for 28 days; l-asparaginase, 6000 IU/m2 IM 3 times a week for 9 doses; intrathecal cytarabine on day 0; and intrathecal methotrexate on day 14.
IM indicates intramuscular.
Patients with CNS disease at diagnosis did not receive intrathecal methotrexate on days 15 and 22 of consolidation therapy.
During the first 2 weeks of consolidation therapy, patients without CNS disease at diagnosis received 1800 cGy of cranial radiotherapy in 10 fractions; patients with CNS disease at diagnosis received 2400 cGy to the cranial midplane in 12 fractions and 600 cGy to the spinal cord in 3 fractions. In the augmented therapy group, patients with testicular enlargement at diagnosis received 2400 cGy bilateral testicular radiation in 8 fractions.
The cycles of maintenance therapy were repeated until the total duration of therapy, beginning with the first interim maintenance period, reached 2 years for girls and 3 years for boys.
Comparison of planned cumulative drug doses during induction
| . | CCG 1882 . | CALGB . |
|---|---|---|
| Prednisone, mg/m2 | 1680 | 1260 |
| Vincristine, mg | 8 | 8 |
| l-Asparaginase, U/m2 | 54 000 | 36 000 |
| Daunorubicin, mg/m2 | 100 | 135-240* |
| Cyclophosphamide, mg/m2 | 1200 | |
| IT-methotrexate, d | 14 | |
| IT-cytarabine, d | 0 |
| . | CCG 1882 . | CALGB . |
|---|---|---|
| Prednisone, mg/m2 | 1680 | 1260 |
| Vincristine, mg | 8 | 8 |
| l-Asparaginase, U/m2 | 54 000 | 36 000 |
| Daunorubicin, mg/m2 | 100 | 135-240* |
| Cyclophosphamide, mg/m2 | 1200 | |
| IT-methotrexate, d | 14 | |
| IT-cytarabine, d | 0 |
*In CALGB 19802, all patients younger than 60 years received daunorubicin at 80 mg/m2 per day for 3 days.
Comparison of planned cumulative drug doses during postremission therapy
| . | CCG 1882-BFM, n = 118 . | CCG 1882 A-BFM, n = 39 . | CALGB, n = 112 . |
|---|---|---|---|
| Dexamethasone, mg/m2 | 210 | 420 | 140 |
| Vincristine, mg/m2 | 22.5 | 45 | 14 mg total |
| l-Asparaginase, U/m2 | 90 000 | 318 000 | 48 000 |
| Doxorubicin, mg/m2 | 75 | 150 | 90 |
| Cytarabine, mg/m2 | 1800 | 2400 | 1200* |
| Cyclophosphamide, mg/m2 | 3000 | 4000 | 3000 |
| 6-Thioguanine, mg/m2 | 840 | 1680 | 840 |
| 6-Mercaptopurine, mg/m2 | 4080 | 4080 | 5040 |
| Methotrexate (IV or oral), mg/m2 | 90 | 1000 | 100† |
| Intrathecal methotrexate and cranial radiotherapy | 132 mg + 1800 cGy or 216 mg + no radiotherapy | 132 mg, 1800 cGy | 105 mg‡, 2400 cGy |
| . | CCG 1882-BFM, n = 118 . | CCG 1882 A-BFM, n = 39 . | CALGB, n = 112 . |
|---|---|---|---|
| Dexamethasone, mg/m2 | 210 | 420 | 140 |
| Vincristine, mg/m2 | 22.5 | 45 | 14 mg total |
| l-Asparaginase, U/m2 | 90 000 | 318 000 | 48 000 |
| Doxorubicin, mg/m2 | 75 | 150 | 90 |
| Cytarabine, mg/m2 | 1800 | 2400 | 1200* |
| Cyclophosphamide, mg/m2 | 3000 | 4000 | 3000 |
| 6-Thioguanine, mg/m2 | 840 | 1680 | 840 |
| 6-Mercaptopurine, mg/m2 | 4080 | 4080 | 5040 |
| Methotrexate (IV or oral), mg/m2 | 90 | 1000 | 100† |
| Intrathecal methotrexate and cranial radiotherapy | 132 mg + 1800 cGy or 216 mg + no radiotherapy | 132 mg, 1800 cGy | 105 mg‡, 2400 cGy |
In CALGB 19802, patients received a 10-fold higher cumulative dose of 12 000 mg/m2 of cytarabine.
