Abstract
Second messenger-mediated inside-out activation of integrin αIIbβ3 is a key step in platelet aggregation. We recently showed strongly impaired but not absent αIIbβ3-mediated aggregation of CalDAG-GEFI–deficient platelets activated with various agonists. Here we further evaluated the roles of CalDAG-GEFI and protein kinase C (PKC) for αIIbβ3 activation in platelets activated with a PAR4 receptor–specific agonist, GYPGKF (PAR4p). Compared with wild-type controls, platelets treated with the PKC inhibitor Ro31-8220 or CalDAG-GEFI–deficient platelets showed a marked defect in aggregation at low (< 1mM PAR4p) but not high PAR4p concentrations. Blocking of PKC function in CalDAG-GEFI–deficient platelets, how-ever, strongly decreased aggregation at all PAR4p concentrations, demonstrating that CalDAG-GEFI and PKC represent separate, but synergizing, pathways important for αIIbβ3 activation. PAR4p-induced aggregation in the absence of CalDAG-GEFI required cosignaling through the Gαi-coupled receptor for ADP, P2Y12. Independent roles for CalDAG-GEFI and PKC/Gαi signaling were also observed for PAR4p-induced activation of the small GTPase Rap1, with CalDAG-GEFI mediating the rapid but reversible activation of this small GTPase. In summary, our study identifies CalDAG-GEFI and PKC as independent pathways leading to Rap1 and αIIbβ3 activation in mouse platelets activated through the PAR4 receptor.
Introduction
Integrins are heterodimeric cell-surface receptors that mediate cell adhesion to the extracellular matrix and cell-cell interactions. Probably the best-studied integrin is αIIbβ3, the platelet receptor for fibrinogen. Activation of αIIbβ3 in platelets is largely dependent on the generation of the second messengers Ca2+ and diacylglycerol (DAG), and the concomitant activation of members of the Gαi family of heterotrimeric G proteins. Ca2+ and DAG are generated by phospholipase Cβ (PLCβ) downstream of G protein-coupled receptors (GPCRs), such as PAR receptors (thrombin receptors) or P2Y1 (ADP receptor), or by PLCγ downstream of the activating collagen receptor, GPVI.1,2 Signaling via Gαi proteins in platelets is selectively coupled to various agonist receptors: Gαz preferentially couples to the α2A adrenergic receptor for epinephrine,3 whereas Gαi2 is preferred by the ADP receptor P2Y12.4 Gαi signaling leads to the activation of phosphoinositide 3-kinase (PI3K), Akt, Rap1B, and the inhibition of adenylyl cyclase but has little, if any, ability to activate PLC.5 Studies with PKC inhibitors and calcium chelators identified separate PKC-dependent and Ca2+-dependent signaling pathways that synergize with Gαi signaling in the activation of αIIbβ3.6,7 In addition, signaling by both PKC and Ca2+ plays a key role in granule secretion from activated platelets.8,9
The Rap family of small GTPases has recently gained much attention as a central player in integrin activation downstream of second messengers.10 In mammals, the Rap family consists of 2 rap1 genes and 2 rap2 genes encoding proteins that are approximately 65% homologous. Platelets express significant amounts of Rap1B and Rap2B, with Rap1B accounting for approximately 90% of the total Rap protein.11 Rap proteins cycle between an inactive GDP-bound and an active GTP-bound form. The GDP-GTP cycle is regulated by guanine nucleotide exchange factors (GEFs), which facilitate the release of GDP and allow GTP to bind. GTPase-activating proteins (GAPs) facilitate the hydrolysis of bound GTP to complete the cycle. On platelet stimulation, Rap1B activation is mediated via Ca2+-dependent and -independent mechanisms, the latter being dependent on Gαi signaling and the subsequent activation of PI3K.12-15 The importance of Rap1B for αIIbβ3 activation has recently been demonstrated in Rap1B-deficient mice.16
Ca2+ and diacylglycerol regulated guanine nucleotide exchange factor I (CalDAG-GEFI, RasGRP2) is a member of the CalDAG-GEF/RasGRP family of intracellular signaling molecules involved in the activation of small G proteins of the Ras superfamily.17,18 CalDAG-GEFI contains binding sites for Ca2+ and DAG and a GEF domain that predominantly activates Rap1.18 We have previously shown that CalDAG-GEFI plays a key role in the activation of Rap1, αIIbβ3 integrin, and β1 integrins in platelets19,20 as well as Rap1, β1, and β2 integrins in neutrophils.20 Platelets from mice deficient in CalDAG-GEFI did not aggregate when stimulated with ADP, thromboxane A2 (TxA2), or Ca2+ ionophore. In contrast, aggregation in response to collagen and, especially, thrombin was only slightly affected. CalDAG-GEFI−/− mice were characterized by a prolonged bleeding time and protection against experimental thrombosis, demonstrating the importance of this molecule for thrombus formation in vivo.19,20
In the present study, we have evaluated how CalDAG-GEFI signaling cooperates with PKC and Gαi signaling in platelet aggregation. We show that CalDAG-GEFI and PKC can independently promote the activation of Rap1 and integrin αIIbβ3 in mouse platelets specifically activated through the major thrombin receptor, PAR4. Whereas CalDAG-GEFI mediates the rapid but reversible activation of Rap1, PKC ensures sustained Rap1 activation through its central role in the release of ADP. In platelets lacking CalDAG-GEFI, integrin activation required cosignaling by PKC and Gαi.
Methods
Reagents and antibodies
Lovenox (enoxaparin sodium; Sanofi-Aventis, Bridgewater, NJ), heparin-coated microcapillaries (VWR, West Chester, PA), bovine serum albumin (BSA), prostacyclin (PGI2), apyrase, 2′-deoxy-N6-methyl adenosine 3′,5′-diphosphate diammonium salt (MRS2179, P2Y1 inhibitor), 2-methylthio-AMP triethylammonium salt hydrate (2-MesAMP, P2Y12 inhibitor), epinephrine, serotonin (all from Sigma-Aldrich, St Louis, MO), protein kinase C (PKC) inhibitor Ro31-8220 (EMD Chemicals, Gibbstown, NJ), ADP (Bio/Data, Horsham, PA), 14C-serotonin (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom), PAR4 receptor-activating peptide H-GYPGKF-NH2 (Advanced Chemtech, Louisville, KY), calcium-sensing dye Fluo-3 (Invitrogen, Carlsbad, CA), RalGDS-RBD coupled to agarose beads (Upstate Biotechnology, Charlottesville, VA), were purchased. Monoclonal antibody directed against the activated form of murine αIIbβ3 integrin, JON/A-PE, was purchased from emfret Analytics (Wuerzburg, Germany). Polyclonal antibodies to CalDAG-GEFI were described recently19 and antibodies to Rap1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Mice
CalDAG-GEFI−/−19 and littermate control (wild-type, WT) mice were received from the mouse facility at the Massachusetts Institute of Technology and were bred in the mouse facilities of the Immune Disease Institute and Thomas Jefferson University. Experimental procedures were approved by the Animal Care and Use Committees of the Immune Disease Institute and of Thomas Jefferson University.
