Abstract
Glycosylation of the antibody Fc fragment is essential for Fc receptor–mediated activity. Carbohydrate heterogeneity is known to modulate the activity of effector cells in the blood, in which fucosylation particularly affects NK cell–mediated killing. Here, we investigated how the glycosylation profile of 2F8, a human IgG1 monoclonal antibody against epidermal growth factor receptor in clinical development, impacted effector function. Various 2F8 batches differing in fucosylation, galactosylation, and sialylation of the complex-type oligosaccharides in the Fc fragment were investigated. Our results confirmed that low fucose levels enhance mononuclear cell–mediated antibody-mediated cellular cytotoxicity (ADCC). In contrast, polymorphonuclear cells were found to preferentially kill via high-fucosylated antibody. Whole blood ADCC assays, containing both types of effector cells, revealed little differences in tumor cell killing between both batches. Significantly, however, high-fucose antibody induced superior ADCC in blood from granulocyte colony-stimulating factor–primed donors containing higher numbers of activated polymorphonuclear cells. In conclusion, our data demonstrated for the first time that lack of fucose does not generally increase the ADCC activity of therapeutic antibodies and that the impact of Fc glycosylation on ADCC is critically dependent on the recruited effector cell type.
Introduction
Monoclonal antibodies constitute a growing class of therapeutics, with major indications in oncology, infectious diseases, and autoimmunity.1 In oncology, antibody-mediated cellular cytotoxicity (ADCC) is considered a particularly relevant mechanism of action for therapeutic antibodies.2 Evidence for this is mainly derived from studies with the CD20 antibody rituximab, the most intensively investigated antibody in this regard. For example, rituximab lost most of its therapeutic efficacy against xenotransplanted human tumors in mice lacking activating Fc receptors by knockout of the common FcRγ-chain.3 Syngeneic B-cell depletion by murine CD20 antibodies has furthermore been correlated with antibody isotypes and with their respective binding to activating Fcγ receptors, compared with inhibitory Fcγ receptors.4,5 In patients, rituximab's therapeutic efficacy has been correlated with well-defined FcR polymorphisms affecting binding of human IgG and the ability to induce ADCC in vitro.6,7 These and other observations stimulated studies exploring opportunities to improve antibodies' capacity to trigger ADCC.8,9 This can be achieved by increasing antibody binding to activating cellular Fc receptors, such as NK cell–expressed FcγRIIIa, and by decreasing binding to the inhibitory FcγRIIb isoform. At least 2 different methodologies have been established: one modifying the protein structure of the antibody Fc region by mutating the respective cDNAs10,11 and the other based on technologies altering the glycosylation profile of antibodies.12-15
Posttranslational modifications, such as glycosylation, are increasingly recognized as altering the functional activity of biopharmaceuticals.16,17 For antibodies, glycosylation of Asn297 in the CH2 domain has long been recognized as critical for complement activation18 and Fc receptor binding.19,20 More detailed analyses revealed that lack of fucose at this glycosylation site selectively improved binding to FcγRIIIa, whereas binding to a number of other Fcγ receptors appeared to be unaffected by this modification.12-14 Crystallographic studies demonstrated that sugar moieties on antibody Fc formed only minor interactions with the amino acids of FcγRIIIa,21 suggesting that the sugars act indirectly by conferring subtle conformational alterations in a limited region of Fc and possibly by decreasing the mobility of the CH2 domain.22,23 Further functional analyses revealed enhanced ADCC by isolated mononuclear effector cells for low-fucosylated compared with high-fucosylated antibodies.12-14,24 More recent studies suggested that, in addition to Fc fucosylation, sialylation affected FcR binding and antibody function.25,26 Antibody galactosylation, on the other hand, was demonstrated to impact complement activation via the lectin pathway27 but not Fc receptor–mediated functions.28 However, to the best of our knowledge, none of the previous studies addressed the impact of antibody glycosylation on polymorphonuclear cell (PMN) function. PMNs constitute the first line of defense against invading bacteria29 and may significantly contribute to tumor rejection,30 at least for some antibodies, such as those against the epidermal growth factor receptor (EGF-R).31,32
EGF-R is a tyrosine kinase receptor, which is expressed on common solid cancers, such as colon, lung, head, and neck, as well as select nonepithelial tumors such as glioblastomas.33 Because activation of this receptor is associated with accelerated tumor cell proliferation and progression to a more malignant tumor phenotype, EGF-R constitutes an attractive molecule for targeted therapies. Consequently, several EGF-R–directed monoclonal antibodies have been developed for clinical applications, 2 of which have obtained FDA approval.34 In this manuscript, we describe effector functions of glycosylation variants of a fully human IgG1 EGF-R antibody.35 These variants bound comparably to EGF-R, inhibited EGF-R phosphorylation, and efficiently blocked proliferation of EGF-R–expressing tumor cells. Only fucosylation and not sialylation or galacotosylation was found to impact ADCC. As expected, low-fucosylated variants were more efficient in binding to FcγRIIIa and in the recruitment of mononuclear cells (MNCs) as effector cells for ADCC. Notably, however, high-fucosylated batches were more effective in triggering PMN, which mediated tumor cell lysis via FcγRII and significantly contributed to the whole blood ADCC activity of these antibodies.
Methods
Experiments reported here were approved by the Ethical Committee of the Christian Albrechts University, Kiel, Germany, in accordance with the Declaration of Helsinki. Blood donors were randomly selected from healthy volunteers, or from granulocyte colony-stimulating factor (G-CSF)–primed hematopoietic stem cell donors, who gave written informed consent before analyses.
Culture of eukaryotic cells
A431 and HEK-293 cells (ATCC, Manassas, VA) were cultured in RPMI 1640- or Dulbecco modified Eagle medium–Glutamax-I medium (both Invitrogen, Carlsbad, CA), respectively, both containing 10% fetal calf serum, penicillin (100 U/mL), and streptomycin (100 μg/mL).
Production and analysis of antibodies
Human IgG1κ monoclonal antibody (mAb) 2F8 against EGF-R was generated by immunizing HumAb mice (Medarex, Milpitas, CA)36 alternately with A431 cells and purified EGF-R (Sigma-Aldrich, St Louis, MO). mAb 2F8 was selected for its potency to block the interaction between EGF-R and its ligands, EGF and TGF-α. mAb 2F8-H was produced by the original 2F8 monoclonal hybridoma cells (derived from SP2/0). mAb 2F8-C was produced in a transfectoma cell line (derived from CHO-DG44 cells). Culture supernatants of both cell lines were purified using protein A affinity chromatography, followed by size exclusion chromatography on an HR200 column (Pfizer, New York, NY), and were formulated in phosphate-buffered saline (PBS) containing Tween 80 and mannitol. 2F8 Fab fragments were made by papain digestion. Human IgG1κ, specific for keyhole limpet hemocyanin, developed using the same mouse strain, served as isotype control. Purity and monomerity were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), isoelectric focusing, and high-performance size-exclusion chromatography.
