Abstract
Histone deacetylase 6 (HDAC6) is a heat shock protein 90 (hsp90) deacetylase. Treatment with pan-HDAC inhibitors or depletion of HDAC6 by siRNA induces hyperacetylation and inhibits ATP binding and chaperone function of hsp90. Treatment with 17-allylamino-demothoxy geldanamycin (17-AAG) also inhibits ATP binding and chaperone function of hsp90, resulting in polyubiquitylation and proteasomal degradation of hsp90 client proteins. In this study, we determined the effect of hsp90 hyperacetylation on the anti-hsp90 and antileukemia activity of 17-AAG. Hyperacetylation of hsp90 increased its binding to 17-AAG, as well as enhanced 17-AAG–mediated attenuation of ATP and the cochaperone p23 binding to hsp90. Notably, treatment with 17-AAG alone also reduced HDAC6 binding to hsp90 and induced hyperacetylation of hsp90. This promoted the proteasomal degradation of HDAC6. Cotreatment with 17-AAG and siRNA to HDAC6 induced more inhibition of hsp90 chaperone function and depletion of BCR-ABL and c-Raf than treatment with either agent alone. In addition, cotreatment with 17-AAG and tubacin augmented the loss of survival of K562 cells and viability of primary acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) samples. These findings demonstrate that HDAC6 is an hsp90 client protein and hyperacetylation of hsp90 augments the anti-hsp90 and antileukemia effects of 17-AAG.
Introduction
Among the stress-inducible molecular chaperones, hsp90 is an abundantly expressed (as much as 2% of total cellular protein), homodimeric, ATP-dependent protein.1-3 Hsp90 is required for the maintenance of native and functionally active conformation of important signaling protein kinases and transcription factors, also known as hsp90 client proteins.1-3 Hsp90-based chaperone complex interacts with its client proteins in an iterative manner, in which hsp90 cycles between ATP- or ADP-bound conformations mediated through multiple rounds of ATP binding and hydrolysis.4,5 Replacement of ADP by ATP in hsp90 alters hsp90 conformation, thereby releasing cochaperones p60HOP and hsp70/hsp40 complex, while simultaneously recruiting another set of cochaperones including p23 and cyclophillin 40 (when the client protein is a steroid nuclear hormone receptor) or p50cdc37 (when the client protein is a signaling protein kinase).2,3,6-9 Conversely, ATP hydrolysis due to its intrinsic ATPase activity creates the ADP-bound conformation of hsp90, which directs the misfolded client protein to a covalent linkage with polyubiquitin and subsequent degradation by the 26S proteasome.2,3
Benzoquinone ansamycin–derived antibiotic geldanamycin (GA) and its analogs (eg, 17-AAG and 17-DMAG) bind to the ATP/ADP-binding pocket with higher affinity than the nucleotide, replacing the nucleotide and inhibiting the chaperone function of hsp90.2,3 By blocking ATP binding, treatment with 17-AAG stabilizes ADP-bound hsp90 into a conformation that recruits hsp70-based cochaperone complex, which allows polyubiquitylation of the misfolded client protein by specific E3 ubiquitin ligase and directs the client proteins to proteasomal degradation.2,3 A growing number of hsp90 client proteins have been recognized in human acute leukemia cells.2,3 These include conformationally metastable signaling protein kinases and mutated oncoprotein kinases (eg, FLT-3, Bcr-Abl, NPM-ALK, c-Raf, AKT, and CDK4).1-3,10-14 Treatment with 17-AAG has been shown to deplete hsp90 client proteins, which are essential for growth and survival of transformed cells. Consequently, 17-AAG exerts lethal in vitro and in vivo effects against human leukemia cells.10,15 Recently, posttranslational modifications such as hyperphosphorylation, S-nitrosylation, and reversible hyperacetylation of lysine residues have been demonstrated to affect cochaperone association, ATP binding, and chaperone function of hsp90.2 The predominantly cytosolic, class IIB HDAC family member HDAC6 was shown to be the deacetylase for hsp90, α-tubulin, and cortactin.16-18 Depletion of HDAC6 levels, or the inhibition of its deacetylase activity with pan-histone deacetylase inhibitors (HDIs), was shown to result in reversible hyperacetylation of hsp90.16,19 This was associated with inhibition of ATP and client protein binding to hsp90, inhibition of hsp90 chaperone function and proteasomal degradation of hsp90 client proteins.16,19 It is noteworthy that since the mutant forms of oncoprotein kinases (eg, Bcr-Abl and FLT-3) may be more dependent on the chaperone function of hsp90, inhibition of hsp90 may be particularly effective in depleting the levels of the mutant Bcr-Abl and FLT-3.16,19,20 Furthermore, we have previously demonstrated that cotreatment with 17-AAG and HDI exerts synergistic cytotoxic effects against Bcr-Abl– and FLT-3–expressing human leukemia cells.13 It is likely that there are multiple possible mechanisms underlying the synergistic antileukemia effects of the combination of HDI and 17-AAG. However, in the present study, we focused on how hsp90 hyperacetylation influences 17-AAG–mediated inhibition of hsp90. Specifically, we have determined that induction of hsp90 hyperacetylation (1) enhances the binding of 17-AAG to hsp90, (2) augments 17-AAG–mediated inhibition of ATP and cochaperone binding to hsp90, and (3) promotes 17-AAG–mediated depletion of hsp90 client proteins. Our findings also show that HDAC6 is an hsp90 client protein, and treatment with 17-AAG promotes the degradation of HDAC6 by the proteasome.