Aggregometry
Platelet-rich plasma (PRP) was obtained from heparinized whole blood by centrifugation at 100g for 10 minutes. Light transmission was measured by using PRP adjusted to 3 × 108 platelets per milliliter with modified Tyrode's buffer (137 mM NaCl, 0.3 mM Na2HPO4, 2 mM KCl, 12 mM NaHCO3, 5 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 5 mM glucose, pH 7.3) containing 0.35% BSA and 1 mM CaCl2. Inhibitors and agonists were added at the indicated concentrations, and transmission was recorded over 12 minutes on a Chrono-log 4-channel optical aggregation system (Chrono-log, Havertown, PA).
Flow cytometry
PRP was centrifuged at 700g in the presence of PGI2 (2 μg/mL) for 7 minutes at room temperature. After 2 washing steps, pelleted platelets were resuspended in modified Tyrode's buffer.
Calcium flux measurement.
Washed platelets were incubated with 5 μM of the calcium-sensing dye Fluo-3 for 15 minutes, activated with the indicated concentrations of PAR4p, and immediately analyzed on a FACScalibur. Data were analyzed with FlowJo software (TreeStar, Ashland, OR).
Integrin activation.
Platelets were diluted in Tyrode buffer containing 1 mM CaCl2, activated with PAR4p, epinephrine, or serotonin in the presence of JON/A-PE21 for 10 minutes, and studied immediately by flow cytometry. Inhibitors to PKC (Ro31-8220), P2Y12 (2-MesAMP), or P2Y1 (MRS2179) were added before stimulation to block the respective signaling pathways.
Rap1 activation
Amounts of activated Rap1 in platelets were measured using a protocol similar to the one previously described.19 Platelets were activated for various times under static or stirring conditions in the presence or absence of inhibitors and immediately lysed with ice-cold lysis buffer (25 mM Tris-HCl at pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 5 mM MgCl2, 5% glycerol, and complete protease inhibitor cocktail lacking ethylenediaminetetraacetic acid; Roche Applied Science, Indianapolis, IN). A total of 30 μL of sample was immediately withdrawn from the lysates for the determination of total Rap1 levels. Detection of activated Rap1 (Rap1-GTP) in platelet lysates was done according to the instructions of the manufacturer. Briefly, Rap1-GTP was precipitated from lysates using RalGDS-RBD beads. Precipitated proteins were separated on a 15% SDS-PAGE gel and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Rap1 was detected with rabbit polyclonal antibodies followed by antirabbit antibodies conjugated to horseradish peroxidase (Vector Laboratories, Burlingame, CA). Immunoreactivity was detected by Western Lightning enhanced chemiluminescence (PerkinElmer Life and Analytical Sciences, Waltham, MA).
Serotonin release
PRP was incubated in a 37°C water bath for 15 minutes with 14C-serotonin (57 mCi/mmol per mL of PRP), and diluted with modified Tyrode buffer containing 1 mM of CaCl2. Platelets were activated under stirring conditions (standard aggregometry), and activation was terminated by adding 1 volume of 4% ice-cold paraformaldehyde solution. The samples were then centrifuged at 2500 g for 5 minutes, and the supernatants were used for scintillation counting of 14C-serotonin. Total or 100% 14C-serotonin secretion was defined as the 14C-serotonin in samples lysed with 0.5% Triton X-100.
Results
CalDAG-GEFI and PKC represent independent pathways leading to activation of αIIbβ3
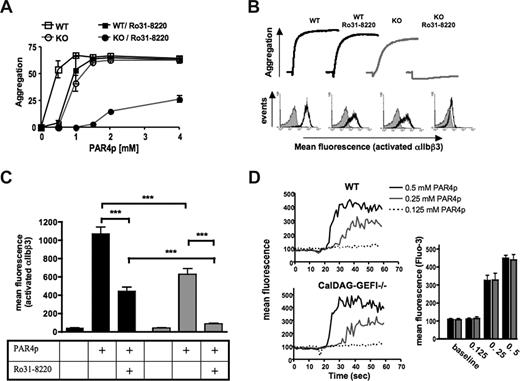
We have previously shown that CalDAG-GEFI−/− mouse platelets show impaired aggregation in response to stimulation by low-dose thrombin, whereas no such defect was observed at higher concentrations of the agonist.19 These results indicated the existence of a CalDAG-GEFI-independent pathway downstream of PLCβ activation that is sufficient to activate αIIbβ3. To evaluate whether PKC is involved in this signaling pathway, PKC function was inhibited in WT and CalDAG-GEFI–deficient platelets with Ro31-8220, an inhibitor of conventional and novel PKC isoforms22 (Figure 1). Activation of platelets through the major activating thrombin receptor on mouse platelets, PAR4,23,24 was induced with the peptide GYPGKF (PAR4p). Both WT platelets pretreated with Ro31-8220 and CalDAG-GEFI–deficient platelets showed a significantly impaired aggregation response when stimulated with 0.5 mM PAR4p (P < .001 and .001, respectively), a concentration sufficient to induce full aggregation of WT platelets (Figure 1A). When stimulated with 1 mM of PAR4p, aggregation of CalDAG-GEFI-deficient (P < .01) but not WT/Ro31-8220 platelets was significantly reduced. No significant difference in maximum aggregation was observed between WT platelets and WT/Ro31-8220 or CalDAG-GEFI–deficient platelets when the cells were activated with PAR4p concentrations equal or higher than 1.5 mM. In contrast, compared with WT platelets, CalDAG-GEFI–deficient platelets pretreated with Ro31-8220 showed significantly reduced aggregation at all tested PAR4p concentrations (P < .001). Figure 1B (top panel) shows representative aggregation traces for platelets activated with 1.25 mM of PAR4p, a concentration that allows aggregation of platelets lacking either functional PKC or CalDAG-GEFI but that does not cause aggregation in CalDAG-GEFI-deficient platelets treated with Ro31-8220. Shape change as indicated by the initial decrease in light transmission was observed in all samples. Direct assessment of αIIbβ3 activation in platelets stimulated with 1.25 mM of PAR4p, using an antibody against the activated form of the receptor (JON/A-PE21 ), showed significantly reduced activation of αIIbβ3 in both Ro31-8220–treated and CalDAG-GEFI–deficient platelets (Figure 1B bottom panel; Figure 1C). Almost complete inhibition of αIIbβ3 activation (and platelet aggregation), however, was only observed in CalDAG-GEFI–deficient platelets treated with Ro31-8220. Similar results were observed when integrin activation was studied using FITC-labeled fibrinogen (not shown). These data demonstrate a synergistic role of PKC and CalDAG-GEFI in αIIbβ3 activation in platelets.