In CALGB 19802, patients received a total of 6600 mg/m2 of systemic methotrexate.
In CALGB 19802, patients received a total of 120 mg of IT methotrexate; cranial irradiation was omitted.
From 1989 to 1995, the CCG focused on a risk-stratified therapeutic approach for the treatment of ALL. Of the 197 evaluable 16- to 20-year-old patients, 175 enrolled on CCG 1882.16 On this trial, patients were stratified to receive postremission therapy based on the results of an induction day-7 bone marrow examination.16 One hundred rapid early responders (RERs), defined as those patients with less than 25% blasts in the day-7 bone marrow aspirate, received a CCG-modified BFM17 treatment approach published previously16 and outlined in Table 3 with or without cranialirradiation. Slow early responders (SERs), defined as those with more than 25% blasts in the day-7 marrow aspirate, received either the same CCG-modified BFM regimen as RER patients with cranial irradiation (n = 18), or a CCG-augmented BFM regimen (A-BFM)2 with cranial irradiation (n = 39). A-BFM, outlined in Table 3, included higher cumulative doses of vincristine, L-asparaginase, and corticosteroids during the first year of therapy and incorporated intravenous MTX without leucovorin rescue into postremission therapy. A total of 22 patients with lymphomatous features at presentation were enrolled on CCG 1901 using the “New York” regimen,18 which included a 5-drug induction similar to the one used by the CALGB. A total of 3 CCG patients received an allogeneic stem cell transplant in first remission.
Statistical analysis
Statistical analyses were performed by CCG and CALGB statisticians. Chi-square tests for homogeneity of proportions were used to assess the similarities between patients in the CCG and CALGB. Outcome analyses used life table methods and associated statistics. The primary endpoints examined were event-free survival (EFS) and overall survival (OS) from the time of study entry to last contact. The EFS events were relapse at any site, death at any time, or a remission induction failure, whichever occurred first. A complete remission (CR) was defined as recovery of normal blood counts, less than 5% blasts in the bone marrow, and disappearance of all extramedullary disease. Patients who did not achieve a CR after 2 induction cycles of therapy were considered induction failures and taken off study and followed for survival. Data on patients who had not had an event at the time of analysis were censored in the analysis of EFS at the time of the last contact. Life table estimates were calculated by the Kaplan-Meier (KM) procedure, and the confidence interval (CI) was calculated using the standard error obtained from the Peto method of the life table estimate.19,20 The log-rank test was used to compare outcome in treatment or prognostic groups, and estimates (with the associated CI) of the relative hazard rate (RHR) and the relative incidence rate (RIR) were obtained from the Cox regression. The KM life table estimates (with the associated CI) are presented for the 7-year time point unless otherwise stated. All patients have had a minimum follow-up of 7 years.
Results
Patient characteristics
Outcome analysis included only those patients entered onto CCG and CALGB trials between the ages of 16 and 20 years, inclusive. The 2 groups were well matched with respect to sex, ethnicity, immunophenotype, and initial WBC count as shown in Table 1. There were no remarkable differences among the patients who had central karyotype review. However, the age distribution differed significantly between the CCG and CALGB patients with median ages of 16 and 19 years, respectively (P < .001). A total of 85% of CCG patients were 16 to 17 years old, whereas 80% of CALGB patients were 18 to 20 years old.
