Abstract
CD8+ T cells contribute to central nervous system inflammation in human T-cell lymphotropic virus type I (HTLV-I)–associated myelopathy/tropical spastic paraparesis (HAM/TSP). We analyzed CD8+ T-cell dysfunction (degranulation and IFN-γ production) and have demonstrated that CD8+ T cells of patients with HAM/TSP (HAM/TSP patients) spontaneously degranulate and express IFN-γ in ex vivo unstimulated culture. CD8+ T cells of HTLV-I asymptomatic carriers and healthy donors did not. Spontaneous degranulation was detected in Tax11-19/HLA-A*201 tetramer+ cells, but not in CMV pp65 tetramer+ cells. Interestingly, degranulation and IFN-γ production in CD8+ T cells was induced by coculture with autologous CD14+ cells, but not CD4+ T cells, of HAM/TSP patients, which correlated with proviral DNA load in CD14+ cells of infected patients. Moreover, the expression of IL-15, which induced degranulation and IFN-γ production in infected patients, was enhanced on surface of CD14+ cells in HAM/TSP patients. Blockade of MHC class I and IL-15 confirmed these results. Thus, CD8+ T-cell dysregulation was mediated by both virus infection and enhanced IL-15 on CD14+ cells in HAM/TSP patients. Despite lower viral expression than in CD4+ T cells, HTLV-I–infected or –activated CD14+ cells may be a heretofore important but under recognized reservoir particularly in HAM/TSP patients.
Introduction
The human T cell lymphotropic virus I (HTLV-I) infects 20 million people worldwide where the majority of infected individuals are asymptomatic carriers (ACs) of the virus.1 However, in a small percentage of infected people this agent has also been demonstrated to be the etiologic agent in patients with adult T-cell leukemia/lymphoma (ATL)2 and a chronic, progressive neurologic disease termed HTLV-I–associated myelopathy/tropical spastic paraparesis (HAM/TSP).3,4 Virologic and immunologic differences between ACs and patients with HAM/TSP (HAM/TSP patients) has been reported, including HTLV-I proviral DNA load, HTLV-I Tax mRNA load, spontaneous T-cell proliferation, frequency of Tax-specific CD8+ T cells, and production of inflammatory cytokine.5-9 Although it is likely that genetic differences also influence the immune response in HTLV-I–infected individuals and thereby contributes to the risk of HAM/TSP,10-14 the mechanism(s) of development of HAM/TSP remain unknown.
Although HTLV-I can infect a wide range of human cell types,15-20 the virus is considered predominantly tropic for CD4+ T cell.21 HTLV-1 infection of high proportions of CD4+ T cells in vivo has been associated with leukemogenesis and reduced regulatory function of CD4+ T cells,22-24 but does not induce severe immune suppression like HIV-1 infection.25 Because HTLV-I proviral loads are significantly elevated in HAM/TSP patients compared with ACs,26 increased expression particularly of the transactivating viral gene encoding HTLV-I Tax has been suggested to play a role in HTLV-I disease progression.7,9,27 Moreover, Tax has been shown in vitro to induce the expression of a variety of cellular genes, including IL-2,28 the α-chain of the IL-2 receptor (IL-2Rα),29 IL-15,30 and IL-15Rα.31 Increased expression of these critical immune mediators caused to dysregulate T-cell activation and proliferation that may contribute to inflammatory central nervous system (CNS) damage in HAM/TSP patients.32
CD8+ T cells play a crucial role in immunity against viruses through their ability to secrete various factors that suppress viral replication and kill infected target cells.33,34 HTLV-I–specific cytotoxic T lymphocytes (CTL) suppressed proviral loads in HTLV-I–infected patients,35 and high frequencies of these effector cells was found in peripheral blood of HAM/TSP patients, with even higher levels in cerebrospinal fluid.6-8,27,36-38 These observations are consistent with an immunopathogenic role for HTLV-I–specific CTL in HAM/TSP patients in which cytotoxicity and the production of inflammatory cytokines including IFN-γ and TNF-α may be associated with damage of the CNS. However, the mechanisms by which virus-specific CD8+ T cells are regulated remain unclear.
Degranulation of activated CD8+ T cells occurs rapidly after TCR stimulation, as a result of the polarized mobilization of microtubules that transport lytic granules toward the immunologic synapse formed between CTL and the target.39 By measuring the mobilization of cytolytic granule membrane proteins (lysosome-associated membrane proteins; CD107) to the cell surface, which occurs as the granule membrane merges with the cell membrane during degranulation, it is possible to identify and quantify antigen-specific CD8+ T-cell degranulation by flow cytometry.40 The ability of CD8+ T cells to degranulate has been directly correlated with cytolytic activity of effector CD8+ T cell and is therefore useful for characterizing CD8+ T-cell lytic activity in various disease studies.41-44 In addition, antiviral CD8+ T-cell effector function is determined primarily by antigen concentration, suggesting that responding CD8+ T cells would produce cytokines and elaborate cytolytic activity in the presence of antigen presenting cells (APCs) expressing high viral antigen levels.45 Based on these observations, we characterized CD8+ T-cell spontaneous degranulation and IFN-γ production in HAM/TSP patients, ACs and HTLV-I–seronegative healthy donors (NDs), ex vivo. We demonstrate significant elevations in the frequency of CD107a mobilizing/IFN-γ–expressing (CD107a/IFN-γ) CD8+ T cells specifically in HAM/TSP patients. Moreover, this spontaneous degranulation and IFN-γ production was clearly correlated with proviral DNA load in CD14+ cells from HTLV-I–infected patients. Mechanistically, enhanced IL-15 expression in CD14+ cells mediated the dysregulation of spontaneously degranulated CD8+ T cells in HAM/TSP patients. These observations add new insights into the pathogenesis of inflammatory disease associated with retroviral infection.