CalDAG-GEFI and PKC synergize in αIIbβ3 activation in platelets activated through the PAR4 receptor. (A) Dose response for PAR4p-induced aggregation of wild-type (WT) and CalDAG-GEFI–deficient (KO) platelets. The data shown represent the percentage aggregation measured 5 minutes after addition of the agonist; n = 6 (P values given in “Results”). (B) WT or CalDAG-GEFI–deficient platelets were stimulated with 1.25 mM PAR4p in the presence or absence of the broad-range PKC inhibitor Ro31-8220 (5 μg/mL). (Top panel) Representative aggregation traces. (Bottom panel) Representative histograms for the activation of integrin αIIbβ3 as measured by flow cytometry. Gray curve represents untreated; black curve, treated with PAR4p. The results are representative of 5 individual experiments. (C) WT ( ) or CalDAG-GEFI–deficient (
) or CalDAG-GEFI–deficient ( ) platelets were stimulated with 1.25 mM of PAR4p in the presence or absence of the broad-range PKC inhibitor Ro31–8220. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001). (D) Calcium flux was measured over time in Fluo-3–loaded WT or CalDAG-GEFI–deficient platelets stimulated with the indicated concentrations of PAR4p. The bar graph shows the maximum fluorescence intensity (Fluo-3) measured in WT (
) platelets were stimulated with 1.25 mM of PAR4p in the presence or absence of the broad-range PKC inhibitor Ro31–8220. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001). (D) Calcium flux was measured over time in Fluo-3–loaded WT or CalDAG-GEFI–deficient platelets stimulated with the indicated concentrations of PAR4p. The bar graph shows the maximum fluorescence intensity (Fluo-3) measured in WT ( ) and CalDAG-GEFI–deficient (
) and CalDAG-GEFI–deficient ( ) platelets within 1 minute after addition of PAR4p; n = 6. No significant difference in Fluo-3 fluorescence signals between WT and KO platelets was observed.
) platelets within 1 minute after addition of PAR4p; n = 6. No significant difference in Fluo-3 fluorescence signals between WT and KO platelets was observed.
CalDAG-GEFI and PKC synergize in αIIbβ3 activation in platelets activated through the PAR4 receptor. (A) Dose response for PAR4p-induced aggregation of wild-type (WT) and CalDAG-GEFI–deficient (KO) platelets. The data shown represent the percentage aggregation measured 5 minutes after addition of the agonist; n = 6 (P values given in “Results”). (B) WT or CalDAG-GEFI–deficient platelets were stimulated with 1.25 mM PAR4p in the presence or absence of the broad-range PKC inhibitor Ro31-8220 (5 μg/mL). (Top panel) Representative aggregation traces. (Bottom panel) Representative histograms for the activation of integrin αIIbβ3 as measured by flow cytometry. Gray curve represents untreated; black curve, treated with PAR4p. The results are representative of 5 individual experiments. (C) WT ( ) or CalDAG-GEFI–deficient (
) or CalDAG-GEFI–deficient ( ) platelets were stimulated with 1.25 mM of PAR4p in the presence or absence of the broad-range PKC inhibitor Ro31–8220. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001). (D) Calcium flux was measured over time in Fluo-3–loaded WT or CalDAG-GEFI–deficient platelets stimulated with the indicated concentrations of PAR4p. The bar graph shows the maximum fluorescence intensity (Fluo-3) measured in WT (
) platelets were stimulated with 1.25 mM of PAR4p in the presence or absence of the broad-range PKC inhibitor Ro31–8220. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001). (D) Calcium flux was measured over time in Fluo-3–loaded WT or CalDAG-GEFI–deficient platelets stimulated with the indicated concentrations of PAR4p. The bar graph shows the maximum fluorescence intensity (Fluo-3) measured in WT ( ) and CalDAG-GEFI–deficient (
) and CalDAG-GEFI–deficient ( ) platelets within 1 minute after addition of PAR4p; n = 6. No significant difference in Fluo-3 fluorescence signals between WT and KO platelets was observed.
) platelets within 1 minute after addition of PAR4p; n = 6. No significant difference in Fluo-3 fluorescence signals between WT and KO platelets was observed.
To rule out the possibility that impaired aggregation of CalDAG-GEFI–deficient platelets at low dose of the agonist was because of a defect in signaling events upstream of CalDAG-GEFI, calcium flux was studied in PAR4p-stimulated platelets. As shown in Figure 1D, a similar calcium flux response of WT and CalDAG-GEFI–deficient platelets to various doses of PAR4p was observed.
