Abstract
We have previously demonstrated that mTOR inhibitors (MTIs) are active in preclinical models of acute lymphoblastic leukemia (ALL). MTIs may increase degradation of cyclin D1, a protein involved in dihydrofolate reductase (DHFR) synthesis. Because resistance to methotrexate may correlate with high DHFR expression, we hypothesized MTIs may increase sensitivity of ALL to methotrexate through decreasing DHFR by increasing turn-over of cyclin D1. We tested this hypothesis using multiple ALL cell lines and nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice xenografted with human ALL. We found MTIs and methotrexate were synergistic in combination in vitro and in vivo. Mice treated with both drugs went into a complete and durable remission whereas single agent treatment caused an initial partial response that ultimately progressed. ALL cells treated with MTIs had markedly decreased expression of DHFR and cyclin D1, providing a novel mechanistic explanation for a combined effect. We found methotrexate and MTIs are an effective and potentially synergistic combination in ALL.
Introduction
Novel and less toxic treatment strategies are needed for patients with acute lymphoblastic leukemia (ALL).1 Previously, we have demonstrated that mTOR inhibitors (MTIs), a class of signal transduction inhibitors, are effective as single agents in preclinical models of ALL.2,3 Combination treatment is the next logical step in the therapeutic use of MTIs. It is important to choose rationally-designed combinations, building on an understanding of the mechanism of action of MTIs and interactions with other agents.
MTIs have been shown to prevent activation and increase degradation of cyclin-dependent kinases, including cyclin D1.4 Cyclin D1 is involved in Rb phosphorylation and release E2Fs which are involved in dihydrofolate reductase (DHFR) synthesis.5-7 Resistance to methotrexate has been shown in tumors that have high DHFR expression.8,9 We hypothesized that MTIs may increase the sensitivity of ALL to methotrexate by decreasing cyclin D1, which would in turn would decrease DHFR.5 We tested this hypothesis using relevant preclinical models.
Methods
In vitro drug testing using ALL cell lines
We used 9 previously characterized ALL cell lines for these experiments, including 4 murine ALL lines (289, 83, 420, and T309) and 5 human ALL lines (Nalm 6, Nalm16, CEM, Molt-4, and Jurkat; the phenotypes are listed in Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).3 Cell lines were maintained in culture using published techniques.3 Cells were treated with chemotherapeutic agents, including sirolimus (Wyeth Pharmaceuticals, Philadelphia, PA), temsirolimus (Wyeth), methotrexate (Mayne Pharmaceuticals, Paramus, NJ), L-asparaginase (Merck, Whithouse Station, NJ), doxorubicin (Bedford Labs, Bedford, OH), vincristine (Mayne), dexamethasone (American Regent, Shirley, NY), cytarabine (American Pharmaceutical Partners, Schaumburg, IL), and etoposide (Sicor Pharmaceuticals, Irvine, CA). For each cell line, 1 to 3 × 105 cells/mL were cultured and exposed to drug(s) in triplicate in at least 2 independent experiments. Cell inhibition was assessed using methylthiazoletetrazolium (MTT) and cell death and apoptosis were assessed using annexin-V and 7-amino-actinomycin D (7-AAD) staining as described.2,10 To determine whether combinations were additive, synergistic, or antagonistic we used Chou and Talalay median effects analysis to calculate a combination index (CI) using CalcuSynv1 software (Biosoft, Cambridge, United Kingdom) as described.11
In vivo drug testing using NOD/SCID xenografts
Under Institutional Animal Care and Use Committee– and Institutional Review Board–approved protocols of the University of Pennsylvania and the Children's Hospital of Philadelphia and informed consent obtained in accordance with the Declaration of Helsinki, we generated nonobese diabetic/severe combined immunodeficient (NOD/SCID) xenografts from 2 primary human ALL samples as described previously.2 Blasts were detected by flow cytometry (FACS) using antibodies recognizing human CD19 and CD45 (Beckman Coulter, Brea, CA). Xenografted mice were randomized to treatment arms as described in “Results and discussion,” and drugs and vehicle were prepared as described previously.2 Response to treatment was measured weekly by monitoring peripheral white blood counts and percent blasts by FACS as described.2 Ill-appearing mice were killed, and tissues were harvested. Immunoblots of harvested cells were performed, and relative protein densities were quantitated as described.2
Results and discussion
MTIs can be effectively combined with multiple cytotoxic chemotherapeutics
As an initial experiment and to demonstrate potential synergy between MTIs and chemotherapy agents, we tested one mTOR inhibitor, sirolimus, in combination with 7 cytotoxics with known activity in ALL in 3 ALL cell lines (289, 420, and Nalm6). All 3 lines were sensitive to every cytotoxic agent. Figure 1A shows results for 1 cell line (289), demonstrating at least an additive effect when combining sirolimus with methotrexate, dexamethasone, doxorubicin, etoposide, or asparaginase and no difference in effect when combined with vincristine or cytarabine. Similar results were seen with all cell lines (data not shown). These experiments demonstrate that MTIs can be effectively combined with many agents used to treat ALL.