Degalactosylated and desialylated 2F8-H and 2F8-C were prepared by treatment with neuraminidase (Acrobacter; Roche Diagnostics, Mannheim, Germany), 1,4 β-galactosidase (Streptococcus pneumoniae; Calbiochem, San Diego, CA), and α-galactosidase (Sigma-Aldrich) for 48 hours at 37°C. Unexpectedly, approximately 30% of the N-glycolylneuraminic acid present on 2F8-H appeared resistant to neuramidase treatment. As a substitute, we used mAb 2F8 produced in HEK-293 cells, which contains comparable levels of fucose as 2F8-H but lacks sialic acids in the N-linked glycan structure. After enzymatic treatment, the material was repurified and formulated as described in “Methods.” Both preparations were analyzed by high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) to confirm removal of galactose and sialic acid. The degalactosylated and desialylated 2F8 produced in CHO cells was designated 2F8-C-(F−/G−/S−), and the 2F8 produced in HEK-293 cells was designated 2F8-HEK-(F+/G−/S−).
Analysis of antibody-attached carbohydrate structures
Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (MS) of released N-linked glycans.
A total of 100 μg IgG was digested with 1 U of N-glycosidase F (Roche Diagnostics) to release the N-linked glycans. In some cases, samples were concomitantly incubated with 3 mU of neuramidase (Acrobacter, Roche Diagnostics) to remove sialic acid residues. Positive ion MALDI-MS was performed without further purification on a Voyager DE Pro mass spectrometer (Applied Biosystems, Foster City, CA) in the reflector mode using 2,5-dihydroxybenzoic acid as a matrix (10 mg/mL in 50:50:0.1 acetonitrile/water/trifluoroacetic acid). Peak assignment to carbohydrate structures was done using GlycoMod software (www.expasy.ch/tools/glycomod).
N-Linked glycosylation profiling by HPAEC-PAD.
For HPAEC-PAD analyses, mAb 2F8 was incubated with N-glycosidase F as described in the previous paragraph. In case of β-galactosidase treatment, 1 mU of β-galactosidase (Streptococcus pneumoniae; Calbiochem) was added during deglycosylation. Samples were centrifuged at 13 000g for 10 seconds before analysis by HPAEC-PAD. HPAEC-PAD analysis was performed on a 2-mm internal diameter CarboPac PA1 column (Dionex, Sunnyvale, CA) with CarboPac PA100 guard, using a linear elution gradient of 0 to 175 mM of sodium acetate in 150 mM of sodium hydroxide at a flow rate of 1 mL/min. PAD occurred with the quadropulse, and peaks were integrated with Chromeleon Software (Dionex). For each profile, 70 μg digest was loaded.
Cloning, expression, and purification of soluble Fc receptors
Plasmid DNA from RZPD (German Resource Center for Genome Research, Berlin, Germany) clones containing cDNA encoding for FcγRI (P12314), FcγRIIa-R131 allotype (P12318), and FcγRIIIa-V158 allotype (P08637) were used as polymerase chain reaction (PCR) templates. For FcγRIIb (P31994), a codon-optimized synthetic construct was synthesized at GeneArt (Regensburg, Germany) and used as PCR template. Specific primers were used that generated fragments encoding each extracellular domain, introduced suitable restriction sites for cloning in the mammalian expression vector pEE13.4 (Lonza Nottingham, Nottingham, United Kingdom), an ideal Kozak sequence and a C-terminal His6 tag. PCR fragments were cloned in pEE13.4, and the correct sequences of positive clones were confirmed by DNA sequencing. The extracellular domain coding sequences of FcγRIIIb-NA1 (AAA35881) and FcγRIIIb-NA2 (O75015) were synthesized at GeneArt, introducing suitable restriction sites, ideal Kozak sequences, and C-terminal His6 tag coding sequences and cloned in pEE13.4.
The FcγRIIIa-F158 and FcγRIIa-H131 allotype sequences were generated by PCR-based site-directed mutagenesis of the FcγRIIIa-V158 or FcγRIIa-R131 cDNA, respectively, using the QuickChange II XL Kit (Stratagene, Heidelberg, Germany). For all FcγR, transmembrane and intracellular domains were replaced by DNA encoding a His6 tag. Consequently, the expected proteins comprised the extracellular domains of the FcγR at their COOH termini and 6× His at amino acid positions as follows: FcγRI: His292; FcγRIIa: Gly217; FcγRIIb: Gly223; FcγRIIIa: Ser200; FcγRIIIb: Ile199 (residue numbers include the signal peptide). Plasmid DNA was transiently transfected in HEK293F cells using 293fectin (both Invitrogen). Proteins were purified from culture supernatant by BD Talon chromatography (BD Biosciences, Palo Alto, CA), and their appropriate molecular weights confirmed by SDS-PAGE.
Measurement of Fc/Fc receptor interactions
Enzyme-linked immunosorbent assay (ELISA) plates were coated overnight with 5 μg/mL mAb 2F8 diluted in PBS. mAb 2F8-C Fab fragments were included as negative control. Next, the plates were washed with PBS, 0.05% (vol/vol) Tween-20 (PBST), incubated with 3-fold serial dilutions of FcγR protein diluted in PBST, 2% (wt/vol) bovine serum albumin (assay buffer) and incubated for 1 hour at room temperature. Plates were then washed again, replenished with ice-cold biotin-conjugated mouse antipoly histidine antibodies (clone AD1.1.10; R&D Systems, Minneapolis, MN), and incubated for 1 hour at 4°C. Subsequently, binding of FcγR was visualized using peroxidase-conjugated streptavidin and 2, 2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) substrate. Absorbance at 405 nm was measured on an EL808 ELISA plate reader (Bio-Tek Instruments, Winooski, VT). Half-maximal effective concentration (EC50) values were calculated with GraphPad Prism 4.03 software using 4-parameter logistic curve fitting. Affinity differences of FcγR for 2F8-H and 2F8-C were expressed as the ratio [EC50(2F8-H)]/[EC50(2F8-C)]. Because of the low affinity interactions between antibodies and some Fcγ receptors, EC50 calculations were not possible when binding curves did not reach upper plateaus.
Inhibition of cell proliferation
Effects on tumor cell growth were evaluated by using AlamarBlue for measuring vital cell mass. mAb 2F8 dilutions were added to A431 cell cultures in 96-well flat-bottom tissue culture plates (500 cells/well). Plates were incubated at 37°C for 5 days, before AlamarBlue solution (BioSource International, Camarillo, CA) was added. Plates were incubated for another 4 hours, transferred to room temperature, and fluorescence of reduced AlamarBlue was measured by exciting at 528 nm and measuring emission at 590 nm on Synergy HT plate reader (Bio-Tek Instruments).
Inhibition of EGF-R phosphorylation
Inhibition of EGF-induced EGF-R phosphorylation was measured using a 2-step assay. Briefly, A431 cells were cultured overnight in serum-deprived medium. Cells were then incubated with serial dilutions of mAb 2F8 at 37°C. After 60 minutes, 50 ng/mL recombinant human EGF (BioSource) was added for an additional 30 minutes. Subsequently, cells were solubilized with lysis buffer (Cell Signaling Technology, Danvers, MA), lysates were transferred to ELISA plates coated with 1 μg/mL of mouse anti–EGF-R antibodies (mAb EGFR1; BD Biosciences PharMingen, San Diego, CA), and incubated for 2 hours at room temperature. Next, the plates were washed and binding of phosphorylated EGF-R was visualized using a europium-labeled mouse mAb, specific for phosphorylated tyrosines (mAb P-Tyr-100; PerkinElmer Life and Analytical Sciences, Waltham, MA). Finally, dissociation-enhanced fluoroimmunoassay enhancement solution was added, and time-resolved fluorescence was measured by exciting at 315 nm and measuring emission at 615 nm on an EnVision plate reader (PerkinElmer Life and Analytical Sciences).