Methods
Informed consent was obtained in accordance with the Declaration of Helsinki. The study was approved by the Medical College of Georgia's Human Assurance Committee (IRB).
Reagents and antibodies
17-AAG was obtained from Developmental Therapeutics Branch of the Cancer Treatment Evaluation Program, National Cancer Institute/National Institutes of Health (NIH; Bethesda, MD). LBH589 and LAQ824 were provided by Novartis Pharmaceuticals (East Hanover, NJ). ATP-Sepharose was purchased from Innova Biosciences (Cambridge, United Kingdom). Anti-hsp90 and -hsp70 antibodies were purchased from StressGen Biotechnologies (Victoria, BC). Monoclonal anti–acetyl lysine antibody was purchased from Cell Signaling Technology (Beverly, MA). Monoclonal anti–acetyl α-tubulin was purchased from Sigma-Aldrich (St Louis, MO). Monoclonal anti-Abl and HDAC6 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture
Human chronic myeloid leukemia (CML) K562 cells, acute myeloid leukemia (AML) HL-60 cells, and the bone marrow stroma HS-5 cells were maintained as previously described.16
Western blot analyses and immunoprecipitation
Western blot analyses of Bcr-Abl, c-Raf-1, AKT, hsp90, hsp70, HDAC6, and β-actin were performed using specific antisera or monoclonal antibodies (listed in “Regents and antibodies”), as described previously.16,19-21 Bcr-Abl and hsp90 were immunoprecipitated as previously described.16,19 The expression of β-actin was used as a loading control.
Acetylation of Hsp90 and its binding to ATP-Sepharose
For hsp90 ATP-binding studies, cell lysates were prepared after drug treatment and hsp90 was affinity precipitated from 200 μg total cellular protein using ATP-Sepharose beads. The hsp90 in the precipitates was assessed by Western blot analysis using a monoclonal anti-hsp90 antibody. For hsp90 acetylation, immunoprecipitated hsp90 was immunoblotted and acetylated hsp90 was detected using an anti–acetyl lysine antibody.16
Preparation of detergent-soluble and -insoluble fractions
Transfection of HDAC6 siRNA vector
K562 cells were transiently transfected as per the manufacturer's instructions, using Amaxa Nucleofector Electroporator (Gaithersburg, MD) with the plasmid vector pBS/U6 with or without the HDAC6 siRNA,16 which had a 21-nucleotide sequence of 5′-GG ATG GAT CTG AAC CTT GAG A- 3′, corresponding to the targeted nucleotide sequence 200-219 in the HDAC6 mRNA (accession no. BC013737).23
Biotinylated-GM–binding assay
Biotinylated-GM binding to hsp90 was assessed as described previously.22 Briefly, 48 hours after transfection, control- and siHDAC6-transfected cell lysates were incubated with increasing concentration of 17-AAG, and biotinylated-GM (B-GM) was added as a competitor to displace 17-AAG from hsp90. This was followed by immunoprecipitation of biotinylated-GM–bound hsp90 using Streptavidin-agarose beads and immunoblotting for hsp90.
Confocal immunofluorescence microscopy to evaluate Ki-67 expression
Following vector or siHDAC6 transfection for 48 hours, cells were fixed with 4% paraformaldehyde for 10 minutes. Following this, the slides were blocked with 3% BSA for 30 minutes and incubated with anti-HDAC6 and anti–Ki-67 antibody.24 After 3 washes with PBS, the slides were incubated in Alexa Fluor 488 and Alexa Fluor 594 secondary antibodies (Molecular Probes, Invitrogen, Eugene, OR) for 1 hour at 1:3000 dilution. After 3 washes with PBS, the cells were counterstained with DAPI using Vectashield mountant with DAPI and imaged using Zeiss LSM510 confocal microscope (Carl Zeiss, Heidelberg, Germany), as previously described.25
Colony growth inhibition, loss of viability, and apoptosis assessment
After vector or siHDAC6 transfection for 48 hours, cells were treated with the designated concentrations of 17-AAG for 24 hours; untreated and drug-treated cells were washed in RPMI 1640 medium. After this, 200 cells treated under each condition were resuspended in 100 μL RPMI 1640 media containing 10% FBS, then plated in duplicate wells in a 12-well plate containing 1.0 mL Methocult media (StemCell Technologies, Vancouver, BC) per well. The plates were placed in an incubator at 37°C with 5% CO2 for 10 days. After this incubation, colonies consisting of 50 or more cells, in each well, were counted by an inverted microscope and percentage of colony growth inhibition compared with the untreated control cells was calculated.16,21 Viability of cells incubated with the drugs was performed using trypan blue exclusion assay after 48 hours. The percentage of apoptotic cells was determined by annexin V/propidium iodide (PI) staining and/or by assessing the fraction of hypodiploid (sub-G1) cells, using flow cytometry as previously described.19,20
Statistical analyses
Data were expressed as means plus or minus SEM. Comparisons used Student t test or ANOVA, as appropriate. Values of P less than .05 were assigned significance.