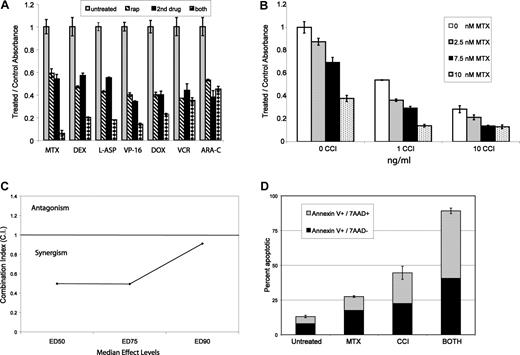
MTIs can be effectively combined with chemotherapy. Aliquots of 2 × 104 cells from 3 ALL lines were treated with sirolimus (rap) and 7 chemotherapeutics for 72 hours. (A) MTT data for each drug combination in 1 representative cell line.  represent untreated, ▧ represent MTI alone, ■ represent cytotoxic alone, and ▩ represent combined effect. All data are normalized to untreated baseline (= 1) with a value greater than 1 representing relative cell proliferation and less than 1 inhibition. Each group of 4 bars represents a combination with a different cytotoxic agent. MTIs had at least an additive effect when combined with methotrexate (MTX), dexamethasone (DEX), L-asparaginase (L-ASP), etoposide (VP-16), and doxorubicin (DOX). The combination of MTIs with vincristine (VCR) and Ara-C (cytarabine) did not add a benefit over either single agent alone. Doses depicted in panel A: sirolimus (0.3 ng/mL), MTX (5 nM), DEX (5 μM), L-ASP (1 μg/μL), VP-16 (1 nM), DOX (1 nM), VCR (1 nM), ARA-C (0.1 μg/mL). Next, aliquots of cells from 9 ALL lines were treated with methotrexate and 2 MTIs (temsirolimus (CCI) and sirolimus). (B) MTT data for temsirolimus and methotrexate in 1 cell line (289), demonstrating a synergistic effect at multiple drug doses. The other cell lines tested showed similar results. (C) Chou and Talalay median effects analysis results for one representative cell line (289), showing a combination index (CI) less than 1 at ED50 (median effective dose to inhibit 50% of cells), ED75, and ED90. (D) A representative example of annexin-V and 7-AAD staining in one cell line (289), demonstrating a synergistic increase in cell death and apoptosis with combined treatment. Doses depicted in panel D: CCI (7.5 ng/mL) and MTX (12.5 nM). Error bars represent SD.
represent untreated, ▧ represent MTI alone, ■ represent cytotoxic alone, and ▩ represent combined effect. All data are normalized to untreated baseline (= 1) with a value greater than 1 representing relative cell proliferation and less than 1 inhibition. Each group of 4 bars represents a combination with a different cytotoxic agent. MTIs had at least an additive effect when combined with methotrexate (MTX), dexamethasone (DEX), L-asparaginase (L-ASP), etoposide (VP-16), and doxorubicin (DOX). The combination of MTIs with vincristine (VCR) and Ara-C (cytarabine) did not add a benefit over either single agent alone. Doses depicted in panel A: sirolimus (0.3 ng/mL), MTX (5 nM), DEX (5 μM), L-ASP (1 μg/μL), VP-16 (1 nM), DOX (1 nM), VCR (1 nM), ARA-C (0.1 μg/mL). Next, aliquots of cells from 9 ALL lines were treated with methotrexate and 2 MTIs (temsirolimus (CCI) and sirolimus). (B) MTT data for temsirolimus and methotrexate in 1 cell line (289), demonstrating a synergistic effect at multiple drug doses. The other cell lines tested showed similar results. (C) Chou and Talalay median effects analysis results for one representative cell line (289), showing a combination index (CI) less than 1 at ED50 (median effective dose to inhibit 50% of cells), ED75, and ED90. (D) A representative example of annexin-V and 7-AAD staining in one cell line (289), demonstrating a synergistic increase in cell death and apoptosis with combined treatment. Doses depicted in panel D: CCI (7.5 ng/mL) and MTX (12.5 nM). Error bars represent SD.