Flow cytometric analyses
For indirect immunofluorescence, cells were incubated with antibodies 2F8-C, 2F8-H, or keyhole limpet hemocyanin at various concentrations in PBS supplemented with 1% bovine serum albumin (Sigma-Aldrich) and 0.1% sodium-azide (PBA buffer) for 30 minutes on ice. After washing, cells were stained with fluorescein isothiocyanate-labeled F(ab′)2 fragments of polyclonal goat antimouse antibodies (Dako Denmark, Glostrup, Denmark). Cells were then analyzed on a flow cytometer (Epics Profile; Beckman Coulter, Fullerton, CA).
Isolation of mononuclear and neutrophil effector cells
Peripheral blood from healthy volunteers or from hematopoietic stem cell donors was layered over a discontinuous Percoll (Biochrom, Berlin, Germany) gradient consisting of 70% and 62% Percoll. After centrifugation, neutrophils were collected at the interface between the 2 Percoll layers, and mononuclear cells from the serum/Percoll interface. Remaining erythrocytes were removed by hypotonic lysis. Purity of neutrophils was determined by cytospin preparations and exceeded 95%. MNCs typically contained approximately 60% CD3-positive T cells, 20% CD56-positive NK cells, and 10% CD14-expressing monocytes, as determined by immunofluorescence staining. Viability of cells tested by Trypan blue exclusion was higher than 95%.
ADCC assays
ADCC assays against 51Cr-labeled target cells were performed as described.37 Whole blood (50 μL), plasma, or isolated effector cells and sensitizing antibodies at various concentrations were added to microtiter plates (Nunc, Neerijse, Belgium). Assays were started by adding effector and target cells at a (E:T) ratio of 80:1, unless otherwise indicated. Isolated PMNs were stimulated by granulocyte-macrophage colony-stimulating factor (50 U/mL). After 3 hours at 37°C, 51Cr-release from triplicates was measured in counts per minute. Percentage of cellular cytotoxicity was calculated using the formula “% specific lysis = (exp cpm − basal cpm)/(maximal cpm) × 100,” with maximal 51Cr release determined by adding perchloric acid (3% final concentration), and basal release measured in the absence of sensitizing antibodies and effector cells. Antibody-independent cytotoxicity (effectors without target antibodies) was observed in whole blood assays, and with mononuclear effector cells, but not with PMNs. For analyses of Fc receptor involvement, F(ab′)2 fragments of blocking antibodies AT10 (FcγRII, CD32), 3G8 (FcγRIII, CD16), or Ox55 (control, rat CD2) were added at 10 μg/mL before adding target cells. All 3 F(ab′)2 fragments were kindly provided by Prof Martin Glennie (Tenovus, Southampton, United Kingdom).
Results
Antibody production, characterization, and glycosylation analyses
To analyze the impact of antibody Fc glycosylation on PMN- and MNC-mediated ADCC, we compared glycosylation variants of a fully human EGF-R antibody of the IgG1 isotype. Two batches of this mAb were produced by hybridoma cells, designated 2F8-H, or by CHO-DG44 cells, designated 2F8-C. For both the hybridoma and the CHO-produced material, we analyzed the protein and the oligosaccharide portions. There were no significant differences observed in SDS-PAGE and high-performance size-exclusion chromatography analyses. Both antibody preparations had the same amino acid sequence and consisted of more than 99% pure, monomeric IgG (data not shown). However, their N-linked oligosaccharide structures, analyzed by HPAEC-PAD and MALDI-MS, showed some major differences (Tables 1 and S1 and Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The analysis of 2F8-C carbohydrate indicated that approximately 25% of the complex-type N-linked glycans were not core-fucosylated on the reducing N-acetylglucosamine (peaks 2, 4, 6, and 7). In contrast, for 2F8 produced in hybridoma cells, non–core-fucosylated glycans (ie, peaks 2 and 4) were not detected, demonstrating that 2F8-H was fully fucosylated.
In addition, both batches showed differences in sialylation and galactosylation. 2F8-C mainly contained nonsialylated complex-type glycans, with limited galactose. Approximately 46% of the glycans did not contain galactose, approximately 35% contained only one galactose, and only 6% were fully galactosylated. We did not observe the presence of α-galactosyl or N-glycolylneuraminic acid (NeuGc) on 2F8-C. Hybridoma-derived 2F8-H contained significantly more sialylated glycans (ie, peaks eluting between 37-43 and 55-58 minutes) compared with CHO-derived mAb 2F8-C. Characterization of these charged peaks revealed that they were mainly core-fucosylated complex-type glycans with one (structures between 37 and 43 minutes) or 2 N-glycolylneuraminic acids (structures between 55-58 minutes). Taken together, although the difference in the fucose content was most prominent, there were also differences in other monosaccharides, such as galactose and sialic acid.
Antigen binding and Fab-mediated direct effector functions
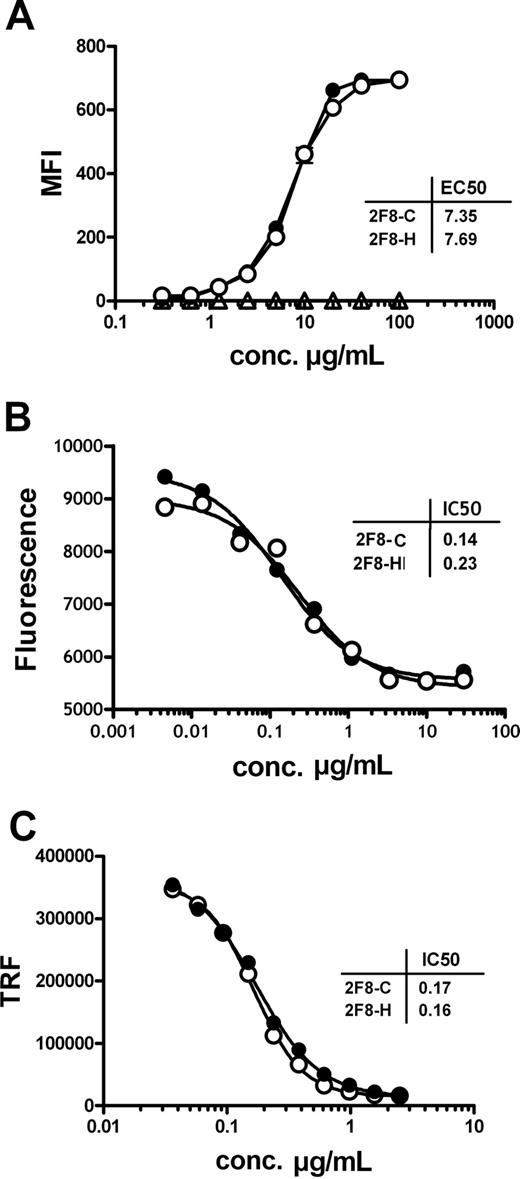
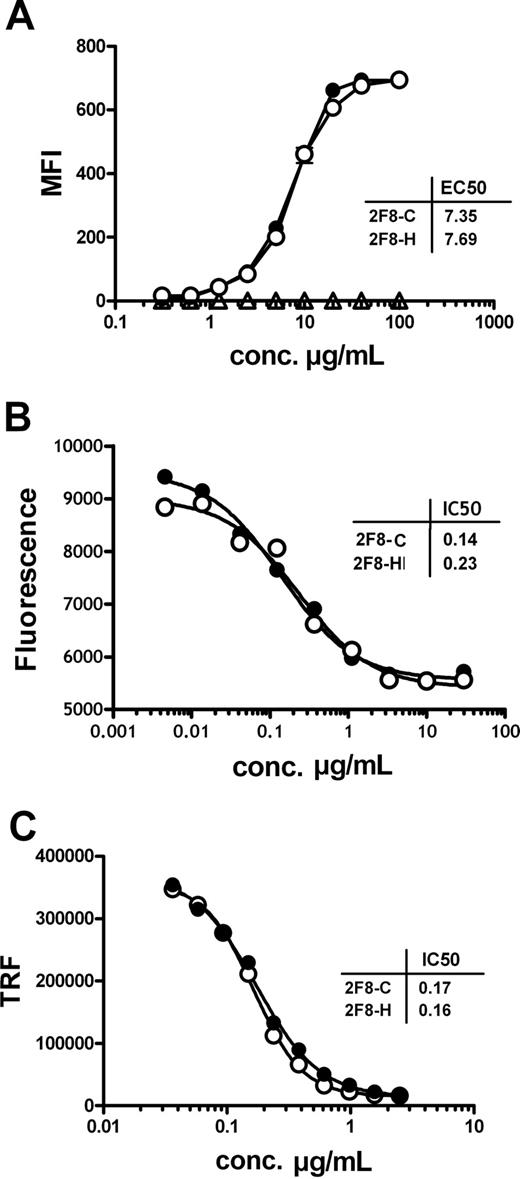