Results
HDAC6 inhibition induces hyperacetylation of hsp90 and hsp70
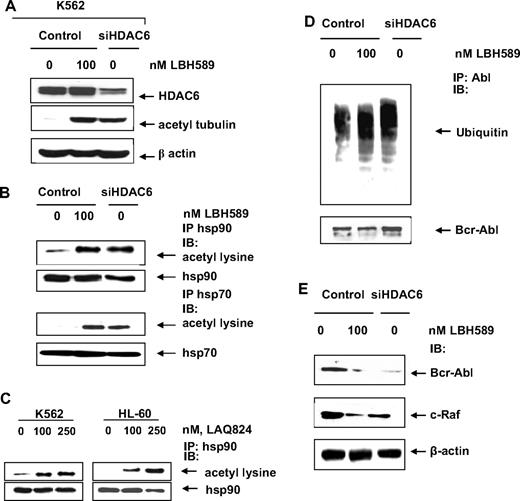
We first compared the effect of the pan-HDAC inhibitor LBH589 and the selective HDAC6 knockdown on hsp90 and hsp70 acetylation in acute leukemia cells. Previous reports had indicated that LBH589 is a potent pan-HDAC, and especially HDAC6 inhibitor.26 Consistent with these reports, treatment with LBH589, or knockdown of HDAC6 protein levels using an siRNA to HDAC6, caused α-tubulin acetylation, as well as induced hyperacetylation of hsp90 and hsp70 (Figure 1A,B). Similar findings were also observed after treatment of human leukemia HL-60 cells with LBH589 (data not shown). Consistent with our previous report,16 similar to treatment with siRNA to HDAC6, LBH589 treatment also increased the amount of polyubiquitylated proteins in the immunoprecipitate with anti–Bcr-Abl antibody (Figure 1D), as well as caused the depletion of Bcr-Abl and c-RAF levels in K562 cells (Figure 1E).
Hydroxamic acid analogues induce acetylation of hsp90 and hsp70. (A) K562 cells were transfected with control or siHDAC6 constructs. After 48 hours, they were exposed to the indicated does of LBH589 for 16 hours and immunoblotted for HDAC6, acetyl-tubulin, or β-actin. (B) After 48 hours of control vector or siHDAC6 transfection and indicated exposure to LBH589, hsp90 and hsp70 were immunoprecipitated and immunoblotted for acetyl lysine or indicated chaperone/cochaperone. (C) K562 and HL-60 cells were exposed to the indicated concentrations of LAQ824 for 16 hours. After this, hsp90 was immunoprecipitated from the cell lysates and immunoblotted with either anti-hsp90 or anti–acetylated lysine antibody. (D) The cell lysates from K562 cells expressing HDAC6 siRNA or from untreated or LBH589-treated K562 cells were immunoprecipitated with anti-Abl antibody, and the immunoprecipitates were immunoblotted with antiubiquitin. The blot was stripped and immunoblotted with anti-Abl antibody. (E) The cell lysates were immunoblotted with anti-Abl or anti–c-Raf antibody. The levels of β-actin served as the loading control.
Hydroxamic acid analogues induce acetylation of hsp90 and hsp70. (A) K562 cells were transfected with control or siHDAC6 constructs. After 48 hours, they were exposed to the indicated does of LBH589 for 16 hours and immunoblotted for HDAC6, acetyl-tubulin, or β-actin. (B) After 48 hours of control vector or siHDAC6 transfection and indicated exposure to LBH589, hsp90 and hsp70 were immunoprecipitated and immunoblotted for acetyl lysine or indicated chaperone/cochaperone. (C) K562 and HL-60 cells were exposed to the indicated concentrations of LAQ824 for 16 hours. After this, hsp90 was immunoprecipitated from the cell lysates and immunoblotted with either anti-hsp90 or anti–acetylated lysine antibody. (D) The cell lysates from K562 cells expressing HDAC6 siRNA or from untreated or LBH589-treated K562 cells were immunoprecipitated with anti-Abl antibody, and the immunoprecipitates were immunoblotted with antiubiquitin. The blot was stripped and immunoblotted with anti-Abl antibody. (E) The cell lysates were immunoblotted with anti-Abl or anti–c-Raf antibody. The levels of β-actin served as the loading control.
Hyperacetylated hsp90 has a higher affinity for 17-AAG
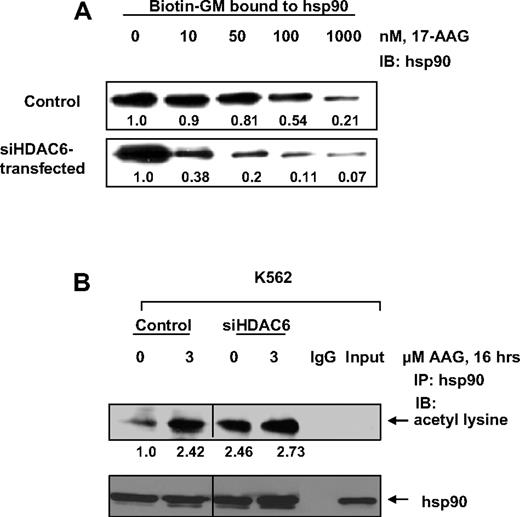
Given that hsp90 from tumor cells has higher affinity for 17-AAG,22 we determined whether knockdown of HDAC6 affects the affinity of hsp90 for 17-AAG. Using the biotinylated geldanamycin (B-GM)–binding assay, we observed that HDAC6 knockdown increased the affinity of 17-AAG for hsp90, as evidenced by the decreased in vitro ability of B-GM to displace 17-AAG from hsp90 (Figure 2A). We further evaluated whether 17-AAG affects hsp90 acetylation in vivo. As shown in Figure 2B, treatment with 3.0 μM 17-AAG for 16 hours alone induced hsp90 acetylation to an extent that is comparable with hsp90 acetylation after HDAC6 knockdown. No significant increase in hsp90 acetylation was detected in cells in which HDAC6 had been knocked down by treatment with 17-AAG, either because the anti–acetyl lysine antibody is unable to detect further increase in acetylation or because the exposure to either condition alone achieved a saturation threshold for hsp90 acetylation. Lower levels of hsp90 acetylation were observed with 1.0 μM 17-AAG (data not shown). These observations suggest that 17-AAG induces hyperacetylation of hsp90 and hyperacetylated hsp90 has increased affinity for 17-AAG.