MTIs can be effectively combined with chemotherapy. Aliquots of 2 × 104 cells from 3 ALL lines were treated with sirolimus (rap) and 7 chemotherapeutics for 72 hours. (A) MTT data for each drug combination in 1 representative cell line.  represent untreated, ▧ represent MTI alone, ■ represent cytotoxic alone, and ▩ represent combined effect. All data are normalized to untreated baseline (= 1) with a value greater than 1 representing relative cell proliferation and less than 1 inhibition. Each group of 4 bars represents a combination with a different cytotoxic agent. MTIs had at least an additive effect when combined with methotrexate (MTX), dexamethasone (DEX), L-asparaginase (L-ASP), etoposide (VP-16), and doxorubicin (DOX). The combination of MTIs with vincristine (VCR) and Ara-C (cytarabine) did not add a benefit over either single agent alone. Doses depicted in panel A: sirolimus (0.3 ng/mL), MTX (5 nM), DEX (5 μM), L-ASP (1 μg/μL), VP-16 (1 nM), DOX (1 nM), VCR (1 nM), ARA-C (0.1 μg/mL). Next, aliquots of cells from 9 ALL lines were treated with methotrexate and 2 MTIs (temsirolimus (CCI) and sirolimus). (B) MTT data for temsirolimus and methotrexate in 1 cell line (289), demonstrating a synergistic effect at multiple drug doses. The other cell lines tested showed similar results. (C) Chou and Talalay median effects analysis results for one representative cell line (289), showing a combination index (CI) less than 1 at ED50 (median effective dose to inhibit 50% of cells), ED75, and ED90. (D) A representative example of annexin-V and 7-AAD staining in one cell line (289), demonstrating a synergistic increase in cell death and apoptosis with combined treatment. Doses depicted in panel D: CCI (7.5 ng/mL) and MTX (12.5 nM). Error bars represent SD.
represent untreated, ▧ represent MTI alone, ■ represent cytotoxic alone, and ▩ represent combined effect. All data are normalized to untreated baseline (= 1) with a value greater than 1 representing relative cell proliferation and less than 1 inhibition. Each group of 4 bars represents a combination with a different cytotoxic agent. MTIs had at least an additive effect when combined with methotrexate (MTX), dexamethasone (DEX), L-asparaginase (L-ASP), etoposide (VP-16), and doxorubicin (DOX). The combination of MTIs with vincristine (VCR) and Ara-C (cytarabine) did not add a benefit over either single agent alone. Doses depicted in panel A: sirolimus (0.3 ng/mL), MTX (5 nM), DEX (5 μM), L-ASP (1 μg/μL), VP-16 (1 nM), DOX (1 nM), VCR (1 nM), ARA-C (0.1 μg/mL). Next, aliquots of cells from 9 ALL lines were treated with methotrexate and 2 MTIs (temsirolimus (CCI) and sirolimus). (B) MTT data for temsirolimus and methotrexate in 1 cell line (289), demonstrating a synergistic effect at multiple drug doses. The other cell lines tested showed similar results. (C) Chou and Talalay median effects analysis results for one representative cell line (289), showing a combination index (CI) less than 1 at ED50 (median effective dose to inhibit 50% of cells), ED75, and ED90. (D) A representative example of annexin-V and 7-AAD staining in one cell line (289), demonstrating a synergistic increase in cell death and apoptosis with combined treatment. Doses depicted in panel D: CCI (7.5 ng/mL) and MTX (12.5 nM). Error bars represent SD.