In a first set of functional experiments, the antigen binding characteristics of the 2 antibody preparations were investigated. 2F8-C was compared with its highly fucosylated counterpart 2F8-H. Binding to purified EGF-R and to native EGF-R on the cell surface of A431 cells was analyzed by ELISA and indirect immunofluorescence staining, respectively. Both antibodies bound specifically and with similar EC50 to purified EGF-R (data not shown) and to cell-surface expressed EGF-R (Figure 1A), demonstrating that the differences in glycosylation did not influence antigen binding characteristics of the 2 antibody preparations.
The glycosylation profile of 2F8 does not influence antigen binding and Fab-mediated inhibition of EGF-R signaling.(A) Binding of 2F8-C (●) and 2F8-H (○), or a human IgG1 control antibody (Δ), to EGF-R on A431 cells was analyzed by flow cytometry. A431 cells were incubated with various concentrations (0.016-100 μg/mL) of 2F8 and stained with polyclonal fluorescein isothiocyanate-conjugated rabbit anti–human IgG serum. Each data point represents the mean plus or minus SEM of triplicates (error bars are smaller than symbols and therefore not visible in the graph). The capacity of 2F8-C (●) and 2F8-H (○) to inhibit tumor cell growth (B) or EGF-induced EGF-R phosphorylation (C) was evaluated. (B) A431 cells were seeded in the presence of various concentrations of antibody (0.002-30 μg/mL). After 5 days, vital cell mass was analyzed by measuring fluorescence of reduced AlamarBlue. Each data point represents the mean of duplicate wells. (C) A431 cells were seeded in the presence of various concentrations of antibodies (0.04-2.5 μg/mL) and subsequently stimulated with 50 ng/mL EGF. EGF-R phosphorylation was measured in cell lysates of treated cells using a phospho-EGF-R–specific ELISA and time-resolved fluorescence (TRF). Each data point represents the mean plus or minus SEM of triplicates (error bars are smaller than symbols). Every experiment was performed at least 2 times.
The glycosylation profile of 2F8 does not influence antigen binding and Fab-mediated inhibition of EGF-R signaling.(A) Binding of 2F8-C (●) and 2F8-H (○), or a human IgG1 control antibody (Δ), to EGF-R on A431 cells was analyzed by flow cytometry. A431 cells were incubated with various concentrations (0.016-100 μg/mL) of 2F8 and stained with polyclonal fluorescein isothiocyanate-conjugated rabbit anti–human IgG serum. Each data point represents the mean plus or minus SEM of triplicates (error bars are smaller than symbols and therefore not visible in the graph). The capacity of 2F8-C (●) and 2F8-H (○) to inhibit tumor cell growth (B) or EGF-induced EGF-R phosphorylation (C) was evaluated. (B) A431 cells were seeded in the presence of various concentrations of antibody (0.002-30 μg/mL). After 5 days, vital cell mass was analyzed by measuring fluorescence of reduced AlamarBlue. Each data point represents the mean of duplicate wells. (C) A431 cells were seeded in the presence of various concentrations of antibodies (0.04-2.5 μg/mL) and subsequently stimulated with 50 ng/mL EGF. EGF-R phosphorylation was measured in cell lysates of treated cells using a phospho-EGF-R–specific ELISA and time-resolved fluorescence (TRF). Each data point represents the mean plus or minus SEM of triplicates (error bars are smaller than symbols). Every experiment was performed at least 2 times.
To address whether also biologic activity mediated by the antigen-binding Fab domains were unaffected, growth inhibition of EGF-R-expressing tumor cells by 2F8-C and 2F8-H was analyzed. A431 cells, which express high levels of EGF-R, were incubated at increasing concentrations of 2F8-C or 2F8-H, and tumor cell proliferation was measured. Both antibody preparations efficiently inhibited tumor cell growth in vitro at a half-maximal inhibitory (IC50) concentration of 0.19 μg/mL (95% confidence interval [CI], 0.10-0.40 μg/mL). No significant differences were observed between 2F8-C and 2F8-H (Figure 1B). Further, inhibition of ligand-induced EGF-R phosphorylation was analyzed. A431 cells were incubated with serial dilutions of 2F8-C or 2F8-H and subsequently stimulated with recombinant EGF. Both 2F8-C and 2F8-H inhibited ligand-induced EGF-R phosphorylation similarly, at an IC50 concentration of 0.17 μg/mL (95% CI, 0.16-0.19 μg/mL; Figure 1C). Both 2F8-C and 2F8-H, therefore, showed no significant differences in their ability to modulate EGF-R signaling, which demonstrated that differences in Fc fragment glycosylation did not impact the function of the antigen combining site.
Fc-mediated effector functions
It is well established that the Fc glycosylation profile of antibodies may affect Fc-mediated effector functions, such as complement-dependent cytotoxicity38 and ADCC.12-14 However, in complement-dependent cytotoxicity assays with A431 tumor cells as targets and human plasma as source of complement, none of the tested antibody preparations triggered significant complement-mediated lysis (not shown). This is probably attributable to the high expression levels of membrane-bound complement regulators (eg, CD55 and CD59) on most solid tumor cells.