Depletion of HDAC6 increases the affinity of acetylated hsp90 for 17-AAG. (A) K562 cells were transfected with control vector or siRNA to HDAC6. After 48 hours, the cells were harvested and lysed. The lysates were incubated with or without indicated doses of 17-AAG for 30 minutes at 4°C, and then incubated with biotin-GM for 1 hour at 4°C. To this, washed Streptavidin-agarose beads were added and incubated overnight at 4°C. The immunoprecipitates were washed and proteins were eluted with SDS sample loading buffer before the immunoblot analyses with specific antibody against hsp90. Bands in the Western blots were quantified by densitometry using ImageQuant5.2 (GE Healthcare, Piscataway, NJ). (B) Vector of siHDAC6-transfected cells were exposed to indicated dose of 17-AAG after 48 hours of transfection. Hsp90 acetylation was assessed as described in “Acetylation of Hsp90 and its binding to ATP-Sepharose.” Vertical line has been inserted to indicate a repositioned lane from the same gel.
Depletion of HDAC6 increases the affinity of acetylated hsp90 for 17-AAG. (A) K562 cells were transfected with control vector or siRNA to HDAC6. After 48 hours, the cells were harvested and lysed. The lysates were incubated with or without indicated doses of 17-AAG for 30 minutes at 4°C, and then incubated with biotin-GM for 1 hour at 4°C. To this, washed Streptavidin-agarose beads were added and incubated overnight at 4°C. The immunoprecipitates were washed and proteins were eluted with SDS sample loading buffer before the immunoblot analyses with specific antibody against hsp90. Bands in the Western blots were quantified by densitometry using ImageQuant5.2 (GE Healthcare, Piscataway, NJ). (B) Vector of siHDAC6-transfected cells were exposed to indicated dose of 17-AAG after 48 hours of transfection. Hsp90 acetylation was assessed as described in “Acetylation of Hsp90 and its binding to ATP-Sepharose.” Vertical line has been inserted to indicate a repositioned lane from the same gel.
Hyperacetylation of hsp90 increases 17-AAG–mediated depletion of ATP and p23-binding to hsp90
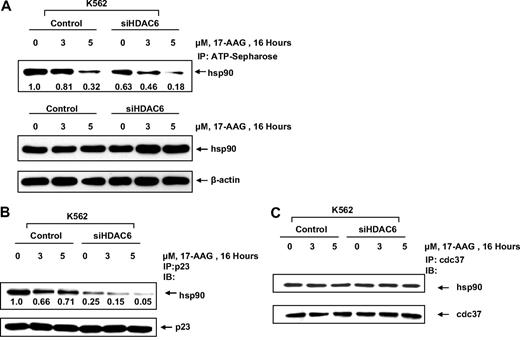
We next evaluated whether increased binding of 17-AAG to hyperacetylated hsp90 affects 17-AAG–mediated decline in the chaperone function of hsp90, which is dependent on its ATP and cochaperone binding. Control vector or siHDAC6-transfected cells were treated with 17-AAG for 16 hours and the level of ATP-bound hsp90 was determined. Our results indicate that the hyperacetylation of hsp90 mediated by HDAC6 knockdown depleted ATP binding to hsp90 (Figure 3A). Cotreatment with 17-AAG and siHDAC6 reduced ATP binding to a lower level than either of the treatments alone (Figure 3A). Knockdown of HDAC6 slightly increased 17-AAG–mediated induction of the total hsp90 levels. In addition, hsp90 hyperacetylation was associated with reduced binding of hsp90 to the cochaperone p23 but not cdc37 (Figure 3B,C). Reduced binding of p23 to hyperacetylated hsp90 is consistent with its reduced binding to ATP. This is consistent with previous observation that p23 binds only the ATP-bound form of hsp90, which facilitates the maturation of hsp90 client proteins.4,5
Knockdown of HDAC6 expression inhibits binding of ATP to hsp90 and affects the binding of hsp90 to its cochaperone p23. (A) K562 cells expressing HDAC6 siRNA or K562 vector control cells were treated with the indicated dose of 17-AAG for 16 hours. After this treatment, cell lysates were affinity precipitated with ATP-Sepharose and immunoblotted with anti-hsp90 antibody. The levels of hsp90 after 17-AAG treatment are as indicated. (B) Vector control K562 and HDAC6 siRNA knockdown cells were left untreated or treated for 16 hours with 3 and 5 μM 17-AAG. Lysates were immunoprecipitated with anti-p23 antibody followed by immunoblotting with anti-hsp90 and anti-p23 antibody. (C) Alternatively, lysates were immunoprecipitated with anti-cdc37 antibody followed by immunoblotting with anti-hsp90 and anti-cdc37 antibody.