Clinical outcome
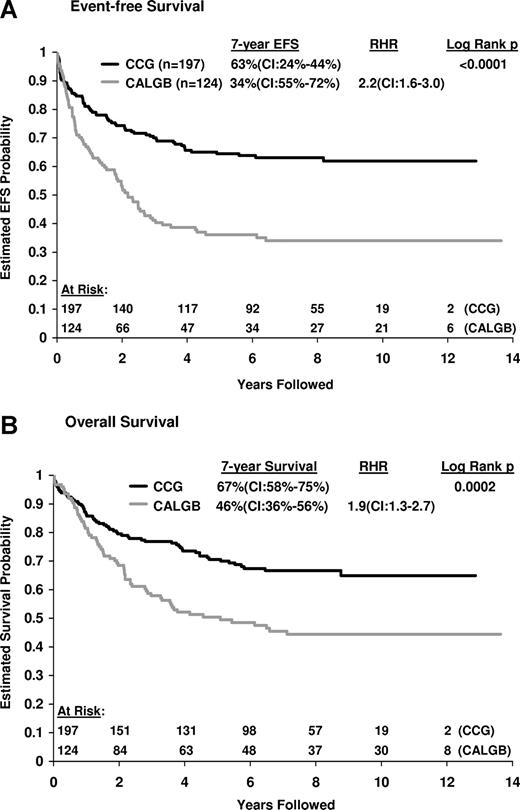
Clinical results are summarized in Table 6 and Figure 1A,B. A total of 152 events occurred; 14 patients failed to achieve CR, 23 patients died in remission, and 115 patients relapsed. CR rates were identical (90%) for both CCG and CALGB patients. However, significant differences in survival between the 2 study populations were noted. CCG patients had a 63% 7-year EFS (CI, 55%-72%) and a 67% OS (CI, 58%-75%). In contrast, for CALGB patients, 7-year EFS was only 34% (CI, 24%-44%; P < .001), the RHR was 2.2 (CI,1.6-3.0), and the OS was 46% (CI, 36%-56%; P < .001), the RHR was 1.9 (CI, 1.3-2.7). As demonstrated by the survival curve in Figure 1B, the median OS for patients on CALGB studies was 4.2 years.
Treatment outcome
| . | CCG, n = 197 . | CALGB, n = 124 . | P . | RHR . |
|---|---|---|---|---|
| Complete remission, no. patients (%) | 177 (90) | 112 (90) | .89 | |
| 7-y EFS, % (CI) | 63 (55-72) | 34 (24-44) | < .001 | 2.2 (1.6-3.0) |
| EFS by phenotype, % (CI) | ||||
| B-lineage | 54 (42-67) | 37 (24-51) | .03 | 1.6 (1.1-2.5) |
| T-lineage | 65 (42-77) | 37 (14-61) | .08 | |
| EFS by WBC, % (CI) | ||||
| Less than 50 × 109/L | 65 (56-75) | 37 (26-48) | < .001 | 2.2 (1.5-3.2) |
| More than 50 × 109/L | 56 (38-74) | 22 (5-40) | .009 | 2.3 (1.2-4.2) |
| 7-y survival, % (CI) | 67 (58-75) | 46 (36-56) | < .001 | 1.9 (1.3-2.7) |
| . | CCG, n = 197 . | CALGB, n = 124 . | P . | RHR . |
|---|---|---|---|---|
| Complete remission, no. patients (%) | 177 (90) | 112 (90) | .89 | |
| 7-y EFS, % (CI) | 63 (55-72) | 34 (24-44) | < .001 | 2.2 (1.6-3.0) |
| EFS by phenotype, % (CI) | ||||
| B-lineage | 54 (42-67) | 37 (24-51) | .03 | 1.6 (1.1-2.5) |
| T-lineage | 65 (42-77) | 37 (14-61) | .08 | |
| EFS by WBC, % (CI) | ||||
| Less than 50 × 109/L | 65 (56-75) | 37 (26-48) | < .001 | 2.2 (1.5-3.2) |
| More than 50 × 109/L | 56 (38-74) | 22 (5-40) | .009 | 2.3 (1.2-4.2) |
| 7-y survival, % (CI) | 67 (58-75) | 46 (36-56) | < .001 | 1.9 (1.3-2.7) |
Comparison of EFS and OS. (A) Comparison of EFS among CALGB (gray line) and CCG (black line) patients. The 7-year RHR for CALGB patients was 2.2 (CI, 1.6-3.0; P < .001). (B) Comparison of OS among CALGB (gray line) and CCG (black line) patients. The 7-year relative hazard ratio (RHR) for death in CALGB patients was 1.9 (CI, 1.3-2.7; P < .001).
Comparison of EFS and OS. (A) Comparison of EFS among CALGB (gray line) and CCG (black line) patients. The 7-year RHR for CALGB patients was 2.2 (CI, 1.6-3.0; P < .001). (B) Comparison of OS among CALGB (gray line) and CCG (black line) patients. The 7-year relative hazard ratio (RHR) for death in CALGB patients was 1.9 (CI, 1.3-2.7; P < .001).