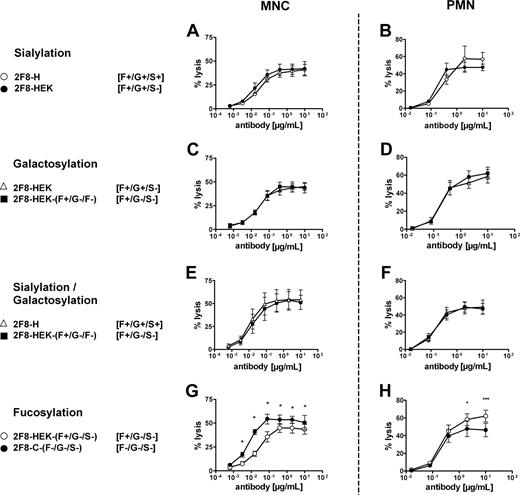
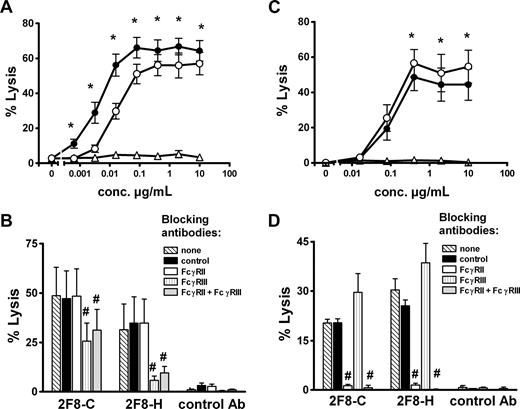
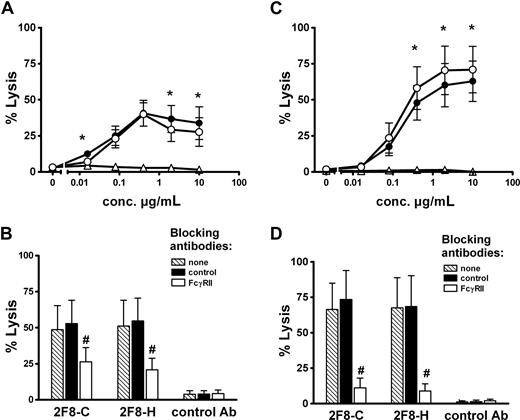
To investigate whether the observed glycosylation differences resulted in altered Fc-mediated biologic effector functions, the 2 glycosylation variants of 2F8 were compared for their ability to trigger ADCC (Figure 2). We first analyzed MNCs, in which NK cells primarily serve as effector cells. Low-fucosylated 2F8-C induced superior ADCC compared with highly fucosylated 2F8-H (Figure 2A). Half-maximal lysis was reached at approximately 4-fold lower antibody concentration with 2F8-C compared with 2F8-H (EC50; 0.004 μg/mL [95% CI, 0.002-0.008] vs 0.016 μg/mL [95% CI, 0.009-0.028]). To address the question of which Fc receptors were engaged in MNC-mediated killing by the 2 2F8 batches, ADCC experiments in the presence of specific Fc receptor blocking antibodies were performed. For these experiments, F(ab′)2fragments of mAb 3G8 (blocking FcγRIII, CD16), AT10 (blocking FcγRII, CD32), or Ox55 (nonbinding control) were used. Killing by isolated MNCs was efficiently blocked by 3G8 F(ab′)2 fragments, demonstrating the involvement of FcγRIIIa on NK cells (Figure 2B). Killing mediated by 2F8-C was inhibited to a lesser extent than lysis by 2F8-H, probably reflecting the higher affinity of 2F8-C for CD16. In contrast, no inhibition of MNC-mediated killing was observed with AT10 F(ab′)2 fragments, demonstrating that FcγRII (CD32) was not involved in 2F8-mediated tumor cell lysis by MNCs (Figure 2B).
MNC-mediated lysis of A431 cells is enhanced by low fucosylated 2F8-C, whereas PMN-mediated lysis is diminished. To analyze Fc-mediated effector functions of 2F8-C and 2F8-H antibodies, both antibody preparations were compared in their capacity to trigger ADCC of A431 cells with isolated (A) MNCs or (C) PMNs as effector cells and variation of antibody concentrations at a fixed E/T ratio (80:1). To define Fc receptors involved in (B) MNC- or (D) PMN-mediated target cell killing, ADCC assays were performed in the presence of F(ab′)2 fragments of Fcγ receptor blocking antibodies AT10 (FcγRII), or 3G8 (FcγRIII), or control F(ab′)2 fragments (all at 10 μg/mL). 2F8-C (●), 2F8-H (○), human IgG1 control antibody (Δ). Data from experiments with 6 (A,C) or 3 (B,D) different donors, respectively, are presented as means plus or minus SEM. *Significant differences in killing between 2F8-C and 2F8-H. #Significant blockade compared with control treated samples (P < .05).
MNC-mediated lysis of A431 cells is enhanced by low fucosylated 2F8-C, whereas PMN-mediated lysis is diminished. To analyze Fc-mediated effector functions of 2F8-C and 2F8-H antibodies, both antibody preparations were compared in their capacity to trigger ADCC of A431 cells with isolated (A) MNCs or (C) PMNs as effector cells and variation of antibody concentrations at a fixed E/T ratio (80:1). To define Fc receptors involved in (B) MNC- or (D) PMN-mediated target cell killing, ADCC assays were performed in the presence of F(ab′)2 fragments of Fcγ receptor blocking antibodies AT10 (FcγRII), or 3G8 (FcγRIII), or control F(ab′)2 fragments (all at 10 μg/mL). 2F8-C (●), 2F8-H (○), human IgG1 control antibody (Δ). Data from experiments with 6 (A,C) or 3 (B,D) different donors, respectively, are presented as means plus or minus SEM. *Significant differences in killing between 2F8-C and 2F8-H. #Significant blockade compared with control treated samples (P < .05).
Next, we performed ADCC with PMN effector cells (Figure 2C). Notably, both 2F8-C and 2F8-H antibodies mediated efficient and significant ADCC by PMNs. Compared with MNCs, which triggered maximum lysis at low antibody concentrations, higher antibody concentrations were required for efficient ADCC by PMNs. Interestingly, high-fucosylated 2F8-H antibody demonstrated enhanced maximum lysis compared with its low-fucosylated counterpart, but no significant differences in the EC50 values of 2F8-C and 2F8-H were observed. Thus, also PMN-mediated ADCC was affected by the glycosylation profiles of the antibody preparations (Figure 2C). In contrast to MNC-mediated lysis, PMN-mediated killing was completely blocked by AT10 F(ab′)2 fragments against FcγRII (CD32), demonstrating that FcγRII engagement was necessary for efficient tumor cell lysis by PMNs. Interestingly, PMN-mediated killing was not inhibited but rather stimulated by 3G8 F(ab′)2 fragments against FcγRIII (CD16; Figure 2D). These results with blocking antibodies and PMN effector cells were independent of the glycosylation status of the targeting antibodies.