Methods
Patient samples
Blood samples were obtained from 9 HAM/TSP patients (HAM#1-9), 5 ACs (AC#1-5) and 3 NDs (ND#1-3). Diagnosis of HAM/TSP was based on World Health Organization (WHO) diagnostic criteria. Three HAM/TSP patients (HAM#2, 4 and 5) and 3 ACs (AC#1, 2 and 4) were HLA-A*201+. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (Lonza Walkersville, Walkersville, MD) centrifugation. The PBMCs obtained from HTLV-I–infected patients or NDs were cryopreserved in liquid nitrogen until use. Informed consent was obtained from each subject. The study was reviewed and approved by the National Institute of Neurologic Disorders and Stroke (NINDS) Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Antibodies and reagents
Mouse anti–human CD3, CD8, CD4, CD14, CD107a, IFN-γ, perforin, granzyme B, CD28 and CD49d were purchased from BD Biosciences (San Jose, CA). Anti–human MHC class I (HLA-ABC) was purchased from AbD Serotec (Oxford, United Kingdom). Tax 11-19/HLA-A201 tetramer was provided by National Institute of Allergy and Infectious Diseases (NIAID) Major Histocompatibility Complex (MHC) Tetramer Core Facility (Atlanta, GA). CMV pp65/HLA-A201 tetramer was purchased from Beckman Coulter (San Diego, CA). Anti–Tax monoclonal antibody (Lt-4) was kindly provided by Dr Y. Tanaka (University of the Ryukyus, Okinawa, Japan). Anti–IL-2Rα (anti-Tac) and anti–IL-2/15Rβ (Mikβ1) were kindly provided by Dr T. Waldmann (National Institutes of Health [NIH], Bethesda, MD). Anti-IL-15 was purchased from R&D Systems (Minneapolis, MN). Recombinant human IL-15 (rhIL-15) was purchased from Peprotech (Rocky Hill, NJ).
CD107a mobilization assay
PBMCs of NDs or HTLV-I–infected patients were suspended at 106 cells/mL in RPMI media (RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin sulfate, and 2 mM l-glutamine). One-milliliter aliquots of the cells were cultured in 24-well plates in a 5% CO2 incubator at 37°C. To detect Tax11-19– or CMV pp65–specific responses, PBMCs were stimulated with the appropriate peptide (either HTLV Tax11-19 LLFGYPVYV or CMV pp65 NLVPMVATV) and 1 μg/mL each of CD28 and CD49d for 5 hours. In blocking experiments, 5 μg/mL of each antibody (anti–MHC class I, anti-Tac, anti–IL-15, and Mikβ1) was added singly or in combination for 24 hours. In IL-15 stimulation, 10 ng/mL rhIL-15 were added into the culture for 12 hours. Conjugated CD107a antibody, 0.7 μL/mL GoldiStop (BD Biosciences), and 1 μg/mL brefeldin A (Sigma-Aldrich, St Louis, MO) were added into the culture for 5 hours before the time point for detection.
Immunofluorescent staining
Expression of CD107a/IFN-γ, intracellular Tax protein, and IL-15 in the cultured PBMCs were examined at each time point by flow cytometric analysis. First, PBMCs were surface-stained with specific antibodies. In the case of combination staining with tetrameric complexes, PBMCs were stained with either Tax11-19– or CMV pp65–specific tetramer before surface staining. After fixation and permeabilization with Fixation/Permeabilization solution (BD Biosciences) according to the manufacturer's instructions, the cells were intracellularly stained with anti–IFN-γ, anti-Tax, or anti–IL-15 for each experiment. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (BD Biosciences). The data were analyzed using FlowJo software (TreeStar, San Carlos, CA).
Coculture of CD8+ T cell with CD4+ T cells or CD14+ cells
CD8+ and CD4+ T cells were magnetically isolated from PBMCs by negative selection with a CD8+ T-cell isolation kit II and a CD4+ T-cell isolation kit II (Miltenyi, Bergisch Gladbach, Germany), respectively, according to the manufacturer's instructions. The purities of CD8+ and CD4+ T cells were confirmed by anti-CD3, anti-CD8, and anti-CD4 as greater than 90%. To collect mononuclear phagocytes (MPs) from PBMCs, CD14+ cells were magnetically isolated using CD14 MicroBeads (Miltenyi). The purity of CD14+ cells was confirmed by anti-CD14 and anti-CD11b as approximately 95%. CD8+ T cells (2 × 105 cells) were cultured with an equal number of CD4+ T cells or CD14+ cells, and CD107a/IFN-γ expression was analyzed on a CD107a mobilization assay.
Tax mRNA detection
The cultured PBMCs were collected at each time point and stored at −80°C until use. For Tax mRNA detection in CD14+ cells, PBMCs were cultured in a teflon flask (Nalge Nunc International, Rochester, NY) to avoid loss of CD14+ cells on the culture plate, and CD14+ cells were magnetically isolated from cultured PBMCs using CD14 MicroBeads (Miltenyi). Total RNAs were extracted from the cell pellets by an RNeasy Mini Kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. Synthesis of cDNA and measurement of HTLV-I Tax mRNA load were performed as previously described,24 using an ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA).
HTLV-I proviral DNA load
Total PBMCs and isolated CD4+ T cells and CD14+ cells were stored at −80°C until use. DNA was extracted from the cells using QIAamp DNA Blood Mini Kit (Qiagen) and HTLV-1 proviral DNA load was measured using TaqMan system as previously described.24
Statistical analysis
Box plot analysis was used to compare CD107a/IFN-γ expressions of CD8+ T cells in HAM/TSP patients and ACs. Simple regression analysis was used to test the correlation between HTLV-1 proviral DNA load in total PBMCs and isolated CD4+ T cells and CD14+ cells, and CD107a/IFN-γ expression of CD8+ T cells in HAM/TSP patients and ACs.