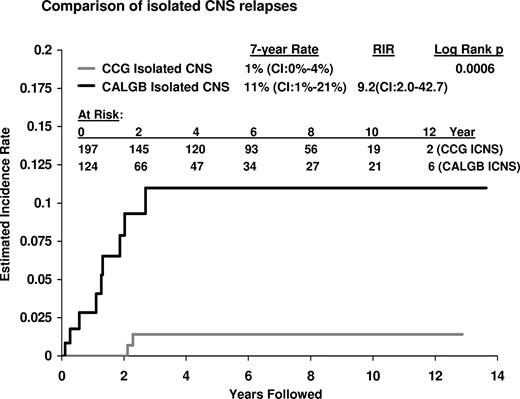
A difference in pattern of relapse was also noted. Among CALGB patients, 9 had an isolated CNS relapse in contrast to only 2 isolated CNS relapses among CCG patients, resulting in a significantly higher CNS relapse rate at 7 years of 11% for CALGB patients and only 1% for CCG patients (RIR, 9.2 [CI, 2.0-43]; P = .006; Figure 2). There were only very rare relapses at any site in either group after 4 years; 3 occurred among CALGB patients (with one eventual death) and 6 (with 5 eventual deaths) occurred among CCG patients. No cases of therapy-related AML have been reported to date.
Estimated incidence of isolated CNS relapses. The 7-year estimated incidence of isolated CNS relapses (ICNS) of 11% for CALGB patients (9 events) was significantly higher than the ICNS rate of 1.4% (2 events) for CCG patients (P < .001); RIR, 9.2 (CI, 2.0-42.7).
Estimated incidence of isolated CNS relapses. The 7-year estimated incidence of isolated CNS relapses (ICNS) of 11% for CALGB patients (9 events) was significantly higher than the ICNS rate of 1.4% (2 events) for CCG patients (P < .001); RIR, 9.2 (CI, 2.0-42.7).
In aggregate, women fared slightly better than men with a RHR of 0.73 (CI, 0.5-1.0) for OS (P = .08). There were no significant differences in outcome for the different racial groups in either CCG or CALGB populations. In both CALGB and CCG, patients with a presenting WBC count of 50 × 109/L or greater fared worse than those presenting with a WBC of less than 50 × 109/L. Within either the CCG or the CALGB, a comparison of EFS according to immunophenotype showed no significant differences between outcomes for precursor B- or T-lineage subsets. For the very small group of 7 patients (5 in CALGB and 2 in CCG) with a t(9;22), none of whom received imatinib, the aggregate analysis showed a poor outcome, with EFS of 29% and a higher RHR of 2.5 (CI, 1.0-6.1) for relapse (P = .05) compared with the overall group, and with relapses occurring much earlier for these patients in both the CCG and CALGB series.
Because the age distributions between the 2 populations were so different, with the percentages skewed in opposite directions for CCG and CALGB, individual age group comparisons were performed to determine any possible influence of age distribution within the 16- to 20-year-old groups of the 2 patient populations (Figure 3). Overall, there was no evidence of an age effect on outcome for patients enrolled on the CCG studies (P = .44). For the CALGB patients, however, there was evidence for an age effect. EFS at 7 years for the 25 CALGB patients aged 16 to 17 years was 55% (CI, 32%-77%). This was not worse than CCG patients (P = .49), and was significantly better than CALGB patients aged 18 to 20 years, for whom the 7-year EFS was only 29% (CI, 19%-39%). In contrast, EFS of 57% (CI, 46%-68%) was noted in CCG 18- to 20-year-olds (P = .01; RHR, 2.1 [CI,1.2-3.8]).
Age effect on outcome. CALGB patients aged 16 to 17 years had a 7-year EFS of 55%, which was similar to a 64% EFS for 16- to 17-year-old CCG patients (P = .49). CALGB patients aged 18 to 20 years old had a 7-year EFS of 29%, which was significantly worse than that for the younger CALGB patients and the 57% EFS for 18- to 20-year-olds on CCG trials (P = .01; RHR, 2.1 [CI, 1.2-3.8]).