Knockdown of HDAC6 expression inhibits binding of ATP to hsp90 and affects the binding of hsp90 to its cochaperone p23. (A) K562 cells expressing HDAC6 siRNA or K562 vector control cells were treated with the indicated dose of 17-AAG for 16 hours. After this treatment, cell lysates were affinity precipitated with ATP-Sepharose and immunoblotted with anti-hsp90 antibody. The levels of hsp90 after 17-AAG treatment are as indicated. (B) Vector control K562 and HDAC6 siRNA knockdown cells were left untreated or treated for 16 hours with 3 and 5 μM 17-AAG. Lysates were immunoprecipitated with anti-p23 antibody followed by immunoblotting with anti-hsp90 and anti-p23 antibody. (C) Alternatively, lysates were immunoprecipitated with anti-cdc37 antibody followed by immunoblotting with anti-hsp90 and anti-cdc37 antibody.
HDAC6 is an hsp90 client protein
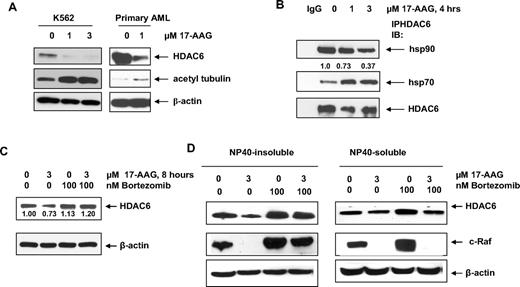
Having established that 17-AAG induces hsp90 acetylation (Figure 2A) and enhances the loss of hsp90 chaperone function after HDAC6 inhibition (Figure 3A,B), we hypothesized that HDAC6 binds to and is chaperoned by hsp90, making it a bona fide hsp90 client protein. Indeed, 17-AAG–mediated abrogation of hsp90 chaperone function was associated with a decline in the levels of HDAC6 in K562 cells, as well as in the primary AML cells (Figure 4A), very similar to the other bona fide hsp90 client proteins. This was accompanied by increased acetylation of α-tubulin in both cell types. Earlier studies have shown that inhibition hsp90 function shifts the chaperone association of client proteins from hsp90 to hsp70 and results in their proteasomal degradation.1-3 Treatment of K562 cells with 17-AAG for 4 hours reduced the binding of hsp90 to HDAC6 and reciprocally increased its association with hsp70 (Figure 4B). 17-AAG–mediated depletion of HDAC6 over 8 hours was due to the proteasomal degradation of HDAC6, since its level was restored by cotreatment with the proteasome inhibitor bortezomib (Figure 4C). This was seen not only in the total cell lysates of K562 cells, but also in the detergent (NP40)–insoluble or the –soluble fraction of K562 cells (Figure 4D). Collectively, these findings indicate that HDAC6 is an hsp90 client protein.
HDAC6 has chaperone association with hsp90. (A) K562 cells or primary AML sample were treated with indicated doses of 17-AAG for 16 hours and 24 hours, respectively. After this, the resulting lysates were probed for HDAC6, acetyl-tubulin, and β-actin. (B) HDAC6 was immunoprecipitated from the cells incubated for 4 hours with indicated doses of 17-AAG and immunoblotted with anti-hsp90, anti-hsp70, or anti-HDAC6 antibody. (C) K562 cells were exposed to indicated concentrations of drugs for 8 hours and the resulting cell lysates were immunoblotted for HDAC6 and β-actin. (D) K562 cells were exposed to indicated concentration of drugs for 8 hours and NP-40–insoluble and –soluble fractions were prepared from the cell lysates. The resulting fractions were immunoblotted with anti-HDAC6 or anti–c-Raf antibodies.
HDAC6 has chaperone association with hsp90. (A) K562 cells or primary AML sample were treated with indicated doses of 17-AAG for 16 hours and 24 hours, respectively. After this, the resulting lysates were probed for HDAC6, acetyl-tubulin, and β-actin. (B) HDAC6 was immunoprecipitated from the cells incubated for 4 hours with indicated doses of 17-AAG and immunoblotted with anti-hsp90, anti-hsp70, or anti-HDAC6 antibody. (C) K562 cells were exposed to indicated concentrations of drugs for 8 hours and the resulting cell lysates were immunoblotted for HDAC6 and β-actin. (D) K562 cells were exposed to indicated concentration of drugs for 8 hours and NP-40–insoluble and –soluble fractions were prepared from the cell lysates. The resulting fractions were immunoblotted with anti-HDAC6 or anti–c-Raf antibodies.