Results
Spontaneous degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients
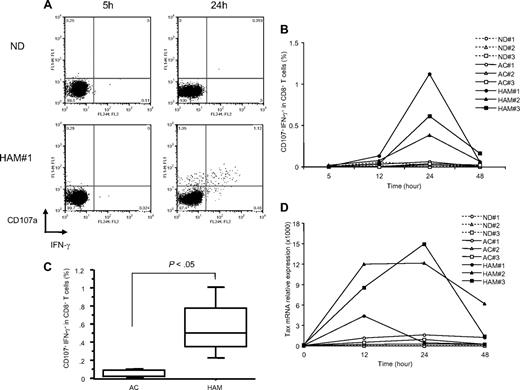
CD8+ T-cell degranulation in HAM/TSP patients and NDs were examined by the CD107a mobilization assay. The frequency of CD107a/IFN-γ in CD8+ T cells during short term ex vivo PBMC culture was determined by flow cytometric analysis. As there were no exogenous stimulators such as anti-CD3 or viral antigens in these cultures, these responses were considered as spontaneous degranulation. A representative dot plot is shown in Figure 1A. After 5 hours culture, CD8+ T cells from both a HAM/TSP patient and an ND did not express CD107a/IFN-γ (Figure 1A top right quadrant). After 24 hours of culture, the expression of CD107a dramatically increased in the HAM/TSP patient, and was also associated with an increase of IFN-γ production. In contrast, ND CD8+ T cells demonstrated no CD107a/IFN-γ expression in the culture. Both degranulation and IFN-γ production was detected specifically in CD8+ T cells and not in CD4+ T cells of HAM/TSP patients (data not shown).
Spontaneous degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients. (A) Flow cytometric analysis of CD107a/IFN-γ expression in CD8+ T cells of an ND and a representative HAM/TSP patient (HAM#1) after culture for 5 and 24 hours. Cells were cultured in the absence of any additional stimulators. (B) Time course of CD107a/IFN-γ expression in CD8+ T cells of NDs (ND#1-3) and HTLV-I–infected patients (AC#1-3 and HAM#1-3). PBMCs were cultured for 5 to 48 hours, and analyzed by flow cytometry at each time point. (C) Comparison of CD107a/IFN-γ expression in CD8+ T cells of HTLV-I–infected patients at 24 hours culture. The data were obtained from 5 ACs and 9 patients with HAM/TSP. The horizontal line represents the median, the box represents the 25th percentile, and the whiskers represent the 75th percentile. (D) Time course of Tax mRNA expression in NDs (ND#1-3) and HTLV-I–infected patients (AC#1-3 and HAM#1-3). This experiment was performed at the same time points as with Figure 1B, and total RNAs were isolated from cultured cells at each time point.
Spontaneous degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients. (A) Flow cytometric analysis of CD107a/IFN-γ expression in CD8+ T cells of an ND and a representative HAM/TSP patient (HAM#1) after culture for 5 and 24 hours. Cells were cultured in the absence of any additional stimulators. (B) Time course of CD107a/IFN-γ expression in CD8+ T cells of NDs (ND#1-3) and HTLV-I–infected patients (AC#1-3 and HAM#1-3). PBMCs were cultured for 5 to 48 hours, and analyzed by flow cytometry at each time point. (C) Comparison of CD107a/IFN-γ expression in CD8+ T cells of HTLV-I–infected patients at 24 hours culture. The data were obtained from 5 ACs and 9 patients with HAM/TSP. The horizontal line represents the median, the box represents the 25th percentile, and the whiskers represent the 75th percentile. (D) Time course of Tax mRNA expression in NDs (ND#1-3) and HTLV-I–infected patients (AC#1-3 and HAM#1-3). This experiment was performed at the same time points as with Figure 1B, and total RNAs were isolated from cultured cells at each time point.
To determine whether spontaneous degranulation and IFN-γ production in CD8+ T cells was associated with specific disease states in HTLV-I infection, CD107a/IFN-γ expression was compared in CD8+ T cells of HAM/TSP patients (HAM#1-3), ACs (AC#1-3) and NDs (ND#1-3) over time. In CD8+ T cells from patients with HAM/TSP, CD107a/IFN-γ expression was slightly detectable at 12 hours of culture, peaking to maximal levels by 24 hours (Figure 1B). By contrast, ACs and NDs did not express CD107a/IFN-γ during 48 hours of ex vivo culture (< 0.1%). Figure 1C shows box plot analysis of CD107a/IFN-γ expression in CD8+ T cells at 24 hours culture from 5 ACs and 9 patients with HAM/TSP. CD107a/IFN-γ expression was significantly elevated in CD8+ cells of HAM/TSP patients (P < .05). These results suggested that spontaneous degranulation and IFN-γ production is a distinguishing feature of CD8+ T cells from patients with HAM/TSP.
To examine the correlation of this increased CD107a/IFN-γ expression in ex vivo culture from CD8+ T cells of HAM/TSP patients with HTLV-I viral production, HTLV-I Tax mRNA expression in PBMCs of HAM/TSP patients and ACs were quantified. HAM/TSP patients showed the spontaneous increase of Tax mRNA expression after the culture with a peak of Tax mRNA level at 12 to 24 hours (Figure 1D). Unlike HAM/TSP patients, the expression of Tax mRNA was significantly lower in ACs as previously reported9 and correlated with the absence of spontaneous degranulation in CD8+ T cells from ACs (Figure 1B,D). Collectively, these results suggested that the expression of HTLV-I viral products in cultured PBMCs was related to CD107a/IFN-γ expression in CD8+ T cells specifically in patients with HAM/TSP.