Age effect on outcome. CALGB patients aged 16 to 17 years had a 7-year EFS of 55%, which was similar to a 64% EFS for 16- to 17-year-old CCG patients (P = .49). CALGB patients aged 18 to 20 years old had a 7-year EFS of 29%, which was significantly worse than that for the younger CALGB patients and the 57% EFS for 18- to 20-year-olds on CCG trials (P = .01; RHR, 2.1 [CI, 1.2-3.8]).
Comparison of outcome by regimen
EFS and OS were also examined according to treatment arm and study. AYAs on both CCG 1882 and 1901 had virtually identical outcomes (P = .97). In CCG 1882, RER patients achieved a 76% 7-year EFS; SER patients were randomized to either A-BFM or CCG-modified BFM (with cranial irradiation) and achieved 52% and 61% 7-year EFSs, respectively. A total of 22 patients with lymphomatous features assigned to CCG protocol 1901 had a 62% (CI, 37%-84%) 7-year EFS and 65% (CI, 40%-90%) 7-year OS. In CALGB, there was more evidence of some heterogeneity in outcome, with AYA patients enrolled on CALGB 8811 and 9311 faring a bit better than AYA patients on 9111, 9511, and 19802; nevertheless, a log-rank test for differences in either EFS or OS outcomes was not significant (P = .63). The 7-year EFS and OS for AYA patients entered on sequential CALGB protocols were variable. For 8811, EFS was 43% (CI, 27%-60%) and OS was 59% (CI, 43%-75%). On 9111, EFS was 24% (CI, 9%-40%) and OS was 36% (CI, 18%-54%), while for 9311, EFS was 46% (CI, 17%-76%) and OS was 54% (CI, 25%-83%). More recent studies showed similar trends: for 9511, EFS was 32% (CI, 8.7%-55%) and OS was 36% (CI, 15%-58%); for 19802, 5-year EFS was 26% (CI, 0.8%-52%) and OS was 39% (CI, 13%-66%).
Comparison of planned cumulative drug dosage
Cumulative planned doses of the drugs administered during induction and consolidation therapy (excluding long-term maintenance therapy) from the CCG 1882 and CALGB regimens were compared and are listed in Table 4 and 5. Because only 22 (10%) of the 197 CCG AYA patients were treated according to the 1901 regimen (with no significant difference in EFS or OS compared with those enrolled on CCG 1882), this regimen is not included in this comparison. CALGB patients received more myelosuppressive therapy with daunorubicin and cyclophosphamide therapy during induction therapy compared with the CCG 1882 patients. Of note, in CALGB 19802, the further intensification of daunorubicin and cytarabine dosing during induction and postremission therapy failed to improve outcome for CALGB AYA patients over any of the earlier CALGB regimens. In contrast, CCG 1882 included higher cumulative doses of the nonmyelosuppressive drugs, including glucocorticoids (prednisone and dexamethasone), vincristine, and L-asparaginase during induction and/or postremission therapy compared with the CALGB regimens. CCG patients also received earlier CNS-directed therapy with intrathecal chemotherapy beginning during induction therapy and a higher cumulative dose of intrathecal therapy. In addition, CNS therapy was continued throughout CCG maintenance in contrast to CALGB patients, for whom there was no CNS-directed therapy during this last phase of treatment. Overall, CCG patients received maintenance therapy for a longer period than CALGB patients. In the CCG, men received 3 years of maintenance therapy measured from the time of beginning the first interim maintenance cycle (women received 2 years); in contrast, all CALGB patients regardless of sex were assigned to continue maintenance therapy to complete a total of 2 years of treatment from diagnosis.
Adherence to the prescribed treatment
To assess the potential influence of delays in treatment due to variances from protocol-prescribed therapy, we analyzed EFS based on the ability of patients to begin long-term maintenance therapy within the specified timeframe of the protocol. Only 75 (63% of the 112 who achieved a CR) CALGB AYA patients began maintenance therapy. A total of 35 CR patients did not begin maintenance therapy for the following reasons: 17 had early relapses, one patient underwent allogeneic stem cell transplantation, 6 died of treatment-related complications, 4 had significant treatment-related toxicity, 4 withdrew consent, 2 were removed from protocol therapy by the physician for noncompliance, and one patient was lost to follow-up. Of the 75 patients who did begin maintenance therapy, 57 (76%) reached this treatment landmark within 30 days of what was recommended by strict adherence to the protocol scheme. EFS, measured from the time of beginning maintenance therapy, was not different for these 58 patients compared with the 18 (24%) patients who had a delay of more than 30 days to beginning maintenance therapy (P = .4).