αIIbβ3 activation in the absence of functional PKC or CalDAG-GEFI depends on Gαi signaling provided by secreted ADP
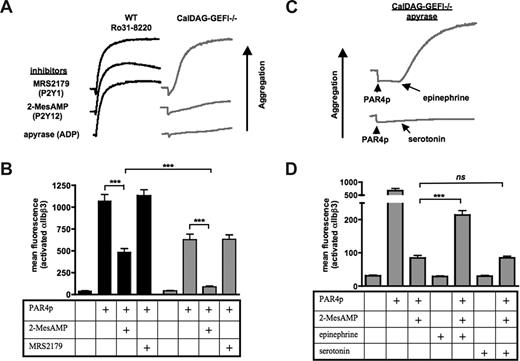
In a next step, the dependency of αIIbβ3 activation in the absence of functional PKC or CalDAG-GEFI on the second wave mediators ADP and/or TxA2 was investigated. Whereas inhibition of TxA2 formation by aspirin did not affect aggregation of WT/Ro31-8220 or CalDAG-GEFI–deficient platelets activated with 1.25 mM of PAR4p (not shown), preincubation of platelets with the ADP scavenger apyrase inhibited the aggregation of CalDAG-GEFI–deficient but not WT/ Ro31-8220 platelets (Figure 2A). The same inhibitory effect was observed when platelets were preincubated with 2-MesAMP, a specific inhibitor of Gαi-coupled P2Y12 receptors. In contrast, preincubation with MRS2179, an inhibitor of Gq-coupled P2Y1 receptors, did not affect the aggregation of PAR4p-stimulated CalDAG-GEFI–deficient platelets. Using JON/A-PE as a probe for activated αIIbβ3, we observed a significant inhibitory effect of 2-MesAMP but not MRS2179 on PAR4p-induced αIIbβ3 activation in WT and CalDAG-GEFI–deficient platelets (Figure 2B). Almost complete inhibition of αIIbβ3 activation was observed in 2-MesAMP-treated CalDAG-GEFI–deficient platelets.
PAR4p-induced activation of αIIbβ3 in CalDAG-GEFI–deficient platelets requires signaling by PKC and Gαi-coupled receptors. (A) WT platelets pretreated with 5 μg/mL Ro31-8220 or CalDAG-GEFI–deficient (CalDAG-GEFI−/−) platelets were activated with 1.25 mM of PAR4p in the presence of (1) the P2Y1 inhibitor MRS2179 (100 μM), (2) the P2Y12 inhibitor 2-MesAMP (50 μM), or (3) the ADP degrading enzyme apyrase (8 U/mL). Aggregation of platelets was recorded for 10 minutes. (B) WT ( ) or CalDAG-GEFI–deficient (
) or CalDAG-GEFI–deficient ( ) platelets were stimulated with 1.25 mM of PAR4p in the presence or absence of 2-MesAMP or MRS2179. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001). (C) CalDAG-GEFI–deficient platelets pretreated with apyrase (8 U/mL) were activated with 1.25 mM of PAR4p, followed by 10 μM of epinephrine or 10 μM of serotonin. Platelet aggregation was recorded for 10 minutes. (D) CalDAG-GEFI–deficient platelets were preincubated with 2-MesAMP, followed by stimulation with 1.25 mM of PAR4p in combination with epinephrine or serotonin. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001; ns indicates not significant).
) platelets were stimulated with 1.25 mM of PAR4p in the presence or absence of 2-MesAMP or MRS2179. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001). (C) CalDAG-GEFI–deficient platelets pretreated with apyrase (8 U/mL) were activated with 1.25 mM of PAR4p, followed by 10 μM of epinephrine or 10 μM of serotonin. Platelet aggregation was recorded for 10 minutes. (D) CalDAG-GEFI–deficient platelets were preincubated with 2-MesAMP, followed by stimulation with 1.25 mM of PAR4p in combination with epinephrine or serotonin. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001; ns indicates not significant).
PAR4p-induced activation of αIIbβ3 in CalDAG-GEFI–deficient platelets requires signaling by PKC and Gαi-coupled receptors. (A) WT platelets pretreated with 5 μg/mL Ro31-8220 or CalDAG-GEFI–deficient (CalDAG-GEFI−/−) platelets were activated with 1.25 mM of PAR4p in the presence of (1) the P2Y1 inhibitor MRS2179 (100 μM), (2) the P2Y12 inhibitor 2-MesAMP (50 μM), or (3) the ADP degrading enzyme apyrase (8 U/mL). Aggregation of platelets was recorded for 10 minutes. (B) WT ( ) or CalDAG-GEFI–deficient (
) or CalDAG-GEFI–deficient ( ) platelets were stimulated with 1.25 mM of PAR4p in the presence or absence of 2-MesAMP or MRS2179. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001). (C) CalDAG-GEFI–deficient platelets pretreated with apyrase (8 U/mL) were activated with 1.25 mM of PAR4p, followed by 10 μM of epinephrine or 10 μM of serotonin. Platelet aggregation was recorded for 10 minutes. (D) CalDAG-GEFI–deficient platelets were preincubated with 2-MesAMP, followed by stimulation with 1.25 mM of PAR4p in combination with epinephrine or serotonin. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001; ns indicates not significant).
) platelets were stimulated with 1.25 mM of PAR4p in the presence or absence of 2-MesAMP or MRS2179. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001). (C) CalDAG-GEFI–deficient platelets pretreated with apyrase (8 U/mL) were activated with 1.25 mM of PAR4p, followed by 10 μM of epinephrine or 10 μM of serotonin. Platelet aggregation was recorded for 10 minutes. (D) CalDAG-GEFI–deficient platelets were preincubated with 2-MesAMP, followed by stimulation with 1.25 mM of PAR4p in combination with epinephrine or serotonin. Binding of JON/A-PE was measured to determine the level of αIIbβ3 activation by flow cytometry; n = 6 (***P < .001; ns indicates not significant).