Hyperacetylation of hsp90 increases 17-AAG–mediated depletion of Bcr-Abl, AKT, and c-Raf
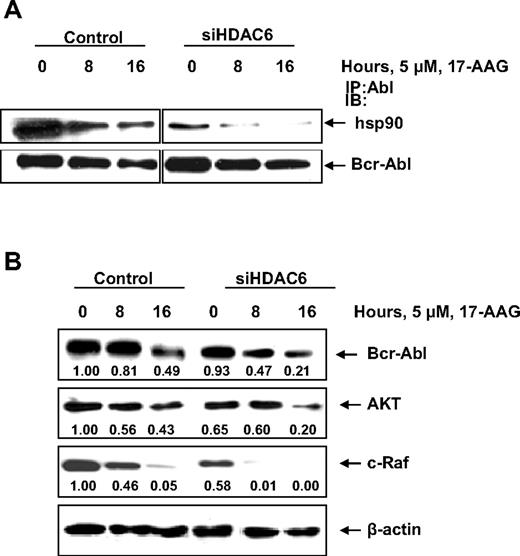
Next, we determined whether hyperacetylation of hsp90 by HDAC6 knockdown would increase 17-AAG–mediated abrogation of the chaperone association of hsp90 with Bcr-Abl. Figure 5A demonstrates that hyperacetylation of hsp90 enhanced 17-AAG–induced disruption in the chaperone association of Bcr-Abl with hsp90 in a time-dependent manner. This resulted in more depletion of Bcr-Abl levels due to 17-AAG treatment of K562 cells treated with the siRNA to HDAC6, compared with those cells treated with the control siRNA (Figure 5B). Importantly, 17-AAG treatment also caused more depletion of Bcr-Abl, AKT, and c-Raf in those cells in which HDAC6 had been knocked down and hsp90 was hyperacetylated, compared with the control cells (Figure 5B).
Diminishing the levels of HDAC6 in leukemic cells not only reduces the binding of hsp90 to its client protein (Bcr-Abl) but also causes partial depletion of the client protein levels. (A) K562 cells were transfected with vector control or HDAC6 siRNA for 48 hours. After this, the cells were treated with 5 μM 17-AAG for 0, 8, and 16 hours and cell lysates were immunoprecipitated with anti-Abl antibody, and the immunoprecipitates were either immunoblotted with anti-hsp90 or anti-Abl antibody. (B) Alternatively, the cell lysates were immunoblotted with anti-Abl, AKT, or anti–c-Raf antibody. The levels of β-actin served as the loading control.
Diminishing the levels of HDAC6 in leukemic cells not only reduces the binding of hsp90 to its client protein (Bcr-Abl) but also causes partial depletion of the client protein levels. (A) K562 cells were transfected with vector control or HDAC6 siRNA for 48 hours. After this, the cells were treated with 5 μM 17-AAG for 0, 8, and 16 hours and cell lysates were immunoprecipitated with anti-Abl antibody, and the immunoprecipitates were either immunoblotted with anti-hsp90 or anti-Abl antibody. (B) Alternatively, the cell lysates were immunoblotted with anti-Abl, AKT, or anti–c-Raf antibody. The levels of β-actin served as the loading control.
Hyperacetylation of hsp90 increases 17-AAG–induced loss of clonogenic survival of K562 cells
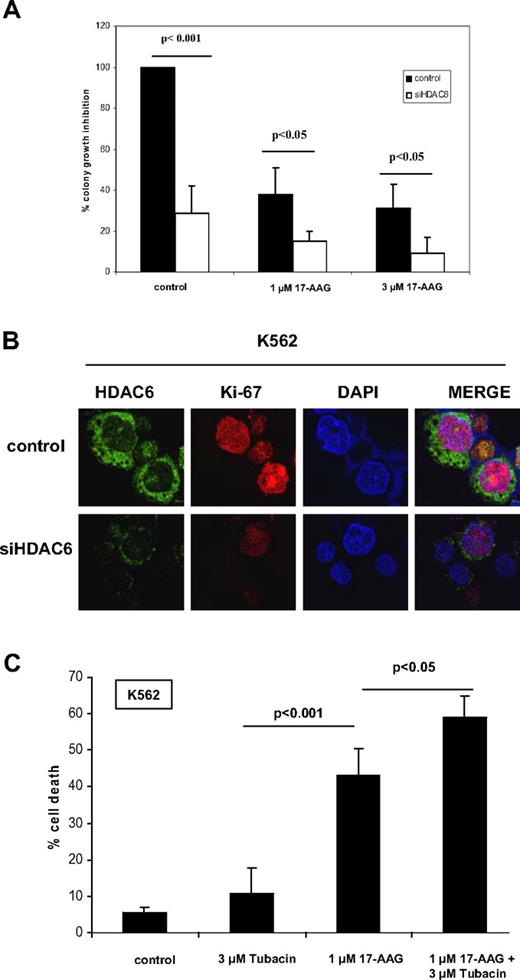
Next, we assessed the effect of HDAC6 depletion and hsp90 hyperacetylation on 17-AAG–mediated loss of clonogenic survival of K562 cells. Figure 6A demonstrates that knockdown of HDAC6 alone markedly inhibited colony growth of K562 cells. Treatment with 17-AAG alone also inhibited the clonogenic survival of K562 cells. In addition, treatment with the siRNA to HDAC6 significantly sensitized K562 cells to 17-AAG–mediated loss of clonogenic survival (P < .05). Knockdown of HDAC6 by treatment with siRNA to HDAC6 also depleted Ki-67 expression in K562 cells, as determined by immunofluorescent confocal microscopy (Figure 6B). This was not associated with induction of apoptosis (sub-G1 fraction or annexin V staining) or with alteration in the percentage of cells in the S phase of the cell cycle (as determined by propidium iodide staining and flow cytometry; data not shown). Cotreatment of K562 cells with 17-AAG and tubacin, an HDAC6-specific inhibitor,27,28 also significantly enhanced the sensitivity of K562 cells to 17-AAG–mediated loss of viability (Figure 6C). We also determined the effects of treatment with tubacin and/or 17-AAG on 3 primary samples each procured from patients with relapsed imatinib refractory CML and relapsed AML, as well as CD34+ normal bone marrow progenitor cells. Table 1 shows that cotreatment with tubacin and 17-AAG caused more loss of cell viability than treatment with either agent alone in each of the CML or AML samples tested. Furthermore, the superior effect of the combination of the 2 drugs was also observed when 2 CML and AML samples each were grown on the human bone marrow–derived HS-5 stromal cells (Table 1). These findings suggest that treatment with tubacin and/or 17-AAG may be able to partially overcome the protective mechanism(s) mediated by the bone marrow stroma. Table 1 also shows that the combination of tubacin and 17-AAG exerts less toxicity against the normal CD34+ progenitor cells.