Tax11-19–specific degranulation and IFN-γ production in CD8+ T cells of HTLV-I–infected patients
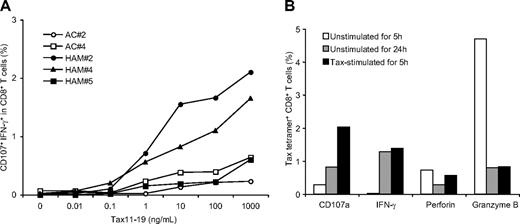
To confirm whether spontaneous degranulation and IFN-γ production could be demonstrated in antigen-specific CD8+ T-cells responses, we examined induction of CD107a/IFN-γ expression from HLA A*201 CD8+ T cells from HTLV-I-infected patients by stimulation with a known immunodominant HLA A2 binding HTLV-I Tax11-19 peptide.46 Three HAM/TSP patients (HAM#2, 4, and 5) and 2 ACs (AC# 2and 4) who were subtyped as HLA-A*201 and had relatively high levels of Tax11-19 tetramer+CD8+ T cells, were selected. Three HAM/TSP patients demonstrated 4.78% (HAM#5), 9.19% (HAM#4) and 21.1% (HAM#2) Tax11-19 tetramer+ cells. Although ACs have been reported to have lower Tax11-19 tetramer+ cells than HAM/TSP patients,8,37 we identified 2 ACs that were 3.56% (AC#2) and 5.04% (AC#4) Tax11-19 tetramer+. Stimulation of Tax11-19 peptide induced CD107a/IFN-γ expression in CD8+ T cells from both HAM/TSP patients and ACs, depending on the concentration of Tax11-19 peptide (Figure 2A). Moreover, the magnitude of CD107a/IFN-γ expression corresponded to the frequency of Tax11-19 tetramer+ cells in HTLV-I–infected patients. Thus, antigen-specific degranulation and IFN-γ production depended on the concentration of immunodominant viral peptide stimulation and correlated with the frequency of antigen-specific tetramer+ cells in HTLV-I–infected individuals.
Evaluation of degranulation and IFN-γ production in CD8+ T cells of HTLV-I–infected patients. (A) Titration of Tax11-19 peptide stimulation to induce degranulation and IFN-γ production in CD8+ T cells of 2 HLA-A*201 ACs (AC#2 and 4) and 3 HLA-A*201 HAM/TSP patients (HAM#2, 4 and 5). CD107a/IFN-γ expression was analyzed after 5 hours stimulation with peptide. (B) CD107a mobilization and expression of intracellular IFN-γ, perforin and granzyme B in HTLV-I–specific CD8+ T cells of a HAM/TSP patient. The graph shows the percentage of Tax tetramer+ CD8+ T cells in unstimulated culture for 5 hours (□), unstimulated for 24 hours ( ), and stimulated with Tax11-19 peptide for 5 hours (■).
), and stimulated with Tax11-19 peptide for 5 hours (■).
Evaluation of degranulation and IFN-γ production in CD8+ T cells of HTLV-I–infected patients. (A) Titration of Tax11-19 peptide stimulation to induce degranulation and IFN-γ production in CD8+ T cells of 2 HLA-A*201 ACs (AC#2 and 4) and 3 HLA-A*201 HAM/TSP patients (HAM#2, 4 and 5). CD107a/IFN-γ expression was analyzed after 5 hours stimulation with peptide. (B) CD107a mobilization and expression of intracellular IFN-γ, perforin and granzyme B in HTLV-I–specific CD8+ T cells of a HAM/TSP patient. The graph shows the percentage of Tax tetramer+ CD8+ T cells in unstimulated culture for 5 hours (□), unstimulated for 24 hours ( ), and stimulated with Tax11-19 peptide for 5 hours (■).
), and stimulated with Tax11-19 peptide for 5 hours (■).
We demonstrated whether CD107a/IFN-γ expression in CD8+ T cells of HAM/TSP patients was correlated with a loss of lytic granules, perforin and granzyme B, in CD8+ T cells. As shown in Figure 2B, tax11-19 tetramer+ CD8+ T cells from unstimulated PBMCs cultured for 24 hours from an HLA-A*201+ patient (HAM#2) demonstrated increased CD107a/IFN-γ expression but decreased levels of intracellular perforin and granzyme B, compared with Tax11-19 tetramer+ CD8+ T cells unstimulated for 5 hours. These results were comparable with an increase of CD107a/IFN-γ expression and a loss of intracellular perforin and granzyme B in Tax11-19 tetramer+ CD8+ T cells after stimulation with Tax11-19 peptide. Because CD107a/IFN-γ expression in CD8+ T cells was clearly correlated with loss of lytic granules in Tax11-19 tetramer+ CD8+ T cells, we confirmed that spontaneous degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients were antigen-specific CTL responses.
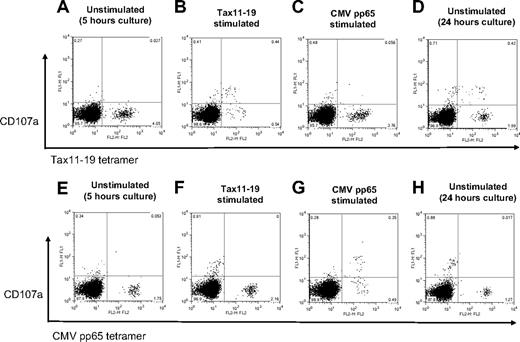
The antigen-specificity of spontaneous degranulation and IFN-γ production was further characterized in CD107a expression from Tax11-19– and CMV pp65–specific CD8+ T cells in a HAM/TSP patient (HAM#5) that had detectable levels of both Tax11-19 (4.78%) and CMV pp65 (1.76%) tetramer+ cells. Figure 3A,E show that both Tax and CMV tetramer+ cells did not express CD107a in unstimulated culture for 5 hours. After Tax11-19 peptide stimulation for 5 hours, CD107a expression could be demonstrated from Tax11-19 tetramer+ CD8+ T cells but not from CMV pp65 tetramer+ cells (Figure 3B,F). Conversely, after CMV pp65 peptide stimulation for 5 hours, CD107a expression could be shown in CMV tetramer+ CD8+ T cells but not from Tax11-19 tetramer+ cells (Figure 3C,G). The decreased number of tetramer+ cells after specific antigen stimulation resulted from TCR down-regulation and degranulation. Thus, each tetramer+ CD8+ T-cell population showed antigen-specific degranulation in the presence of their respective specific antigen and demonstrated no cross reactivity in antigen-induced degranulation. When PBMCs from this HAM/TSP patient were placed in unstimulated culture for 24 hours, CD107a expression was only detected from Tax11-19 tetramer+ cells but not in CMV pp65 tetramer+ cells (Figure 3D,H). Interestingly, CD107a expression was also detected from Tax11-19 tetramer− cells (Figure 3D), suggesting that HTLV-I antigenic stimulator(s) other than the immunodominant Tax11-19 peptide might be also involved in spontaneous degranulation in HLA A*201 HAM/TSP patient.