For the 175 AYAs enrolled on CCG 1882, 126 (81% of the 157 who achieved CR) began long-term maintenance therapy. A total of 31 CR patients did not begin maintenance therapy for the following reasons: 11 had early relapses, 2 died of treatment-related complications, 2 had treatment-related toxicity (infections), 6 withdrew consent, 5 were removed from protocol therapy by their physician, 2 underwent allogeneic stem cell transplantation, 2 had a prolonged break in protocol therapy, and one patient was lost to follow-up. Similar to the CALGB patients, there was no significant difference (P = .6) in EFS for the CCG patients who began maintenance therapy within 30 days of the protocol-prescribed time point in comparison to those who had a longer delay in beginning long-term maintenance therapy. While approximately half of the CCG patients (48 of 92 total) receiving the modified CCG-BFM began maintenance within 30 days of the protocol-designated time point, only 2 of the 25 AYA patients who received the A-BFM were able to begin maintenance therapy within the 30-day timeframe.
Discussion
Our retrospective comparison of treatment strategies and treatment outcomes of 321 ALL patients aged 16 to 20 years who received treatment on pediatric (CCG) or adult (CALGB) cooperative group trials demonstrates that despite identical CR rates of 90% in both groups, EFS and OS were significantly better for AYA patients enrolled on CCG treatment trials. The long-term follow-up reported here confirms our initial observations of these striking outcome differences.21 Subsequent to our initial report, similar results have also been reported by several European adult and pediatric cooperative group studies in retrospective comparisons of AYA outcomes.22-26 Despite considerable differences in the treatment plans between these studies, the outcome data are remarkably similar to those reported here. Each has shown improved outcomes for AYAs treated by the pediatric group compared with the adult group.
There are several potential explanations for these striking differences in outcomes. These include clinical and demographic differences in adolescents receiving treatment at pediatric compared with adult centers, clear differences in protocol design and dose intensity, and potential variations in the degree of adherence to protocol drug administration by adult compared with pediatric oncologists and by the patients treated.
Our 2 groups of patients appeared to be well matched with respect to clinical and biologic features except for age, and both groups used intensive multiagent induction therapy and postremission intensification without hematopoietic cell transplantation. Comparison of drug dosing and scheduling identified some potentially important differences. CCG patients received considerably more treatment with nonmyelosuppressive drugs, including glucocorticoids (both dexamethasone and prednisone), vincristine, and L-asparaginase (Table 4,5). CNS prophylaxis was also administered earlier and with greater frequency during CCG treatment.
Remission rates were identical at 90% for both CCG and CALGB patients. The CCG patients received a minimum cumulative dose of 1680 mg/m2 prednisone during induction compared with 1260 mg/m2 for all CALGB patients. Of note, the cumulative L-asparaginase dose of 54 000 U/m2 given during CCG induction therapy was 44% higher than the cumulative CALGB induction dose of 36 000 U/m2. In contrast, CALGB patients received higher cumulative doses of the myelosuppressive agents: daunorubicin, ranging from 135 to 240 mg/m2 compared with 100 mg/m2 for CCG; and cyclophosphamide, 1200 mg/m2 (none in CCG) during induction.15,27
The protocol schedules for postremission therapy in the CCG and CALGB studies were generally similar; however, again, CALGB patients received only 66% as much dexamethasone, 60% as much vincristine, and 53% as much l-asparaginase compared with the “lower intensity” CCG-BFM arm to which most AYA patients were assigned based on their day-7 marrow response. The dosing of the myelosuppressive agents, daunorubicin and cyclophosphamide, during postremission therapy was similar in CCG and CALGB studies. Given these data, it appears that more frequent dosing of the nonmyelosuppressive drugs may be the more important component of a successful treatment strategy in this population.