MTIs are synergistic in combination with methotrexate in vitro
Based on our hypothesis that MTIs and methotrexate are synergistic in combination, we tested the 2 clinically available mTOR inhibitors (sirolimus and temsirolimus) in combination with methotrexate using 9 different ALL cell lines. Figure 1B depicts results for 1 cell line (289), demonstrating a synergistic effect when combining methotrexate and temsirolimus as assessed by MTT. We found similar results with combinations of all drug doses in 7 of 9 cell lines using both MTIs. With 2 lines (Molt-4 and Jurkat) we found synergy in combination with all doses except using high doses of both drugs (> IC75). For 5 of the cell lines (289, 83, T309, Nalm 6, and CEM), we performed more extensive experiments and used Chou and Talalay median effects analysis to determine whether the combined effect(s) were mathematically synergistic. We found they were synergistic (CI < 1.0: range 0.33-0.98) in all 5 of the cell lines. Figure 1C depicts a representative example in 1 cell line (289). We assessed apoptosis using 4 cell lines (289, 420, Nalm 6, and CEM) with annexin V staining, comparing cells treated with combinations to individual agents. We found a marked and statistically significant (P < .05) increase in apoptotic (annexin-V+/7-AAD−) and dead (annexin-V+/7-AAD+) cells with combined treatment compared with either single agent. Figure 1D depicts a representative example in 1 cell line (289). These results confirm that MTIs and methotrexate are synergistic in in vitro models of ALL.
MTIs and methotrexate produce a complete remission in xenografted ALL
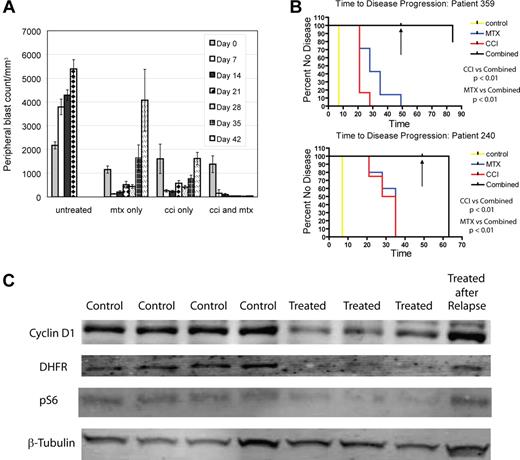
To determine whether MTIs and methotrexate are effective in combination in vivo, we generated xenografts from 2 primary human ALL patient samples (240 and 359). We randomized mice to treatment with temsirolimus 5 mg/kg 5 days a week, temsirolimus 20 mg/kg weekly, methotrexate 5 mg/kg weekly, or temsirolimus and methotrexate combined (with both temsirolimus schedules), after establishment of measurable disease (> 5% blasts in peripheral blood). All drugs were given intraperitoneally. Four to 9 mice were treated in each arm (67 total mice). We focused on the MTI temsirolimus because it is a parenteral agent, simplifying delivery in mice. Mice in all treatment arms had reduction in peripheral blasts; however mice treated with either temsirolimus or methotrexate alone experienced disease recurrence on treatment after 4 to 5 weeks (Figure 2A). After 7 weeks, all drugs were discontinued, and one half of the mice were killed. No mouse in the combination arms from either patient had measurable disease, in contrast to the single agent groups. The remaining mice were killed 2 months later, having received no further treatment. At killing, no mouse from patient 359 treated with combination therapy had detectable disease by FACS in blood, bone marrow, or spleen except for one mouse that had disease in bone marrow only. Mice containing cells from patient 240 treated with combination therapy relapsed 2 weeks after discontinuing therapy. Kaplan-Meier analysis of time to progression demonstrated a statistically significant difference, comparing the combination of drugs to methotrexate (P < .01) and temsirolimus (P < .01) in both samples (240 and 359; Figure 2B), suggesting MTIs and methotrexate are synergistic in vivo. Mice treated with both drugs had similar degrees of myelosuppression to mice treated with methotrexate alone (data not shown). This is the first report to document a therapeutic drug combination that leads to multiple leukemic remissions (some durable) in NOD/SCID xenografts.