Antigen-specific degranulation in CD8+ T cells from a HAM/TSP patient. PBMCs of a HAM/TSP patient (HAM#5) were cultured without any stimulation (A,E) or with Tax11-19 peptides (B,F) or CMVpp65 peptides (C,G) for 5 hours. Top or bottom panels show the detection of CD107a with Tax11-19 tetramer and CMV pp65 tetramer staining, respectively. Panels D and H show the results of spontaneous degranulation in CD8+ T cells of unstimulated culture for 24 hours.
Antigen-specific degranulation in CD8+ T cells from a HAM/TSP patient. PBMCs of a HAM/TSP patient (HAM#5) were cultured without any stimulation (A,E) or with Tax11-19 peptides (B,F) or CMVpp65 peptides (C,G) for 5 hours. Top or bottom panels show the detection of CD107a with Tax11-19 tetramer and CMV pp65 tetramer staining, respectively. Panels D and H show the results of spontaneous degranulation in CD8+ T cells of unstimulated culture for 24 hours.
CD14+ cells induced degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients
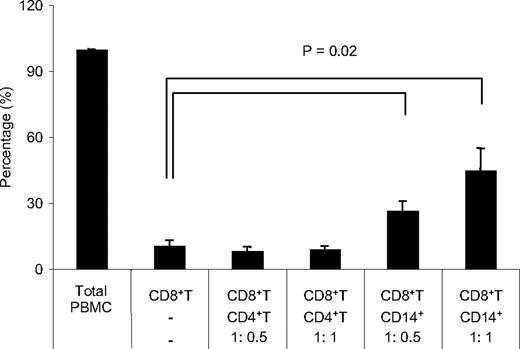
Spontaneous degranulation and IFN-γ production was shown to be an HTLV-I–related response detected only in HAM/TSP patients (Figure 1B), although both HAM/TSP patients and ACs could respond to HTLV-I Tax11-19 stimulation (Figure 2). However, it still remains unknown what strongly regulates spontaneous degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients. CD4+ T cells were likely candidates because they constitute the main reservoir of HTLV-I in vivo and previous study has shown that IFN-γ production can occur during coculture with autologous CD8+ T cells from HTLV-I–infected patients.38 Alternatively, MPs including monocytes, macrophages, and dendritic cells (DCs) could also be involved because they are known to promote CD8+ T-cell function by effectively presenting viral antigens through MHC class I.47 Therefore, CD107a/IFN-γ expression in isolated CD8+ T cells of 5 HAM/TSP patients was examined after coculture with autologous CD4+ T cells or CD14+ cells which mainly included monocytes and macrophages (Figure 4). CD107a/IFN-γ expression in CD8+ T cells in total PBMC culture was normalized to 100%, and the result of each condition was calculated. Isolated CD8+ T cells cultured alone demonstrated minimal CD107a/IFN-γ expression (10%). Unexpectedly, CD8+ T cells cocultured with autologous CD4+ T cells did not induce CD107a/IFN-γ expression compared with total PBMCs. However, after coculture with autologous CD14+ cells, CD8+ T cells expressed CD107a/IFN-γ in a dose-dependent manner (Figure 4). Moreover, the reestablishment of CD107a/IFN-γ expression in CD8+ cells by the addition of CD14+ cells was cell-dependent because the culture supernatant from total PBMCs did not induce CD107a/IFN-γ expression in CD8+ T cells (data not shown). Collectively, these results demonstrated that the loss of CD107a/IFN-γ expression in CD8+ T cells when cultured alone could be restored by the addition of autologous CD14+ cells but not CD4+ T cells, and that cell-to-cell interaction with CD14+ cells was necessary for degranulation and IFN-γ production from CD8+ T cells in HAM/TSP patient.
Coculture of CD8+ T cells with CD14+ cells induced degranulation and IFN-γ production in HAM/TSP patients. CD107a/IFN-γ expression in CD8+ T cells was compared among total PBMCs, isolated CD8+ T cells, and coculture of isolated CD8+ T cells with autologous CD4+ T cells or CD14+ cells of HAM/TSP patients. The isolated CD8+ T cells were cocultured with autologous CD4+ T cells or CD14+ cells at the ratio of 1:0.5 or 1:1. The amount of CD107a/IFN-γ expression of CD8+ T cells in isolated CD8+ T cells and CD8+ T cells cocultured with CD4+ T cells or CD14+ cells were normalized to total PBMC (100%). The graph was prepared from data obtained from 5 HAM/TSP patients. Error bars represent SD.
Coculture of CD8+ T cells with CD14+ cells induced degranulation and IFN-γ production in HAM/TSP patients. CD107a/IFN-γ expression in CD8+ T cells was compared among total PBMCs, isolated CD8+ T cells, and coculture of isolated CD8+ T cells with autologous CD4+ T cells or CD14+ cells of HAM/TSP patients. The isolated CD8+ T cells were cocultured with autologous CD4+ T cells or CD14+ cells at the ratio of 1:0.5 or 1:1. The amount of CD107a/IFN-γ expression of CD8+ T cells in isolated CD8+ T cells and CD8+ T cells cocultured with CD4+ T cells or CD14+ cells were normalized to total PBMC (100%). The graph was prepared from data obtained from 5 HAM/TSP patients. Error bars represent SD.