To confirm that αIIbβ3 activation in the absence of CalDAG-GEFI depends on Gαi signaling, epinephrine was used to specifically stimulate this signaling pathway.3 Addition of epinephrine restored aggregation and significantly increased αIIbβ3 activation in CalDAG-GEFI–deficient platelets pretreated with apyrase (Figure 2C) or 2-MesAMP (Figure 2D), respectively. In contrast, aggregation or αIIbβ3 aggregation could not be restored by addition of serotonin, a specific stimulator of Gαq signaling.25
Impaired dense granule release in CalDAG-GEFI–deficient platelets activated with low- but not high-dose PAR4p
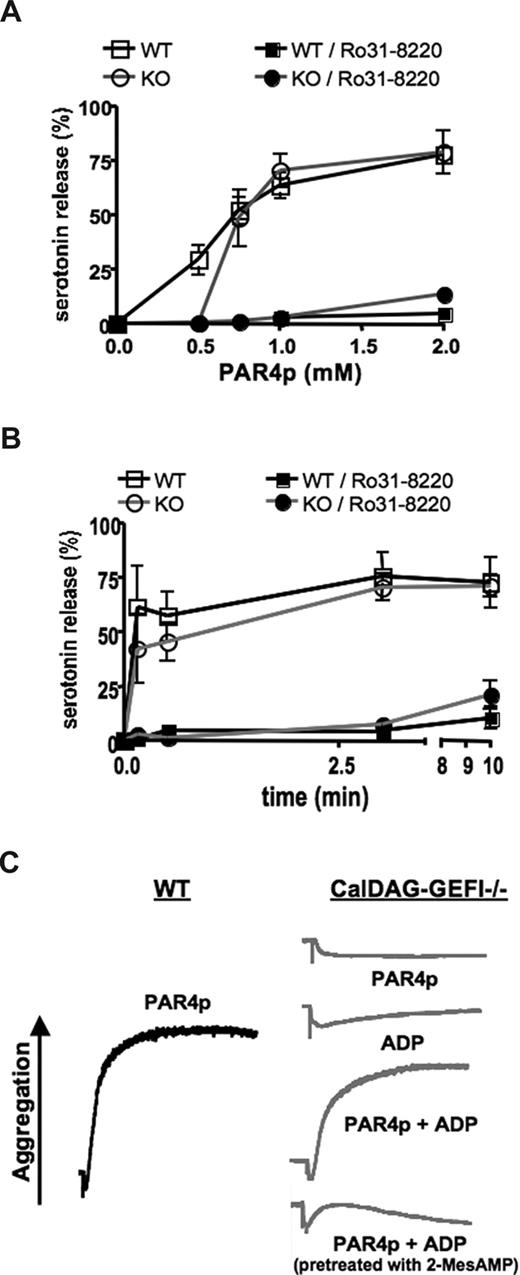
To test whether impaired integrin activation at low PAR4p concentrations could be the result of defective release/cosignaling by ADP, we next studied dense granule release in CalDAG-GEFI–deficient platelets. Release of 14C-labeled serotonin from platelets activated under stirring conditions (standard aggregometry) was measured. Pretreatment of platelets with Ro31-8220 completely inhibited dense granule release in platelets activated with various doses of PAR4p (Figure 3A). In contrast, platelets lacking CalDAG-GEFI showed significantly reduced dense granule release only at low but not high concentrations of the agonist (P < .001 at 0.5 mM PAR4p). No significant differences in the kinetics of granule release were observed between WT and CalDAG-GEFI–deficient platelets activated with 1.25 mM of PAR4p (Figure 3B).
Impaired dense granule release from CalDAG-GEFI–deficient platelets stimulated with low-dose PAR4p. (A) 14C-serotonin-loaded WT or CalDAG-GEFI–deficient (KO) platelets were activated with various concentrations of PAR4p in the presence or absence of Ro31-8220 (5 μg/mL) under stirring conditions. Activation was terminated 10 minutes after addition of the agonist, and 14C-serotonin levels in the supernatant (SN) of activated platelets were determined. 0% indicates 14C-serotonin in SN of resting platelets; 100%, 14C-serotonin levels in platelet samples after lysis; n = 6. (B) Time course of PAR4p-induced serotonin release. The activation process was terminated at the indicated time points after addition of 1.25 mM of PAR4p; n = 6. (C) WT (black line) or CalDAG-GEFI–deficient (gray lines) platelets were activated with 0.5 mM of PAR4p and/or 5 μM of ADP in the presence or absence of 2-MesAMP (50 μM). Results are representative of 4 experiments.
Impaired dense granule release from CalDAG-GEFI–deficient platelets stimulated with low-dose PAR4p. (A) 14C-serotonin-loaded WT or CalDAG-GEFI–deficient (KO) platelets were activated with various concentrations of PAR4p in the presence or absence of Ro31-8220 (5 μg/mL) under stirring conditions. Activation was terminated 10 minutes after addition of the agonist, and 14C-serotonin levels in the supernatant (SN) of activated platelets were determined. 0% indicates 14C-serotonin in SN of resting platelets; 100%, 14C-serotonin levels in platelet samples after lysis; n = 6. (B) Time course of PAR4p-induced serotonin release. The activation process was terminated at the indicated time points after addition of 1.25 mM of PAR4p; n = 6. (C) WT (black line) or CalDAG-GEFI–deficient (gray lines) platelets were activated with 0.5 mM of PAR4p and/or 5 μM of ADP in the presence or absence of 2-MesAMP (50 μM). Results are representative of 4 experiments.
To more directly address whether impaired ADP release accounts for the defect in integrin activation in CalDAG-GEFI–deficient platelets, mutant platelets were costimulated with 0.5 mM of PAR4p and 5 μM of ADP. As shown in Figure 3C, aggregation was restored in CalDAG-GEFI–deficient platelets costimulated with PAR4p and ADP, whereas the individual agonists did not cause aggregation of the mutant cells. Preincubation of CalDAG-GEFI–deficient platelets with 2-MesAMP blocked the costimulatory effect of ADP, demonstrating a key role of Gαi-coupled P2Y12 receptors in this process.
CalDAG-GEFI mediates the rapid but reversible activation of Rap1 in PAR4p-stimulated platelets
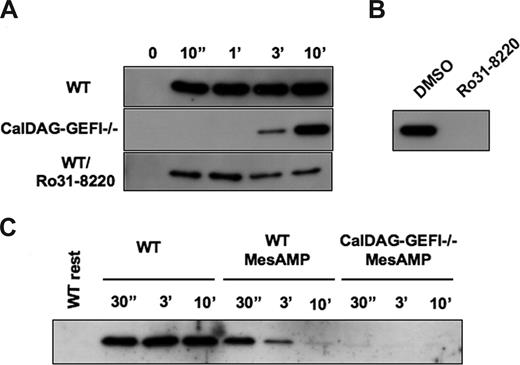
Agonist-induced activation of αIIbβ3 strongly depends on the activation of the small GTPase Rap1.16 CalDAG-GEFI is a key regulator of Rap1 activation in mouse platelets.19 However, CalDAG-GEFI is not the sole activator of Rap1 as significant activation of Rap1 was observed in CalDAG-GEFI–deficient platelets stimulated with thrombin or ADP. In platelets activated with 1.25 mM of PAR4p under nonstirring conditions, CalDAG-GEFI mediated the rapid activation of Rap1 (Figure 4A). Whereas maximum activation of Rap1 in WT platelets was seen already 10 seconds after addition of the agonist, Rap1 activation was completely impaired in CalDAG-GEFI–deficient platelets for more than 1 minute. Weak activation of Rap1 was observed 3 minutes after stimulation with PAR4p, and activation continued to reach almost maximum strength at 10 minutes after stimulation. In contrast, WT platelets treated with Ro31-8220 showed reduced levels of Rap1 activation but no change in the overall kinetics of this process. Pretreatment of CalDAG-GEFI–deficient platelets with Ro31-8220, however, completely abolished Rap1 activation induced by PAR4p (Figure 4B). To determine whether the delayed activation of Rap1 seen in CalDAG-GEFI–deficient platelets is a result of P2Y12-mediated signaling, we tested the effect of 2-MesAMP on this process. Rapid but reversible Rap1 activation was observed in 2-MesAMP-pretreated WT platelets stimulated with PAR4p, whereas no Rap1 activation was observed in 2-MesAMP-pretreated CalDAG-GEFI–deficient platelets (Figure 4C). These data suggest that CalDAG-GEFI mediates the rapid but reversible activation of Rap1 in platelets whereas CalDAG-GEFI–independent stimulation through P2Y12 leads to a delayed but sustained formation of Rap1-GTP. PKC participates in Rap1 activation through its central role in the release of ADP from activated platelets.