Impact of sialic acid, galactose, and fucose removal
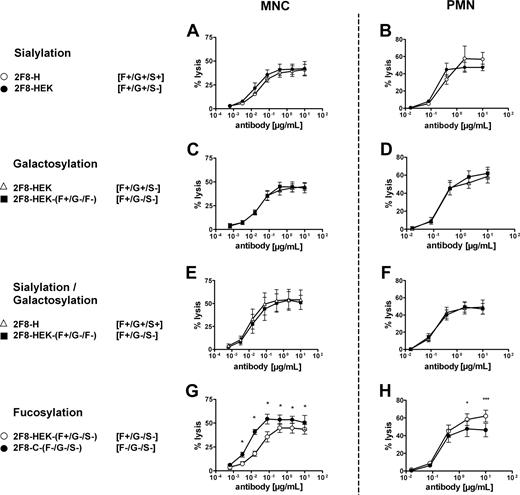
Although the difference in fucose content between the 2 antibody batches was most prominent, we also observed differences in other monosaccharides, such as galactose and sialic acid, as discussed in “Antibody production, characterization, and glycosylation analyses” (Table 1; Figure S1A). To verify whether the observed differences in effector cell–mediated killing were primarily caused by differences in the fucose content or by differences in galactosylation and sialylation, we prepared enzymatically degalactosylated and desialylated batches. Unexpectedly, approximately 30% of the N-glycolylneuraminic acid present on 2F8-H appeared resistant to neuramidase treatment. As a substitute, we generated mAb 2F8 in HEK-293 cells. 2F8 material produced in HEK-293 cells contained comparable levels of fucose as 2F8-H but lacked sialic acids in the N-linked glycan structure. HPAEC-PAD confirmed that the obtained exoglycosidase-treated batches lacked galactose and sialic acid (Table 1; Figure S1B). The exoglycosidase-treated material of 2F8 produced in CHO cells was designated 2F8-C-(F−/G−/S−) and 2F8 produced in HEK-293 cells was designated 2F8-HEK-(F+/G−/S−). For the 2F8-C and 2F8-H, we confirmed that also the HEK-produced batches were unaltered in EGF-R binding and inhibition of signal induction (not shown).
These control batches were then used in ADCC experiments to analyze the impact of sialylation, galactosylation, and fucosylation on MNC- and PMN-mediated ADCC. Importantly, removal of sialic acid, galactose, or both sialic acid and galactose demonstrated no influence on the efficiency of human MNC- or PMN-mediated killing (Figure 3A-F). Furthermore, these experiments confirmed that only the lack of fucose (Figure 3G), but not sialic acid (Figure 3A) or galactose (Figure 3C), was responsible for enhanced ADCC activity by MNC effector cells, as also published by others.12,14 However, lack of fucose from the carbohydrate structure negatively affected PMN-mediated killing (Figure 3H). To our knowledge, this is the first report that reduced levels of fucose in antibody Fc may adversely affect antibody-mediated effector functions.
Removal of sialic acid, galactose, or both was compared with the impact of fucose levels on MNC- or PMN-mediated ADCC by 2F8. To analyze the contribution of sialic acid, galactose and fucose levels to MNC- or PMN-mediated tumor cell killing, ADCC experiments with nonsialylated and degalactosylated in comparison to mock-treated 2F8 batches were performed. (A,B) Contribution of sialic acid. (C,D) Contribution of galactose. (E,F) Contribution of sialic acid and galactose (in combination). (G,H) Contribution of fucose. ADCC experiments were performed as described in “ADCC assays.” Compared batches are indicated, with glycosylation status in brackets. Data from experiments with at least 3 different donors are presented as means plus or minus SEM. *Significant differences in killing between batches (P < .05).
Removal of sialic acid, galactose, or both was compared with the impact of fucose levels on MNC- or PMN-mediated ADCC by 2F8. To analyze the contribution of sialic acid, galactose and fucose levels to MNC- or PMN-mediated tumor cell killing, ADCC experiments with nonsialylated and degalactosylated in comparison to mock-treated 2F8 batches were performed. (A,B) Contribution of sialic acid. (C,D) Contribution of galactose. (E,F) Contribution of sialic acid and galactose (in combination). (G,H) Contribution of fucose. ADCC experiments were performed as described in “ADCC assays.” Compared batches are indicated, with glycosylation status in brackets. Data from experiments with at least 3 different donors are presented as means plus or minus SEM. *Significant differences in killing between batches (P < .05).
Fc receptor binding of glycosylation variants
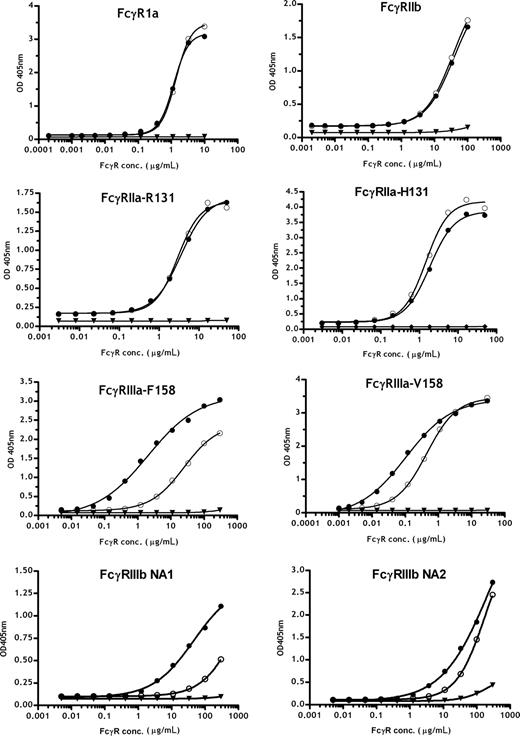
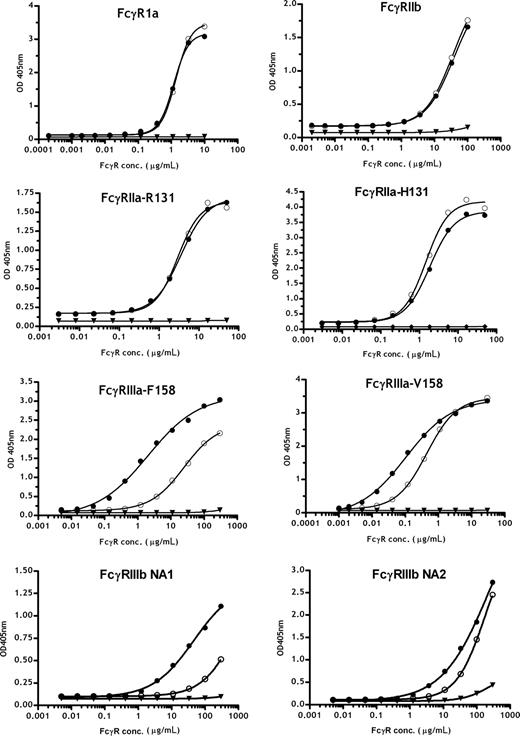
To further analyze the Fc receptor interactions of the 2F8 glycosylation variants in detail, extracellular domains of the human FcγRIa, FcγRIIa, FcγRIIb, FcγRIIIa, and the FcγRIIIb receptors were produced as soluble recombinant proteins. For FcγRIIa, FcγRIIIa, and FcγRIIIb, both common allotypes, FcγRIIa-H131 and -R131, FcγRIIIa-V158 and -F158, FcγRIIIb-NA1 and -NA2, were investigated. To analyze the interactions of the recombinant receptors with differently glycosylated 2F8 variants, antibodies were immobilized on ELISA plates, and increasing concentrations of the different Fcγ-receptor preparations were added. Low affinity interactions of antibodies and Fc receptors led to some assay-to-assay variation (especially for FcγRIIIb), resulting in differences in measured absorbance maxima. Nevertheless, direct binding comparisons of different 2F8 batches to the tested FcγR were possible and provided relative affinity differences of the glycosylation variants for FcR. Thus, low-fucosylated 2F8-C demonstrated a 5-fold increased affinity for FcγRIIIa-V158 and approximately 15-fold enhanced binding to FcγRIIIa-F158 compared with the high-fucosylated 2F8-H. Therefore, the major affinity difference of fully fucosylated 2F8-H for FcγRIIIa-V158 versus -F158 was minimized for low-fucosylated 2F8-C, consistent with previous findings from others.14,39 In addition, binding of low-fucose antibodies to FcγRIIIb was enhanced; more pronounced differences were observed for the FcγRIIIb-NA1 allotype. Interestingly, we observed an approximately 2-fold higher affinity of human IgG1 for FcγRIIa-H131 compared with -R131, which was not influenced by fucosylation levels. Binding to FcγRIIa, FcγRIa, and FcγRIIb was not significantly affected by altering