Correlation of spontaneous degranulation and IFN-γ production with HTLV-I proviral DNA load and viral expression
Given the CD14+ cell dependence of spontaneous degranulation, we asked whether CD14+ cells themselves were infected. Although CD4+ T cells represent the major reservoir of HTLV-I infection, HTLV-I proviral DNA was also detected in monocytes of HTLV-I–infected patients.17 Therefore, we analyzed HTLV-I proviral DNA load in total PBMCs, CD4+ T cells and CD14+ cells isolated from HTLV-I–infected patients and analyzed their relationship to spontaneous CD8+ T-cell degranulation. Proviral DNA loads in total PBMCs and CD4+ T cells were correlated with CD107a/IFN-γ expression of CD8+ T cells in HTLV-I–infected patients (Figure 5Ai,ii). Although HAM/TSP patients had higher proviral DNA loads than ACs, the correlation with CD107a/IFN-γ expression was variable (Figure 5Ai) and some ACs showed high proviral DNA loads in CD4+ T cells without any CD107a/IFN-γ expression of CD8+ T cells (Figure 5Aii). By comparison, the proviral DNA load from isolated CD14+ cells demonstrated significantly better correlation with CD107a/IFN-γ expression of CD8+ T cells in HTLV-I–infected patients (Figure 5Aiii; P < .001, R2 = 0.748). Although proviral DNA load in CD14+ cells were lower than those in CD4+ T cells as reported,17 HAM/TSP patients had higher viral loads in CD14+ cells than ACs. These results suggested that HTLV-I infection of CD14+ cells appear to be a major determinant of spontaneous degranulation in HAM/TSP patients.
Correlation of spontaneous degranulation and IFN-γ production in CD8+ T cells with proviral DNA loads of HTLV-I–infected patients, and HTLV-I viral expression in cultured cells of HAM/TSP patients. (A) Correlation of spontaneous CD107a and IFN-γ expressions in CD8+ T cells and proviral DNA loads in total PBMCs (i), isolated CD4+ T cells (ii), and isolated CD14+ cells (iii) from HTLV-I–infected patients were determined. The data obtained from 5 ACs (○) and 7 HAM/TSP patients (●) are plotted. (B) Expression of intracellular Tax protein in ND CD4+ T cells (○) and CD14+ cells (△), and CD4+ T cells (●) and CD14+ cells (▲) of a HAM/TSP patient after the culture. (C) Expression of Tax mRNA in total PBMCs and CD14+ cells before and after culture for 5 hours. CD14+ cells were magnetically isolated from the cultured PBMCs. The graph was prepared from data obtained from 3 HAM/TSP patients. Error bars represent SD.
Correlation of spontaneous degranulation and IFN-γ production in CD8+ T cells with proviral DNA loads of HTLV-I–infected patients, and HTLV-I viral expression in cultured cells of HAM/TSP patients. (A) Correlation of spontaneous CD107a and IFN-γ expressions in CD8+ T cells and proviral DNA loads in total PBMCs (i), isolated CD4+ T cells (ii), and isolated CD14+ cells (iii) from HTLV-I–infected patients were determined. The data obtained from 5 ACs (○) and 7 HAM/TSP patients (●) are plotted. (B) Expression of intracellular Tax protein in ND CD4+ T cells (○) and CD14+ cells (△), and CD4+ T cells (●) and CD14+ cells (▲) of a HAM/TSP patient after the culture. (C) Expression of Tax mRNA in total PBMCs and CD14+ cells before and after culture for 5 hours. CD14+ cells were magnetically isolated from the cultured PBMCs. The graph was prepared from data obtained from 3 HAM/TSP patients. Error bars represent SD.
To determine viral production in CD14+ cells, the expression of HTLV-I Tax protein was examined in CD14+ cells of HAM/TSP patients during culture, compared with CD4+ T cells. Figure 5B shows representative results regarding intracellular Tax protein expression in a HAM/TSP patient and an ND. As previously reported,48 Tax proteins were dramatically increased in CD4+ T cells of a HAM/TSP patient after short-term culture. Although, Tax protein was detectable in CD14+ cells at 16 hours of culture, this level of HTLV-I expression in CD14+ cells was significantly lower than CD4+ T cells (Figure 5B). Similarly, low level of HTLV-I Tax mRNA expression was also detected in CD14+ cells after short-term culture for 5 hours although total PBMCs showed much higher expression (Figure 5C). Thus, HTLV-I viral production was detected in CD14+ cells of HAM/TSP patients after culture, with CD4+ T cells showing more rapid and higher levels of Tax expression.
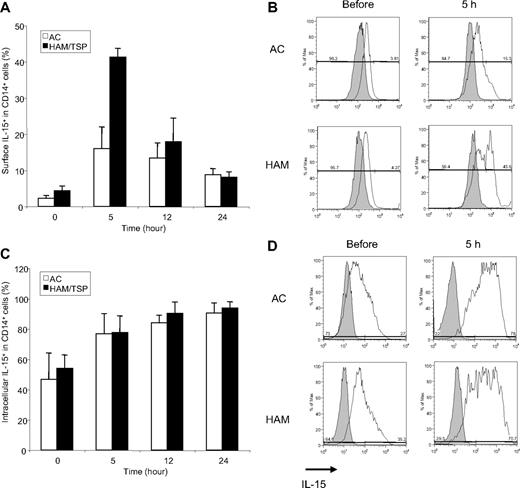
Enhanced IL-15 expression on surface of CD14+ cells in patients with HAM/TSP
HTLV-I infection may lead to altered cytokine expression, manifested by increased spontaneous T-cell proliferation and associated with the inflammatory disease progression.32 Up-regulation of IL-15 expression associated with HTLV-I infection is of particular interest, because recent reports suggested that IL-15 plays a key role in CD8+ T-cell function49-51 and is in trans presented on MPs to neighboring cells, such as CD8+ T cells.52-55 Therefore, to determine whether altered IL-15 expression in CD14+ cells of HAM/TSP patients mediate spontaneous degranulation and IFN-γ production of CD8+ T cells, we temporally analyzed surface and intracellular IL-15 expression in CD14+ cells of each of 3 ACs and HAM/TSP patients (Figure 6A,C, respectively). Representative histograms of surface and intracellular IL-15 expression were shown in Figure 6B and 6D, respectively. Both ACs and HAM/TSP patients expressed an average of 2.23% and 4.37% of IL-15 on the surface of CD14+ cells before the culture (Figure 6A,B). After 5-hour culture, surface IL-15 expression dramatically increased in HAM/TSP patients compared with ACs (Figure 6A,B), which gradually declined over time. Intracellular IL-15 expression also increased over time in CD14+ cells from HTLV-I–infected patients, but without significant differences between ACs and HAM/TSP patients (Figure 6C,D). Consistent with previous reports, lymphocytes expressed relatively low levels of IL-15 (approximately 1%-3%) and the expression level did not change during 24 hours of culture (data not shown). Thus, relatively greater surface expression, rather than production, of IL-15 on CD14+ cells during ex vivo culture distinguished HAM/TSP patients from ACs. Because surface bound IL-15 on monocytes is biologically more active, compared with its soluble counterpart,56 our finding suggests that enhanced IL-15 on the surface of CD14+ cells may contribute to spontaneous degranulation and IFN-γ production in CD8+ T cells of patients with HAM/TSP.