Rap1 activation in platelets stimulated with PAR4p requires CalDAG-GEFI and P2Y12-mediated signaling. (A) Time course of Rap1 activation in WT (± 5 μg/mL Ro31-8220) or CalDAG-GEFI−/− platelets stimulated with 1.25 mM of PAR4p. (B) Rap1-GTP detected in lysates from CalDAG-GEFI–deficient platelets at t = 10 minutes after addition of PAR4p. Rap1 activation was completely inhibited in the presence of Ro31-8220. (C) Effect of 2-MesAMP on Rap1 activation in WT or CalDAG-GEFI-deficient platelets. Platelets were pretreated with 50 μM 2-MesAMP for 5 minutes followed by stimulation with 1.25 mM of PAR4p.
Rap1 activation in platelets stimulated with PAR4p requires CalDAG-GEFI and P2Y12-mediated signaling. (A) Time course of Rap1 activation in WT (± 5 μg/mL Ro31-8220) or CalDAG-GEFI−/− platelets stimulated with 1.25 mM of PAR4p. (B) Rap1-GTP detected in lysates from CalDAG-GEFI–deficient platelets at t = 10 minutes after addition of PAR4p. Rap1 activation was completely inhibited in the presence of Ro31-8220. (C) Effect of 2-MesAMP on Rap1 activation in WT or CalDAG-GEFI-deficient platelets. Platelets were pretreated with 50 μM 2-MesAMP for 5 minutes followed by stimulation with 1.25 mM of PAR4p.
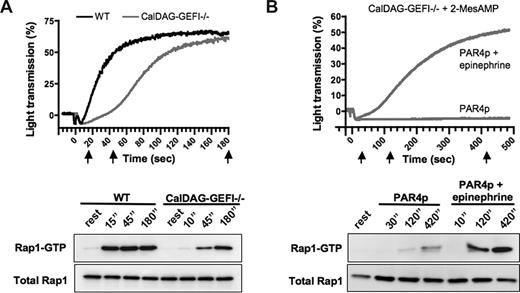
To directly compare the kinetics of Rap1 activation and aggregation/αIIbβ3 activation in CalDAG-GEFI–deficient platelets, we studied the generation of Rap1-GTP in aggregating platelets activated with 1 mM PAR4p, a threshold concentration for the activation of the mutant platelets (Figure 5A). In WT platelets, both aggregation and Rap1 activation occurred within seconds after the addition of PAR4p. In contrast, both aggregation and Rap1 activation were almost completely inhibited in CalDAG-GEFI–deficient platelets 15 seconds after addition of PAR4p. Aggregation in the mutant platelets started approximately 45 seconds after stimulation, a time point when weak Rap1 activation was observed. Full aggregation and strong Rap1 activation were observed at t = 3 minutes. Using the same experimental approach, we also compared the kinetics of Rap1 activation and aggregation in 2-MesAMP–treated CalDAG-GEFI–deficient platelets activated with PAR4p and/or epinephrine (Figure 5B). At all time points tested, Rap1 activation and aggregation were strongly inhibited in the absence of epinephrine. In contrast, platelets activated with the combination of PAR4p and epinephrine showed a strong aggregation response and a marked Rap1 activation, both occurring with similar kinetics.
Similar kinetics for Rap1 activation and aggregation in platelets stimulated with PAR4p. (A) Time course for the aggregation response (top panel) and for Rap1 activation (bottom panel) in WT or CalDAG-GEFI–deficient (CalDAG-GEFI−/−) platelets stimulated with 1.0 mM of PAR4p. Samples for the determination of Rap1-GTP and total Rap1 levels were withdrawn from the aggregation tubes at t = 15, 45, or 180 minutes after addition of the agonist (indicated by black arrows). Results are representative of 3 independent experiments. (B) Time course for the aggregation response (top panel) and for Rap1 activation (bottom panel) in 2-MesAMP-pretreated CalDAG-GEFI−/− platelets stimulated with 1.5 mM of PAR4p in the presence or absence of 10 μM of epinephrine. Samples for the determination of Rap1-GTP and total Rap1 levels were withdrawn from the aggregation tubes at the indicated time points. Results are representative of 3 independent experiments.
Similar kinetics for Rap1 activation and aggregation in platelets stimulated with PAR4p. (A) Time course for the aggregation response (top panel) and for Rap1 activation (bottom panel) in WT or CalDAG-GEFI–deficient (CalDAG-GEFI−/−) platelets stimulated with 1.0 mM of PAR4p. Samples for the determination of Rap1-GTP and total Rap1 levels were withdrawn from the aggregation tubes at t = 15, 45, or 180 minutes after addition of the agonist (indicated by black arrows). Results are representative of 3 independent experiments. (B) Time course for the aggregation response (top panel) and for Rap1 activation (bottom panel) in 2-MesAMP-pretreated CalDAG-GEFI−/− platelets stimulated with 1.5 mM of PAR4p in the presence or absence of 10 μM of epinephrine. Samples for the determination of Rap1-GTP and total Rap1 levels were withdrawn from the aggregation tubes at the indicated time points. Results are representative of 3 independent experiments.