Earlier and more frequent intrathecal chemotherapy injections also appeared to contribute to the significantly lower relapse rate seen in the CCG studies. Only 2 isolated CNS relapses occurred in the CCG patients. This approach contrasted with the CALGB studies, where there were 9 isolated CNS relapses. CALGB patients did not begin CNS-directed therapy until week 5 and did not receive any intrathecal therapy as part of the maintenance schedule. The pediatric groups have demonstrated previously that aggressive CNS therapy not only prevents CNS relapse in children, but also contributes to a reduction in systemic relapses.28,29 In these studies, the CCG also demonstrated that oral administration of dexamethasone resulted in reduced CNS relapse rates compared with prednisone.
Due to differences in referral patterns to pediatric or adult centers, there was a significant skewing of age, with a median age for CCG patients of only 16 years compared with 19 years for CALGB patients. EFS at 7 years did not differ significantly between the “younger” 16- and 17-year olds (64%; SE 5%) and the “older” 18- to 20-year-old patients (57%; SE 11%) who were treated on CCG studies (Figure 3). Interestingly, the “younger” CALGB patients aged 16 to 17 years enjoyed a similar EFS (55%; SE 12%) to all of the CCG patients; this was significantly better than for those CALGB patients aged 18 to 20 years old for whom EFS was only 29% (P = .02). These outcome differences may simply be due to the small sample sizes of 16- to 17-year-old patients on CALGB studies and of 18- to 20-year-old patients on the CCG studies. However, it is intriguing to postulate that the significantly worse outcome seen only in the CALGB patients aged 18 to 20 years could be the result of “emancipated adolescence”—young adults who are living independently and who are removed from family members who might ensure greater compliance with protocol medications and clinical follow-up. In contrast, the 16- to 17-year-old CALGB patients and, quite possibly, all CCG AYAs, may still be living with parents and other family members who provide the necessary support systems to comply with these arduous treatment programs. Prospective studies with appropriate demographic and psychosocial data capture can provide valuable insights into these issues but would still be limited by the confounding issues of referral patterns and differences in adherence to protocol by both physicians and patients. While we cannot exclude the possibility that discrete biologic differences in the pathogenetic mechanisms of ALL occur that may influence drug responsiveness as patients age from 16 to 20 years old, this seems quite unlikely, and these age-specific differences in outcome were only seen in CALGB, and not in CCG patients.30
How might disparities in the practice patterns of pediatric and adult hematologists/oncologists and the attitudes of AYA patients who they treat contribute to these different outcomes? This topic has been discussed heatedly, both at recent hematology meetings and in the literature,31 yet the question remains largely unanswered with little data available. ALL is the most common childhood malignancy. Virtually all children with ALL are referred to pediatric centers and treated on clinical trials by pediatric oncologists who are focused on treatment of ALL and have highly experienced support staff who carefully monitor protocol compliance. In contrast, ALL remains a rare disease for medical oncologists. Some have argued that because adult oncologists more often treat older patients with metastatic solid tumors for palliation, they may be less rigorous in adhering to the strict treatment schedules that may be required to cure ALL. Given the complexity and long duration of these empirical regimens, the degree of adherence to protocol by both physician and patient may vary considerably, and this would likely influence outcome.
While the retrospective nature of the CCG/CALGB comparison prevents a definitive conclusion, we attempted to address one aspect of protocol adherence by comparing the ideal protocol-prescribed treatment interval from the achievement of CR to the beginning of maintenance therapy with the actual treatment duration that was recorded in each patient's medical record. This permitted an estimation of whether adherence to protocol schedule resulted in significant differences in outcome. Only 63% of CALGB AYAs who achieved CR on these studies began maintenance therapy while still on protocol treatment, in contrast to 81% of AYAs achieving CR on CCG 1882. The reasons for withdrawal from protocol therapy in the 2 groups were similar. For patients who did begin maintenance therapy, we analyzed EFS using a somewhat arbitrary cut point defined as beginning maintenance therapy within 30 days of the protocol-prescribed target date. Using this single surrogate parameter, it was somewhat surprising that there was no improvement in EFS for either CALGB or CCG patients who completed the intensive induction and postremission consolidation cycles and began maintenance therapy in a timely fashion. This retrospective analysis could not address the other potentially very important issue of compliance with prescribed drug dosage by both the patient and the treating physician. Because much of ALL treatment relies on self-administered oral medications in the home, it has been suggested that AYAs who find themselves in a medical oncologist's office may be more independent from parental oversight and thus less likely to comply with all of the prescribed oral medications.