IL-15 expression in CD14+ cells of ACs and HAM/TSP patients. (A) Time course of surface IL-15 expression in CD14+ cells from 3 ACs (□) and 3 HAM/TSP patients (■). (B) Representative histograms of surface IL-15 expression on CD14+ cells from ACs and a HAM/TSP patient before and after the culture for 5 hours. Staining with anti–Il-15 (opened histograms) and IgG isotype control (grayed histograms) in CD14+ cells were shown. (C) Time course of intracellular IL-15 expression in CD14+ cells from 3 ACs (□) and 3 HAM/TSP patients (■). This experiment was performed at the same time points as with panel A. (D) Representative histograms of intracellular IL-15 expression on CD14+ cells from ACs and a HAM/TSP patient before and after the culture for 5 hours. Staining with anti–Il-15 (open histogram) and IgG isotype control (grayed histogram) in CD14+ cells were shown.
IL-15 expression in CD14+ cells of ACs and HAM/TSP patients. (A) Time course of surface IL-15 expression in CD14+ cells from 3 ACs (□) and 3 HAM/TSP patients (■). (B) Representative histograms of surface IL-15 expression on CD14+ cells from ACs and a HAM/TSP patient before and after the culture for 5 hours. Staining with anti–Il-15 (opened histograms) and IgG isotype control (grayed histograms) in CD14+ cells were shown. (C) Time course of intracellular IL-15 expression in CD14+ cells from 3 ACs (□) and 3 HAM/TSP patients (■). This experiment was performed at the same time points as with panel A. (D) Representative histograms of intracellular IL-15 expression on CD14+ cells from ACs and a HAM/TSP patient before and after the culture for 5 hours. Staining with anti–Il-15 (open histogram) and IgG isotype control (grayed histogram) in CD14+ cells were shown.
Both MHC class I and IL-15 mediated spontaneous degranulation and IFN-γ production in CD8+ T cells of HTLV-I–infected patients
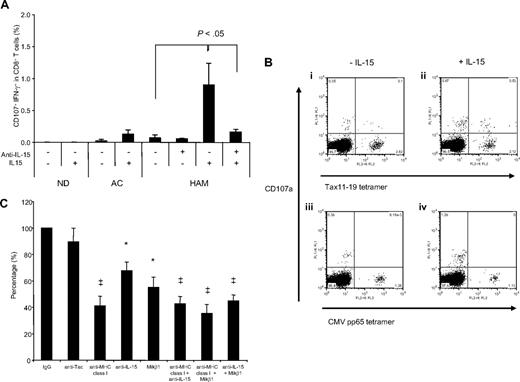
The consequence of increased IL-15 on CD8+ T-cell degranulation was demonstrated by the addition of exogeneous IL-15 during ex vivo culture. The CD107a/IFN-γ expression in CD8+ T-cells from each of 3 NDs, ACs and HAM/TSP patients were examined in the presence of rhIL-15 after 12 hours culture when minimal amounts of CD107a/IFN-γ expression was noted in unstimulated samples (Figure 1B). The addition of rhIL-15 led to several-fold increase in CD107a/IFN-γ expression in CD8+ T cells from HAM/TSP patients (Figure 7A), and also induced in ACs at levels comparable with that seen spontaneously in HAM/TSP patients. CD8+ T cells in NDs demonstrated no CD107a/IFN-γ expression even in the presence of rhIL-15, indicating that the presence of IL-15 alone was not sufficient for CD107a/IFN-γ expression. Interestingly, in combination with specific tetramer staining, CD107a expression was detected in Tax tetramer+ CD8+ T cells but not CMV pp65 tetramer+ cells of a HAM/TSP patient (HAM#5) after culture with rhIL-15 (Figure 7B). These results suggested that IL-15 enhanced CD107a/IFN-γ expression in HTLV-I–specific CD8+ T cells.
IL-15 mediated degranulation and IFN-γ production in CD8+ T cells of HTLV-I–infected patients. (A) CD107a/IFN-γ expression in CD8+ T cells of NDs and HTLV-I–infected patients after culture with rhIL-15. The PBMCs from 3 of each NDs, ACs and HAM/TSP patients were cultured with or without 10 ng/mL rhIL-15 for 12 hours. In HAM/TSP patients, anti–IL-15 was added singly or in combination with rhIL-15 as control. (B) Induction of HTLV-I–specific CD8+ T-cell degranulation by IL-15. The top panels show CD107a mobilization in HTLV-I Tax tetramer+ CD8+ T cells after culture for 12 hours without rhIL-15 (i) and with rhIL-15 (ii). The bottom panels show CD107a mobilization in CMV pp65 tetramer+ CD8+ T cells after the culture for 12 hours without rhIL-15 (iii) and with rhIL-15 (iv). (C) Inhibitory effects of anti–MHC class I, anti–IL-15, anti–IL-2/15Rβ (Mikβ1) and anti–IL-2Rα (anti-Tac) on degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients. The PBMCs were cultured with antibodies singly or in combination for 24 hours. The amounts of CD107a/IFN-γ productions of CD8+ T cells in PBMCs cultured with IgG control were normalized to 100%, and then, those in PBMCs cultured with each antibody were calculated. The graph was prepared from data obtained from 5 HAM/TSP patients. Error bars represent SD. *P < .01, ‡P < .001.