Temsirolimus and methotrexate are synergistic and produce durable remission. NOD/SCID mice were xenografted with human ALL from patient samples. After establishment of ALL, mice were randomized to treatment with vehicle, temsirolimus (CCI), methotrexate, or a combination of temsirolimus and methotrexate. Temsirolimus was dosed with 2 schedules, 5 mg/kg 5 days a week and 20 mg/kg weekly. Disease was evaluated at weekly intervals by FACS of peripheral blood for anti–human CD19 and anti–human CD45. (A) Comparison of arms from xenografts generated from sample 359 showing weekly changes in blast count (WBC in mm3 × % blasts). As similar results were found for both dosing schedules only the 20 mg/kg weekly dosing is depicted in panel A. Each series of vertical bars represents average blast count in animals for a particular treated arm at a given timepoint. Control animals died after 3 weeks. Mice treated with temsirolimus or methotrexate alone had improvement in disease but eventual progression. Mice treated with both drugs had complete resolution of peripheral blasts by day 21. After 49 days (depicted by black arrow in panel B) all drugs were stopped. One-half of the mice were killed and no mouse receiving combination therapy had measurable disease. The remaining mice were followed for 2 months and killed. (B) Time to progression on different arms by Kaplan-Meier analysis from sample 359 (top) and 240 (bottom). (C) Immunoblots of splenocytes from sample 240 mice treated with temsirolimus (“Treated”) or vehicle control (“Control”) for various lengths of time, showing decreased cyclin D1 (top row), DHFR (second row), and phospho-S6 (pS6; third row), comparing treated to control animals. In addition, mice that were treated with temsirolimus until relapse (“Treated after Relapse”) had increased expression of cyclin D1 after relapse. 240 samples depicted in panel C from left to right: control 7 days, control 14 days, control 21 days, control 30 days, treated 7 days, treated 14 days, treated 21 days, and treated 30 days.
Temsirolimus and methotrexate are synergistic and produce durable remission. NOD/SCID mice were xenografted with human ALL from patient samples. After establishment of ALL, mice were randomized to treatment with vehicle, temsirolimus (CCI), methotrexate, or a combination of temsirolimus and methotrexate. Temsirolimus was dosed with 2 schedules, 5 mg/kg 5 days a week and 20 mg/kg weekly. Disease was evaluated at weekly intervals by FACS of peripheral blood for anti–human CD19 and anti–human CD45. (A) Comparison of arms from xenografts generated from sample 359 showing weekly changes in blast count (WBC in mm3 × % blasts). As similar results were found for both dosing schedules only the 20 mg/kg weekly dosing is depicted in panel A. Each series of vertical bars represents average blast count in animals for a particular treated arm at a given timepoint. Control animals died after 3 weeks. Mice treated with temsirolimus or methotrexate alone had improvement in disease but eventual progression. Mice treated with both drugs had complete resolution of peripheral blasts by day 21. After 49 days (depicted by black arrow in panel B) all drugs were stopped. One-half of the mice were killed and no mouse receiving combination therapy had measurable disease. The remaining mice were followed for 2 months and killed. (B) Time to progression on different arms by Kaplan-Meier analysis from sample 359 (top) and 240 (bottom). (C) Immunoblots of splenocytes from sample 240 mice treated with temsirolimus (“Treated”) or vehicle control (“Control”) for various lengths of time, showing decreased cyclin D1 (top row), DHFR (second row), and phospho-S6 (pS6; third row), comparing treated to control animals. In addition, mice that were treated with temsirolimus until relapse (“Treated after Relapse”) had increased expression of cyclin D1 after relapse. 240 samples depicted in panel C from left to right: control 7 days, control 14 days, control 21 days, control 30 days, treated 7 days, treated 14 days, treated 21 days, and treated 30 days.
MTIs down-regulate DHFR expression, increasing sensitivity to methotrexate
We hypothesized MTIs may increase sensitivity of ALL to methotrexate through decreasing DHFR via a decrease in cyclin D1. We treated ALL xenografts from 2 different patient samples (3 treated:3 control from sample 240 and 4 treated:4 control from sample 359) with temsirolimus at 5 mg/kg per day 5 days a week or vehicle control. Mice were exposed for various lengths of time (2 to 21 days) and killed in treatment:control pairs prior to disease relapse. Immunoblots for DHFR and cyclin D1 demonstrated decreased expression of DHFR (> 75%) in all treated animals relative to control animals and decreased expression of cyclin D1 (> 25%) in all treated animals but one over all time periods assessed (Figure 2C). Immunoblots for phospho-S6 were used to confirm down-regulation of a downstream target of mTOR as well (Figure 2C). We treated mice with temsirolimus until after disease relapse (> 28 days) and found DHFR and cyclin D1 expression returned to baseline after relapse.
In conclusion, we have demonstrated a number of chemotherapeutic agents can be effectively combined with MTIs in ALL cells. We have demonstrated methotrexate and MTIs are synergistic in combination in vitro and in vivo through a novel mechanistic interaction. This work has led to a pilot clinical trial investigating the combination in adults with relapsed ALL and suggests that this combination should be investigated in future multiinstitutional clinical trials for patients with relapsed/refractory ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Peter Adamson, MD, for valuable discussions, suggestions, and critical review of this manuscript.