the glycosylation profile of the antibodies (Figure 4). Binding of exoglycosidase-treated batches to FcγRIa, FcγRIIIa (V/F158), FcγRIIa (R/H131), and FcγRIIb in ELISA confirmed that fucose content modulated the affinity to FcγRIIIa (V/F158) but did not affect binding affinity to FcγRIa, FcγRIIa (R/H131), and FcγRIIb. Removal of galactose and sialic acid did not impact the binding of 2F8 to FcγRIA, FcγRIIa (R/H131), and FcγRIIb but slightly decreased the affinity to FcγRIIIa (V/F158; Figure S2). In conclusion, these data underline that MNC-mediated killing was primarily attributable to enhanced FcγRIIIa binding because of the lack of fucose. Because no differences in binding to FcγRIIa were observed, the mechanism of superior PMN killing was not attributable to differences in binding affinity to this receptor. The superior PMN killing by high-fucosylated antibodies may therefore correlate with the lower affinity of high-fucose antibodies to FcγRIIIb. This is in accordance with CD16 blocking experiments demonstrating that blocking FcγRIIIb enhances PMN-mediated ADCC. Together, these findings suggest that high affinity binding to FcγRIIIb partially inhibits PMN-mediated killing (Figure 2D).
Analyses of Fc/Fc receptor interactions using recombinantly expressed extracellular domains of different Fcγ receptors. Binding profiles of 2F8-C (●) and 2F8-H (○) to various Fcγ receptors were measured by ELISA. 2F8-C F(ab′)2 fragments (▼) are shown as a control. The mean values are presented. One representative experiment of 3 is shown.
Analyses of Fc/Fc receptor interactions using recombinantly expressed extracellular domains of different Fcγ receptors. Binding profiles of 2F8-C (●) and 2F8-H (○) to various Fcγ receptors were measured by ELISA. 2F8-C F(ab′)2 fragments (▼) are shown as a control. The mean values are presented. One representative experiment of 3 is shown.
Effector cell recruitment in whole blood ADCC assays
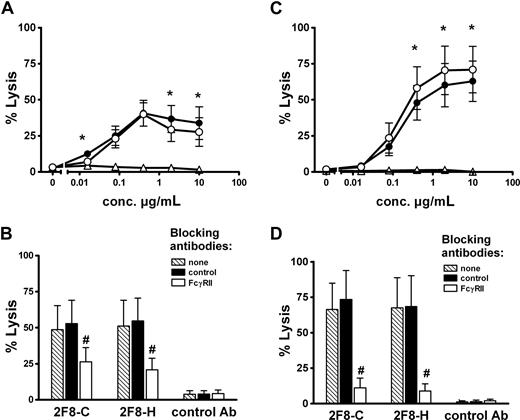
In a further set of experiments, the glycosylation variants 2F8-H and 2F8-C were tested in human whole blood to induce ADCC with a physiologic mixture of effector cells. Interestingly, only small differences in lysis were observed between the 2 antibody variants (Figure 5A). These differences were less pronounced than with isolated MNCs or PMNs (Figure 2A,C). Because the Fc receptor involvement in MNC- and PMN-mediated tumor cell killing was different (MNC via CD16, PMN via CD32), we aimed to investigate the contribution of both effector cell populations to whole blood ADCC. However, because in ADCC assays with isolated effector cells, F(ab′)2 fragments against FcγRIII stimulated PMN-mediated killing (Figure 2D), we could not use this antibody in our whole blood ADCC assay. We therefore performed experiments with AT10 F(ab′)2 fragments to selectively block PMN-mediated killing. In these experiments, blockade of CD32 resulted in partial inhibition of tumor cell lysis. Control F(ab′)2 fragments did not affect whole blood ADCC (Figure 5B). Together, these results clearly demonstrated that PMNs contributed to tumor cell killing in whole blood. To further address the contribution of PMN in 2F8-mediated whole blood killing, we analyzed blood from G-CSF–primed donors. As shown in Figure 5C, ADCC activity in G-CSF–primed blood was significantly enhanced compared with healthy donor blood (Figure 5A). Blocking experiments revealed that killing in G-CSF–primed blood was more efficiently blocked by AT10 F(ab′)2 fragments than was killing in healthy donor blood, indicating the PMN contribution to tumor cell lysis to be enhanced during G-CSF treatment. Notably, high-fucosylated 2F8-H was significantly more effective in blood from G-CSF–treated patients than low-fucosylated 2F8-C.
Impact of glycosylation status on whole blood ADCC and contribution of PMNs. Tumor cell lysis at various antibody concentrations was analyzed with whole blood from healthy (A) or G-CSF–primed donors (C) as a physiologic combination of different effector cell populations. A431 cells served as target cells. To analyze PMN involvement in target cell killing in whole blood, ADCC assays were performed in the presence of F(ab′)2 fragments of FcγRII blocking antibody AT10, or control F(ab′)2-fragments (both at10 μg/mL). 2F8 antibody concentrations were0.4 μg/mL. Blocking experiments with blood from healthy donors (B), or from G-CSF–primed donors (D). 2F8-C (●), 2F8-H (○), or human IgG1 control antibody (Δ). Data from experiments with 5 (healthy donors) and 3 (G-CSF–primed donors) different volunteers, respectively, are presented as means plus or minus SEM. *Significant difference in killing between 2F8-C and 2F8-H. #Significant blockade compared with control treated samples (P < .05).
Impact of glycosylation status on whole blood ADCC and contribution of PMNs. Tumor cell lysis at various antibody concentrations was analyzed with whole blood from healthy (A) or G-CSF–primed donors (C) as a physiologic combination of different effector cell populations. A431 cells served as target cells. To analyze PMN involvement in target cell killing in whole blood, ADCC assays were performed in the presence of F(ab′)2 fragments of FcγRII blocking antibody AT10, or control F(ab′)2-fragments (both at10 μg/mL). 2F8 antibody concentrations were0.4 μg/mL. Blocking experiments with blood from healthy donors (B), or from G-CSF–primed donors (D). 2F8-C (●), 2F8-H (○), or human IgG1 control antibody (Δ). Data from experiments with 5 (healthy donors) and 3 (G-CSF–primed donors) different volunteers, respectively, are presented as means plus or minus SEM. *Significant difference in killing between 2F8-C and 2F8-H. #Significant blockade compared with control treated samples (P < .05).