Knockdown or inhibition of HDAC6 augments loss of clonogenic survival due to treatment with 17-AAG. (A) K562-transfected with control/siHDAC6 construct for 24 hours followed by treatment with indicated doses of 17-AAG for 24 hours and colonies were counted on day 10. Values plotted are means of 3 experiments plus or minus SD. P values were determined by Student t test. (B) K562-transfected with control/siHDAC6 construct for 48 hours, stained with anti-HDAC6 and anti–Ki-67 antibodies, and imaged using confocal immunofluorescent microscope using a 63×/1.2 W correction objective. (C) K562 cells were treated with indicated doses of tubacin with or without 17-AAG for 48 hours, and the loss of survival was assessed by trypan blue exclusion test. Error bars represent SD.
Knockdown or inhibition of HDAC6 augments loss of clonogenic survival due to treatment with 17-AAG. (A) K562-transfected with control/siHDAC6 construct for 24 hours followed by treatment with indicated doses of 17-AAG for 24 hours and colonies were counted on day 10. Values plotted are means of 3 experiments plus or minus SD. P values were determined by Student t test. (B) K562-transfected with control/siHDAC6 construct for 48 hours, stained with anti-HDAC6 and anti–Ki-67 antibodies, and imaged using confocal immunofluorescent microscope using a 63×/1.2 W correction objective. (C) K562 cells were treated with indicated doses of tubacin with or without 17-AAG for 48 hours, and the loss of survival was assessed by trypan blue exclusion test. Error bars represent SD.
Discussion
In this study, we demonstrate for the first time that hyperacetylation of hsp90 induced either by HDAC6 inhibition after LBH589 treatment, or by the depletion of HDAC6 levels by treatment with siRNA to HDAC6, preferentially increased the binding of hsp90 to 17-AAG. Previous studies had demonstrated that inducing hyperacetylation of hsp90 alone was associated with decreased binding of hsp90 to ATP, with disruption of the chaperone association of hsp90 with GR, AR, and ERα,18,29,30 as well as with the other oncoprotein kinases Bcr-Abl, FLT-3, AKT, c-Raf, and HER-2.16,20,31 Present studies extend these observations by demonstrating that hyperacetylation of hsp90 enables 17-AAG to more potently inhibit hsp90 binding to p23, ATP, and the client proteins. This augments 17-AAG–mediated inhibition of hsp90 chaperone function, resulting in depletion of hsp90 client proteins.
Both LBH589 treatment and HDAC6 knockdown decreased hsp90 binding to ATP and the cochaperone p23. This finding is consistent with the notion that p23 binds ATP-bound hsp90 and locks it in an ATP-dependent conformational state that has high affinity for client proteins.32 In contrast, the binding of hsp90 to cdc37, the cochaperone required for the loading of kinase client proteins on to hsp90, remained unimpaired. It is recognized that cdc37 differs from p23 in its ability to associate equally well with both ATP-bound and 17-AAG–bound hsp90.33 Structural data support the model that cdc37 can bind the open, ATP-free conformation of the N-termini of the hsp90 dimer, and that ATP binding converts the hsp90 dimer into a closed or tensed conformation, which is crucial for chaperoning client proteins.14 Therefore, although 17-AAG does not affect cdc37 binding to hsp90, it may prevent ATP-dependent chaperoning of client proteins by hsp90.14
Identification of specific acetylation site(s) that are involved in the reversible hyperacetylation of hsp90 is critical in comprehensively defining the role of this posttranslational modification in chaperone function of hsp90. Recently, K294 has been identified as one of the many acetylation sites that is at the junction of the N-terminal and middle domain of hsp90.34,35 Acetylation-mimetic lysine to glutamine mutant of K294 showed a decrease in the client protein and cochaperone binding.34 Our recent findings have indicated that additional functional acetylation sites are located in the N-terminal and middle domain of hsp90, which regulate the binding of ATP, cochaperone p23, and 17-AAG to hsp90.25,35 These observations highlight that, by modulating its chaperone function, reversible hyperacetylation of hsp90 provides another layer of control on the activity and levels of the signaling hsp90 client proteins, as well as the response of the cell to environmental stimuli.36