Discussion
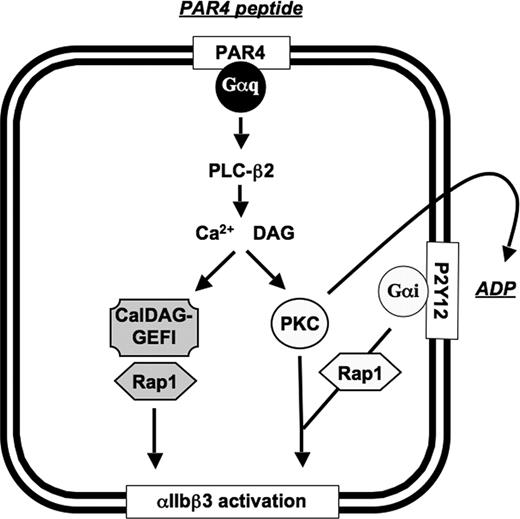
Based on the results of this study, a new model for αIIbβ3 activation in platelets can be proposed (Figure 6): CalDAG-GEFI and PKC represent independent, synergistic signaling pathways leading to αIIbβ3 activation downstream of agonist-induced activation of PLC-β2. In WT platelets, generation of the second messengers calcium and DAG led to the concurrent activation of CalDAG-GEFI and PKC, allowing for αIIbβ3 activation independent of the generation of the second wave mediators TxA2 and ADP. In the absence of CalDAG-GEFI, PKC-mediated activation of αIIbβ3 requires cosignaling by Gαi, provided by platelet-derived ADP, most probably because of its role in the activation of the small GTPase Rap1. In the absence of functional PKC, activation of Rap1 and αIIbβ3 is mediated solely by CalDAG-GEFI, as ADP release is inhibited under such experimental conditions.
Schematic representation of the CalDAG-GEFI–dependent and PKC-dependent signaling pathways leading to αIIbβ3 activation in mouse platelets. PLC-β2 indicates phospholipase C-β2; PAR4, protease activated receptor 4; Gαq/Gαi, heterotrimeric G protein; PKC, protein kinase C.
Schematic representation of the CalDAG-GEFI–dependent and PKC-dependent signaling pathways leading to αIIbβ3 activation in mouse platelets. PLC-β2 indicates phospholipase C-β2; PAR4, protease activated receptor 4; Gαq/Gαi, heterotrimeric G protein; PKC, protein kinase C.
This study was designed to further clarify the role of CalDAG-GEFI in αIIbβ3 activation in platelets stimulated through the activating thrombin receptor PAR4. A specific PAR4 agonist (GYPGKF, PAR4p) was used for these studies because (1) PAR4 is the major activating receptor for thrombin in mouse platelets, whereas PAR3 facilitates the activation through PAR424 ; (2) stimulation of platelets with thrombin would also cause the activation of signaling cascades downstream of GPIbα,26 thus leading to unwanted complexities in the interpretation of the results; and (3) in contrast to thrombin, PAR4p does not activate the coagulation system and can thus be used in PRP.
Synergistic signaling between CalDAG-GEFI and PKC was evident in 2 ways: (1) at high concentrations of PAR4p, where full aggregation was observed in platelets lacking functional PKC or CalDAG-GEFI, preincubation of CalDAG-GEFI–deficient platelets with Ro31-8220 blocked integrin activation/aggregation; and (2) at a low dose of the agonist, where successful activation of αIIbβ3 was observed in WT platelets but not platelets lacking either functional PKC or CalDAG-GEFI (Figure 1).
In addition to PKC, αIIbβ3 activation/aggregation induced by high PAR4p concentrations in the absence of CalDAG-GEFI required signaling by the Gαi-coupled ADP receptor, P2Y12 (Figure 2). Interestingly, our studies also suggest that the aggregation defect of CalDAG-GEFI–deficient platelets stimulated with low-dose PAR4p is the result of impaired ADP/dense granule release in these platelets (Figure 3). These studies with low-dose PAR4p-stimulated mutant platelets cannot rule out the possibility that addition of ADP simply pushes the platelets above the threshold concentration of stimulus required for αIIbβ3 activation. However, aggregation was again dependent on P2Y12 signaling, demonstrating the importance of this signaling pathway as an alternative to CalDAG-GEFI signaling in integrin activation. It is not clear how CalDAG-GEFI-Rap1 signaling affects granule release in platelets stimulated with low-dose PAR4p. Studies in other cell types demonstrated that Rap1 is localized to the surface of secretory granules and that it translocates to the plasma membrane on cellular stimulation, suggesting that it may play a role in granule secretion.27-29 Furthermore, CalDAG-GEFII and CalDAG-GEFIII have been implicated in granule release in mast cells and endocrine tissue, respectively.30,31 CalDAG-GEF–mediated granule release in these cells involved activation of the small GTPase RhoA, and microtubule formation and was, at least in part, independent of PKC function. The latter observation is in contrast to our own findings, which show completely inhibited granule release in PAR4p-activated platelets lacking PKC signaling. The mechanisms by which CalDAG-GEFI affects granule release in platelets will be investigated in further studies.