In conclusion, our retrospective analysis demonstrated that AYAs fared substantially better when treated in pediatric clinics on CCG trials, perhaps as a result of dose intensification of the nonmyelosuppressive drugs that have been the mainstay of pediatric ALL therapy—glucocorticoids, vincristine, and l-asparaginase—plus early and prolonged CNS prophylaxis. To begin to address the many unanswered questions about this important group of patients with ALL in a unified manner, the US adult and pediatric cooperative groups are embarking on a prospective trial focusing specifically on AYAs (Intergroup trial C10403). Newly diagnosed patients with ALL from 16 to 30 years of age will be enrolled on a study that parallels the current Children's Oncology Group (COG) study for adolescents and high-risk children (AALL0232). This study will examine prospectively disease biology, psychosocial disparities between adolescents referred to and treated by pediatric or adult oncologists, and, importantly, the ability to administer in a safe and timely manner the same treatment that is being used by the US pediatric cooperative group. Similar efforts piloted both in the United States and in Europe suggest that using an intensive pediatric approach is feasible for adults up to the age of 40 to 50 years with encouraging, albeit preliminary, improvements in disease-free survival.32-34
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dawn Spearmon for her expert help in preparing this manuscript, and Harland Sather, Meenakshi Devidas, and, in memorium, Richard Dodge for their important contributions to the initial statistical analysis of this dataset.
CALGB is supported by National Institutes of Health (NIH) grant CA 31946; COG is supported by NIH grant CA 98543.
National Institutes of Health
Authorship
Contribution: W.S. developed the research questions, analyzed the data, and wrote the paper; M.L. performed the statistical analysis for the CCG and was the senior statistician coordinating the 2 datasets; B.S. performed the statistical analysis for the CALGB; C.D.B. analyzed the cytogenetics for the CALGB and provided valuable comments on the text; J.W.V. performed the immunophenotypic review for this paper; P.G. was the primary investigator for the CCG 1882 trial; R.A.L. leads the CALGB leukemia committee and provided valuable editing and suggestions for the manuscript; and J.N. helped to design the research question and review the analysis, and was a lead investigator for the CCG leukemia trials. W.S., B.S., C.D.B., J.W.V., R.A.L. are members of CALGB, Chicago, IL; M.L., P.G., and J.N. are members of COG, Arcadia, CA.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wendy Stock, Section of Hematology-Oncology, University of Chicago Medical Center, 5841 South Maryland Avenue, MC 2115, Chicago, IL 60637; e-mail: wstock@medicine.bsd.uchicago.edu.

![Figure 3. Age effect on outcome. CALGB patients aged 16 to 17 years had a 7-year EFS of 55%, which was similar to a 64% EFS for 16- to 17-year-old CCG patients (P = .49). CALGB patients aged 18 to 20 years old had a 7-year EFS of 29%, which was significantly worse than that for the younger CALGB patients and the 57% EFS for 18- to 20-year-olds on CCG trials (P = .01; RHR, 2.1 [CI, 1.2-3.8]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/5/10.1182_blood-2008-01-130237/5/m_zh80160821890003.jpeg?Expires=1769112907&Signature=LTJrSwow12ZBx6Ttjcb8LwfQ-iWpsk7BCM0wOLj8bM6lhKPxYZSQG0lC~0dWECFPQji8FRxcAR~NbWEOM5O8-ZHIZ4GmohEQAOI~R2oYkoOYga8QkTL2QEX5R1RFmz4HJ0Wo-vRt16A0R7XyzKzOz344zHcOMO3QtPHnug5MwOY4KDRXsqUKnFq8iJXA~gSnLXSnL0VRLj~DhWoB3lkKAjNpQeuNFMkBDxnMvM5BvI~4caLBMA9lB7lbsFje4OuboX8pF4hp4ih80i9CKYn5HE-T6nglHx1tazjIF3i6tKyfGmrYVWbrV689D~YMHAVQ6OicUKf~AFzLsZcnX-MHRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)