Discussion
In this work, we investigated the impact of antibody Fc glycosylation on triggering Fc receptor-mediated tumor cell killing by different human effector cell types. Glycoengineering of therapeutic antibodies currently receives great attention because it is thought to represent a promising approach to improve antibody binding to activating Fcγ receptors, compared with inhibitory Fcγ receptors.14,40 As previously reported for antibodies against other target antigens,12-14,24,40 we observed low-fucosylated batches of a human EGF-R–specific antibody to be more effective in binding to recombinant FcγRIIIa than its high-fucosylated variant, which led to enhanced tumor cell killing in ADCC assays with MNC effector cells. Interestingly, low fucosylated 2F8-C showed smaller affinity differences between FcγRIIIa-V158 and -F158 than fully fucosylated 2F8-H. This observation suggests that the clinically relevant FcγRIIIa polymorphism may be less important for low compared with high fucosylated antibodies, as also proposed by others.14,39 However, lower levels of fucose adversely affected PMN-mediated killing, leading to similar killing levels by both antibody batches in human whole blood assays. Similarly, a fully fucosylated HLA class II antibody was more effective in recruiting PMN than its nonfucosylated variant (data not shown), demonstrating that this observation is not dependent on one particular target antigen. Notably, the impact of antibody fucosylation on PMN-mediated killing has not been reported before and may have implications for therapeutic antibodies that recruit PMNs for their in vivo effects.
In addition to fucosylation, antibody sialylation has been reported to impact on antibody efficacy.25 However, these studies were performed in mice, which express Fc receptors with limited homology, different cellular expression patterns, and different Fc-binding preferences compared with the human system.41 With human effector cells, sialylation did not significantly affect antibody efficacy40,42 (and our results), but other observations have also been reported.26 These contradictory results may be explained, for example, by differences in antibody preparations, selected target antigens, or assay conditions. Antibody galactosylation has been observed to affect complement activation27 but did not impact ADCC40,42 (and our results). Corresponding to our functional data, antibody fucose (Figure 4), but not galactose or sialic acid (Figure S2), content affected binding to human FcR.
The contribution of NK cells to cell-mediated killing mechanisms of monoclonal antibodies is widely acknowledged,43 and is strongly supported by clinical studies investigating the contribution of FcR polymorphisms.6,7,44 Because most NK cells express FcγRIIIa as their only IgG receptor, engineering antibody variants with enhanced FcγRIII binding is an attractive approach to improve antibody efficacy. However, the extracellular domains of human FcγRIIIa are highly homologous to FcγRIIIb, which is an abundantly expressed GPI-linked IgG receptor on PMNs. As expected, MNC-mediated killing was blocked by F(ab′)2 fragments against FcγRIII (CD16). However, PMN-mediated killing was blocked by F(ab′)2 fragments against FcγRII (CD32) but rather stimulated by F(ab′)2 fragments against FcγRIII (CD16). EC50 values of both 2F8 variants to the FcγRIIa-H/R131 alloforms were not significantly different, suggesting that the mechanism of higher PMN-mediated killing by 2F8-H was probably not the result of differences in binding affinity to FcγRIIa. Interestingly, both antibody variants bound with 2-fold higher affinity to the FcγRIIa-H131 than the -R131 allele. This suggests that the correlation of FcγRIIa-H131 expression and clinical responses to rituximab, herceptin, or cetuximab may be correlated to this affinity difference.7,45,46 Previous reports did not find this difference,47 probably because of different assays for the determination of Fc/FcR interactions.
Interestingly, binding to FcγRIIIb was severely affected by Fc-glycosylation, with low-fucose antibodies demonstrating higher affinity to FcγRIIIb receptors. Thus, neutrophils express at least 2 classes of Fcγ receptors, which compete for IgG binding. Therefore, enhancing affinity for the noncytotoxic FcγRIII isoform by lower fucosylation levels may actually impede antibody efficacy with PMN, but a role for other Fc-binding receptors cannot be excluded. Recent findings suggested that PMNs express low levels of FcγRIIb,48,49 an inhibitory Fc receptor that is proposed to be involved in the regulation of cytotoxic responses.43 However, in our FcγRIIb binding studies, no significant differences were observed between the low- and high-fucosylated antibody batches. A potential contribution of human PMNs to antibody efficacy is less established,30 but previous work indicated PMNs to constitute a significant effector population for antibodies against HLA class II37,50 and against tyrosine kinase receptors such as HER-2/neu51 or EGF-R.31,32 Results with blood from G-CSF–primed donors (Figure 5) suggested that the relative contribution of PMNs to whole blood ADCC activity can be therapeutically enhanced by G-CSF.
Today, clinical data with glycosylation variants of therapeutic antibodies are limited.52 Results from animal models demonstrated that improving the affinity for activating (A) Fc receptors (FcγRI, FcγRIIIa and FcγRIV), and decreasing the interaction with inhibitory (I) receptors (ie, homolog of human FcγRIIb), thus raising A/I ratios, improved the in vivo activity in mice.4,53 In human whole blood ADCC assays, however, we observed only minor differences in killing between both 2F8 glycosylation variants. Because the efficacy of glycoengineered antibodies in vivo may well depend on the actions of various effector cells, our data suggest that the effector cell type could critically affect the efficacy of glycoengineered antibodies. Because of the limited homology between human and mouse Fcγ receptors, these issues cannot reliably be addressed in animal studies, and results from clinical trials need to demonstrate superior therapeutic efficacy of glycoengineered antibodies compared with unmodified variants. Importantly, the impact of glycosylation may differ between target antigens, and results from these trials may provide indirect evidence for the involvement of different effector cell populations in vivo. In this respect, it is interesting to note that the contributions of FcγR polymorphisms appear to be different between rituximab, herceptin,6,7,45 and cetuximab.46
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Daniela Barths for excellent technical assistance, Prof M. Glennie for providing valuable reagents, and Dr A. Humpe for blood samples from G-CSF–primed donors.
This work was supported by the Deutsche Forschungsgemeinschaft (Va 124/6-3, De 1478/1-1) and by intramural funding from the Christian-Albrechts University (Kiel, Germany).
Authorship
Contribution: M.P., T.S.-M., J.J.L.v.B., W.W.K.B., T.B., M.D., T. Vink, and P.C.H.v.B. performed experiments, analyzed experiments, wrote the manuscript; and R.R., J.G.J.v.d.W., P.W.H.I.P., and T. Valerius planned experiments, analyzed experimental data, and wrote the manuscript.
Conflict-of-interest disclosure: P.C.H.v.B., J.J.L.v.B., T. Vink, W.W.K.B., J.G.J.v.d.W., and P.W.H.I.P. are employees of Genmab. M.D. and T. Valerius received research grants from Genmab. The remaining authors declare no competing financial interests.
Correspondence: Thomas Valerius, Division of Nephrology and Hypertension, Christian-Albrechts-University, Kiel, Schittenhelmstr 12, 24105 Kiel, Germany; e-mail: valerius@nephro.uni-kiel.de.
References
Author notes
*M.P. and J.J.L.v.B. contributed equally to this work.