Our results on the role of CalDAG-GEFI and PKC signaling in platelet integrin activation are well in line with previous studies. Inhibition of PKC signaling was shown to strongly impair dense granule release in thrombin-activated human platelets, whereas it only minimally affects the aggregation of these cells.32-34 Using calcium chelators to remove intracellular calcium, Quinton et al suggested a calcium-regulated pathway that synergizes with PKC signaling in fibrinogen receptor activation in human platelets stimulated through PAR1.6 Our studies suggest that CalDAG-GEFI is the primary signaling molecule mediating calcium-dependent integrin activation in PAR4p-stimulated platelets. Like platelets treated with a calcium chelator, platelets lacking CalDAG-GEFI require PKC signaling and costimulation through Gαi-coupled receptors for successful αIIbβ3 activation (Figures 1,2). Furthermore, as shown previously,19 CalDAG-GEFI–deficient platelets show aggregation defects to agonists other than thrombin, which are very similar to those published for BAPTA-AM–treated platelets. This involves defective aggregation in response to ADP and the TxA2 mimetic U46619 and partially inhibited aggregation in response to collagen. And lastly, calcium ionophore A23187 failed to aggregate CalDAG-GEFI–deficient platelets, whereas the DAG mimetic PMA led to full platelet aggregation.19
The key role of CalDAG-GEFI in the rapid activation of Rap1 further confirms that this molecule is the main signaling molecule involved in calcium-induced activation of integrins. In 1997, Franke et al showed that Rap1 activation in platelets is mediated by a rapid calcium-dependent mechanism.12 In a subsequent study, the same group suggested a model for the sequential activation of Rap1 in thrombin-stimulated platelets.35 In this model, Rap1 activation is initiated by intracellular calcium generated through PLC activity, followed by a second phase of activation requiring PKC. In later years, several groups demonstrated a role for ADP-initiated Gαi signaling in the PKC-mediated pathway of Rap1 activation.13-15 In CalDAG-GEFI–deficient platelets activated with PAR4p under static conditions, Rap1 activation was completely inhibited in the presence of inhibitors of P2Y12 or PKC (Figure 4). Trace amounts of Rap1-GTP were observed in 2-MesAMP-treated mutant platelets activated under stirring conditions (Figure 5B). These data show that CalDAG-GEFI is the dominant molecule in PAR4p-activated platelets allowing for the activation of Rap1 in the absence of Gαi signaling, and that it represents the previously described calcium-dependent pathway involved in the rapid activation of Rap1. So far, CalDAG-GEFI is the only Rap1-GEF with documented activity in the regulation of platelet Rap1. Other identified Rap1-GEFs in platelets include CalDAG-GEFIII,36 PDZ-GEF1,36 and Epac1 (cAMP-GEFI).37 In addition to GEFs, Rap1 activation is regulated by GAPs. In platelets, Rap1GAP2 was recently identified.36 In other cell types, Gαi/o signaling was shown to promote ubiquitination and proteosomal degradation of Rap1GAP2, leading to enhanced Rap1 activity.38 The role of Rap1GAP2 in Gαi-dependent Rap1 activation in platelets has not been addressed.
In addition to identifying CalDAG-GEFI as the central molecule mediating the rapid activation of Rap1, we could demonstrate that Rap1 activation and aggregation follow very similar kinetics in activated platelets (Figure 5). In CalDAG-GEFI–deficient platelets activated with a threshold dose of PAR4p, both aggregation and Rap1 activation were delayed by approximately 30 seconds. Whereas Rap1 activation was weak at the beginning of the aggregation phase, marked Rap1 activation was observed when maximum aggregation was reached. Similarly, the kinetic of Rap1 activation mirrored that of the aggregation response in 2-MesAMP-pretreated CalDAG-GEFI-deficient platelets costimulated with PAR4p and epinephrine. It cannot be concluded, however, that impaired αIIbβ3 activation/aggregation observed in CalDAG-GEFI–deficient platelets pretreated with MesAMP or Ro31-8220 is the direct result of defective Rap1 signaling. Indeed, studies with platelets lacking Rap1b make this assumption rather doubtful as they showed significant αIIbβ3 activation in response to various agonists (including thrombin).16 However, platelets also express other small GTPases shown to be involved in integrin activation, such as Rap239 and R-ras.40,41 These proteins may be regulated by CalDAG-GEFI–dependent and -independent mechanisms and thus facilitate αIIbβ3 activation in the absence of Rap1.
It is important to note that there are significant species-specific differences in platelet responses to thrombin between human and mouse platelets, with mice expressing PAR3 to support cellular activation through PAR4,23 whereas αIIbβ3 activation induced by thrombin in human platelets depends on signaling provided by both PAR1 and PAR4. Recent studies by Holinstat et al demonstrated impaired activation of integrin αIIbβ3 in PAR4-stimulated human platelets pretreated with BAPTA-AM and 2-MesAMP.42 In contrast, pretreatment with BAPTA-AM and 2-MesAMP inhibited αIIbβ3 activation in human platelets stimulated with a low but not high dose of PAR1-stimulating peptide. These findings may be explained by signaling through Gαi/o, which couples to PAR1 but not PAR4.43
Our findings are also important with regard to the diagnosis of patients with leukocyte adhesion deficiency type III (LAD-III), a clinical complication characterized by recurrent infections and a Glanzmann-like bleeding phenotype.44 Studies in mice20 and humans45 strongly suggest that impaired expression/function of CalDAG-GEFI, resulting in defective Rap1 activation and integrin-mediated cell adhesion, is a molecular cause for this disease. Others, however, have not found defects in Rap1 activation in platelets and leukocytes from patients with a similar inflammatory and hemostatic phenotype (designated LAD-I/variant).46,47 It is entirely possible that abnormalities in signaling molecules other than CalDAG-GEFI can lead to a combined defect in β1, β2, and β3 integrins demonstrated for LAD-III (LAD-I/variant) patients. It is important to keep in mind, however, that there are alternative pathways in platelets (and most probably leukocytes), which can facilitate Rap1 activation in the absence of functional CalDAG-GEFI. Based on our studies, we suggest that patients with a LAD-III phenotype be tested for Rap1 activation in platelets activated with weak agonists, such as ADP or low-dose thrombin. If CalDAG-GEFI function is impaired, Rap1 activation will be strongly reduced in these cells. In contrast, Rap1 activation will appear normal in platelets activated with high-dose thrombin, as ADP-induced P2Y12/Gαi signaling will serve as a backup for CalDAG-GEFI under these experimental conditions.
In conclusion, we show that the activation of Rap1 and αIIbβ3 in platelets stimulated through the PAR4 receptor is regulated by independent CalDAG-GEFI– and PKC/ADP-dependent pathways. Our data further demonstrate that CalDAG-GEFI is the dominant signaling molecule allowing for the rapid but reversible activation of Rap1 in PAR4p-stimulated platelets and that CalDAG-GEFI is important for dense granule release in platelets stimulated with low-dose PAR4p.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Crystal Piffath for her excellent technical support throughout the study, Janos Polgar for help with the serotonin release assay and helpful discussions, and Jill Crittenden and Ann Graybiel for providing the mice and for helpful discussions.
This work was supported by a Scientist Development Grant 0630044N from the American Heart Association (W.B.) and the National Heart, Lung, and Blood Institute of the National Institutes of Health (grants R37 HL41002 and P01 HL56949; D.D.W.)
National Institutes of Health
Authorship
Contribution: S.M.C. performed many of the experiments and helped analyze the data; D.D.W. helped design the study and interpret the results; and W.B. designed the study, performed many of the experiments, analyzed the results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wolfgang Bergmeier, Cardeza Foundation and Department of Medicine, Thomas Jefferson University, 803 Curtis, 1015 Walnut Street, Philadelphia, PA 19107; e-mail: wolfgang.bergmeier@jefferson.edu.