As inferred from our preferential displacement of biotinylated GM-binding studies, hyperacetylation of hsp90 induced by siRNA to HDAC6 increased the affinity of hsp90 for 17-AAG. This augmented 17-AAG–mediated inhibition of ATP and cochaperone binding. Enhanced affinity of 17-AAG for hsp90 also increased 17-AAG–mediated disruption of the chaperone function of hsp90 in K562 cells, resulting in greater polyubiquitylation and proteasomal degradation of hsp90 client proteins, including Bcr-Abl, AKT, and c-Raf. This was associated with the observation that the cells cotreated with 17-AAG and the siRNA to HDAC6 showed greater loss of clonogenic survival than due to either agent alone. Notably, HDAC6 knockdown alone with resulting hyperacetylation of hsp90 was sufficient to markedly reduce the colony-forming ability of K562 cells. Associated with depletion of Ki67 expression, this was not associated with induction of apoptosis or with alteration in the percentage of cells in the S phase of the cell cycle. Although in vitro treatment with tubacin was reported to not deplete BrdU-positive S phase population or increase sub-G1 fraction of cells,27 this has not, as yet, been confirmed in the in vivo setting due to the lack of availability of sufficient quantities of HDAC6-specific inhibitor for the in vivo studies. It is tempting to speculate that HDAC6 knockdown-mediated lysine hyperacetylation and alteration of the function of proteins other than hsp90 may also contribute to the increased loss of clonogenic survival of K562 cells.37 Inhibition of HDAC6 activity or depletion of HDAC6 levels also disrupts the protective handling of misfolded and ubiquitylated toxic protein aggregates, which too can affect the clonogenic survival of K562 cells.37 Cotreatment of K562 cells with tubacin and 17-AAG resulted in enhanced loss of cell survival, even though treatment with tubacin alone did not affect the viability of K562 cells, as has also been noted in previous studies.27 A recent report noted that mice lacking HDAC6 have hyperacetylated tubulin but are viable and develop normally.38 It would be important to demonstrate the in vivo response to 17-AAG in these mice. In addition, it has been recently observed that normal cytoplasmic HDAC6 function is required for efficient in vivo oncogenesis in HDAC6 knockout mice (T. P. Yao, Duke University, oral communication, May 19, 2008).
Present studies also show that HDAC6 binds and has chaperone association with hsp90. This is supported by the observation that treatment with 17-AAG shifted the binding of HDAC6 from hsp90 to hsp70, which was associated with the degradation of HDAC6 by the 26S proteasome. Thus, by inhibiting chaperone function of hsp90, 17-AAG also depletes HDAC6 levels. This explains why 17-AAG treatment was also associated with hyperacetylation of hsp90 (Figure 4A). Because HDAC6 shuttles misfolded proteins into the protective aggresome, 17-AAG–mediated depletion of HDAC6 could undermine the protection against misfolded proteins afforded by the aggresome.39 Boyault et al40 and Westerheide and Morimoto41 have recently demonstrated that cellular stress and increased levels of misfolded polyubiquitylated proteins caused by treatment with a proteasome inhibitor trigger the dissociation of a repressive HDAC6/hsp90/HSF1 (heat shock factor 1) complex, leading to phosphorylation, trimerization, nuclear localization, and transcriptional activity of HSF1. The latter induces the expression of the major cellular chaperones. Dissociation of HDAC6 from hsp90 also induces hsp90α acetylation. Collectively, these observations also explain why cotreatment with 17-AAG and the proteasome inhibitor bortezomib results in proteotoxic ER stress, accentuated by simultaneous depletion of HDAC6 along with inhibition of the formation of the protective aggresomes.40,42 Thus, increased ER stress noted after cotreatment with 17-AAG and bortezomib is likely due to 17-AAG–mediated depletion of HDAC6.42-44 This combination is currently being investigated against multiple myeloma, where ER stress is constitutively active and can be accentuated to exert selective anti–multiple myeloma activity.43,44 In the present studies, we also show that targeted knockdown of HDAC6 induces hyperacetylation of hsp70. This suggests that HDAC6 also deacetylates hsp70. However, the mechanistic role that hsp70 hyperacetylation plays in regulating 17-AAG–induced disruption of the chaperone association of hyperacetylated hsp90 with its client proteins remains to be fully elucidated.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We wish to thank Drs Stuart Schreiber and Ralph Mazitschek, and Initiative for Chemical Genetics, NIH (Bethesda, MD) for providing tubacin for the studies.
Authorship
Contribution: R.R. performed the experiments, evaluated the results, and helped in writing the paper; W.F., Y.Y., P.L., R.J., P.F., and A.M. helped in performing the studies; P.A. provided reagents and helped design the experiments; J.E.B. provided important reagents for the studies; and K.B. helped design the experiments, evaluated the data, and wrote the paper.
Conflict-of-interest disclosure: P.A. is an employee of Novartis Institute for Biomedical Research, Inc. K.B. has received clinical and laboratory research grant from Novartis Institute for Biomedical Research, Inc. All other authors declare no competing financial interests.
Correspondence: Kapil Bhalla, Medical College of Georgia Cancer Center, 1120 15th Street, CN2101A, Augusta, GA 30912; e-mail: kbhalla@mcg.edu.