This work was supported by National Institutes of Health (Bethesda, MD) grants CA102646, CA1116660, and ACS RSG0507101; the Sanford Chair of the Children's Hospital of Philadelphia (S.A.G.), the Leukemia & Lymphoma Society (White Plains, NY), ASCO (Alexandria, VA) Young Investigator and Career Development Awards; and the Larry and Helen Hoag Foundation (Downey, CA) Clinical Translational Research Scientist Award (D.T.T.).
National Institutes of Health
Authorship
Contribution: D.T.T. and S.A.G. designed the research and drafted the manuscript; V.I.B., M.C., J.F., and A.S. contributed to experimental design; D.T.T., J.H., T.R., Y.C., R.N., and C.S. performed research; D.T.T., S.A.G., M.C., and C.S. analyzed and interpreted data; D.T.T., and C.S. performed statistical analysis; and all authors were involved in critical revision of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Teachey, MD, Division of Oncology, Children's Hospital of Philadelphia, ARC 902, 3615 Civic Center Boulevard, Philadelphia, PA 19104; e-mail: teacheyd@email.chop.edu.


 represent untreated, ▧ represent MTI alone, ■ represent cytotoxic alone, and ▩ represent combined effect. All data are normalized to untreated baseline (= 1) with a value greater than 1 representing relative cell proliferation and less than 1 inhibition. Each group of 4 bars represents a combination with a different cytotoxic agent. MTIs had at least an additive effect when combined with methotrexate (MTX), dexamethasone (DEX), L-asparaginase (L-ASP), etoposide (VP-16), and doxorubicin (DOX). The combination of MTIs with vincristine (VCR) and Ara-C (cytarabine) did not add a benefit over either single agent alone. Doses depicted in panel A: sirolimus (0.3 ng/mL), MTX (5 nM), DEX (5 μM), L-ASP (1 μg/μL), VP-16 (1 nM), DOX (1 nM), VCR (1 nM), ARA-C (0.1 μg/mL). Next, aliquots of cells from 9 ALL lines were treated with methotrexate and 2 MTIs (temsirolimus (CCI) and sirolimus). (B) MTT data for temsirolimus and methotrexate in 1 cell line (289), demonstrating a synergistic effect at multiple drug doses. The other cell lines tested showed similar results. (C) Chou and Talalay median effects analysis results for one representative cell line (289), showing a combination index (CI) less than 1 at ED50 (median effective dose to inhibit 50% of cells), ED75, and ED90. (D) A representative example of annexin-V and 7-AAD staining in one cell line (289), demonstrating a synergistic increase in cell death and apoptosis with combined treatment. Doses depicted in panel D: CCI (7.5 ng/mL) and MTX (12.5 nM). Error bars represent SD.
represent untreated, ▧ represent MTI alone, ■ represent cytotoxic alone, and ▩ represent combined effect. All data are normalized to untreated baseline (= 1) with a value greater than 1 representing relative cell proliferation and less than 1 inhibition. Each group of 4 bars represents a combination with a different cytotoxic agent. MTIs had at least an additive effect when combined with methotrexate (MTX), dexamethasone (DEX), L-asparaginase (L-ASP), etoposide (VP-16), and doxorubicin (DOX). The combination of MTIs with vincristine (VCR) and Ara-C (cytarabine) did not add a benefit over either single agent alone. Doses depicted in panel A: sirolimus (0.3 ng/mL), MTX (5 nM), DEX (5 μM), L-ASP (1 μg/μL), VP-16 (1 nM), DOX (1 nM), VCR (1 nM), ARA-C (0.1 μg/mL). Next, aliquots of cells from 9 ALL lines were treated with methotrexate and 2 MTIs (temsirolimus (CCI) and sirolimus). (B) MTT data for temsirolimus and methotrexate in 1 cell line (289), demonstrating a synergistic effect at multiple drug doses. The other cell lines tested showed similar results. (C) Chou and Talalay median effects analysis results for one representative cell line (289), showing a combination index (CI) less than 1 at ED50 (median effective dose to inhibit 50% of cells), ED75, and ED90. (D) A representative example of annexin-V and 7-AAD staining in one cell line (289), demonstrating a synergistic increase in cell death and apoptosis with combined treatment. Doses depicted in panel D: CCI (7.5 ng/mL) and MTX (12.5 nM). Error bars represent SD.