IL-15 mediated degranulation and IFN-γ production in CD8+ T cells of HTLV-I–infected patients. (A) CD107a/IFN-γ expression in CD8+ T cells of NDs and HTLV-I–infected patients after culture with rhIL-15. The PBMCs from 3 of each NDs, ACs and HAM/TSP patients were cultured with or without 10 ng/mL rhIL-15 for 12 hours. In HAM/TSP patients, anti–IL-15 was added singly or in combination with rhIL-15 as control. (B) Induction of HTLV-I–specific CD8+ T-cell degranulation by IL-15. The top panels show CD107a mobilization in HTLV-I Tax tetramer+ CD8+ T cells after culture for 12 hours without rhIL-15 (i) and with rhIL-15 (ii). The bottom panels show CD107a mobilization in CMV pp65 tetramer+ CD8+ T cells after the culture for 12 hours without rhIL-15 (iii) and with rhIL-15 (iv). (C) Inhibitory effects of anti–MHC class I, anti–IL-15, anti–IL-2/15Rβ (Mikβ1) and anti–IL-2Rα (anti-Tac) on degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients. The PBMCs were cultured with antibodies singly or in combination for 24 hours. The amounts of CD107a/IFN-γ productions of CD8+ T cells in PBMCs cultured with IgG control were normalized to 100%, and then, those in PBMCs cultured with each antibody were calculated. The graph was prepared from data obtained from 5 HAM/TSP patients. Error bars represent SD. *P < .01, ‡P < .001.
The role of IL-15 mediated spontaneous degranulation and IFN-γ production were further confirmed by the effects of blocking IL-2Rα (anti-Tac), IL-2/15Rβ (Mikβ1), IL-15, and MHC class I, singly or in combination, on CD107a/IFN-γ expression in CD8+ T cells of 5 HAM/TSP patients (Figure 7C). Anti-MHC class I inhibited (58.9%) CD107a/IFN-γ expression in CD8+ T cells from HAM/TSP patients compared with a control isotype IgG. Although anti-IL-15 and Mikβ1 each alone had less pronounced inhibitory effects, 32.4% and 45.0%, respectively, the combination of both anti–IL-15 and Mikβ1 was more effective (55.2%; Figure 7C). While both anti–IL-15 and Mikβ1 significantly inhibited CD107a/IFN-γ expression in CD8+ T cells from HAM/TSP patients, anti-Tac did not. Indeed, Mikβ1 showed blocking effects against IL-15 but not IL-2, as previously reported.57 Blockade of Mikβ1 and MHC class I in combination significantly inhibited CD107a/IFN-γ expression in CD8+ T cells (64.8%). These results demonstrated the involvement of both MHC class I and IL-15 in spontaneous degranulation and IFN-γ production of CD8+ T cells in HAM/TSP patients.
Discussion
Analyses of ex vivo T-cell function, including cell proliferation, cytokine production and cytotoxicity assays, have suggested a role for HTLV-I–specific CD8+ T cells in the pathogenesis of HAM/TSP.6,8,36,38 In this study, we have extended these observations to assess CD8+ T-cell degranulation in HAM/TSP patients and ACs. Using an immunologic marker for cytolytic activity (CD107a) coupled with intracellular IFN-γ expression, the extent of CD8+ degranulation could be measured and colocalized with antigen-specific HLA class I–restricted CTL using tetramers. Our results demonstrated that spontaneous degranulation and IFN-γ production was specifically detected in HAM/TSP patients, but not ACs or NDs. This is consistent with the report demonstrating that without any stimuli CD8+ T cells from ACs and ATL patients did not produce IFN-γ in short-term culture.43 To our knowledge this present study is the first demonstration of spontaneous degranulation in a human disease and is consistent with the known high frequency of virus-specific CD8+ T cells in HAM/TSP patients associated with high HTLV-I proviral loads.9 Because CD107a/IFN-γ expression in CD8+ T cells of HAM/TSP patients was also clearly correlated with release of perforin and granzyme B from Tax tetramer+ CD8+ T cells, our results confirmed that both cytolytic activity and inflammatory cytokine production were induced in CD8+ T cells of HAM/TSP patients, as previously reported.8,19,48 Moreover, the observed spontaneous degranulation and IFN-γ production was specific for HTLV-I–specific CD8+ T cells because CMV-specific CD8+ T cells in HAM/TSP patients did not demonstrate such spontaneous degranulation. Collectively, these data further supports a role for HTLV-I–specific CD8+ T cells in pathogenesis of HAM/TSP.
IFN-γ production in CD8+ T cells requires higher peptide concentrations whereas degranulation can be evoked with lower concentrations of peptide indicating that CTL define 2 activation thresholds for polarized granule secretion and cytokine production.45,58 Differential mechanisms of CD8+ T-cell cytotoxicity are necessary to quickly kill infected cells at early stages of infection, to carefully regulate and control this potent effector cell population, and to avoid destruction of healthy tissues. Our results also indicated that HLA A*201 HTLV-I–specific CD8+ T-cell responses were dependent on the immunodominant Tax11-19 peptide concentration and was inhibited by anti–MHC class I, suggesting that spontaneous degranulation and IFN-γ production in CD8+ T cells of HAM/TSP patients resulted from high density of peptide-MHC complexes on the surface of target cells.
In HAM/TSP patients, HTLV-I–specific CD8+ T-cell dysfunctions have been reported to correlate with HTLV-I proviral load and Tax mRNA expression,7,9,27 suggesting that persistence of HTLV-I in CD4+ T cells would be the stimuli that drive the high frequency of virus-specific T cells in vivo, although it is difficult to detect high viral antigen production in HTLV-I–infected patients. Unexpectedly, CD107a/IFN-γ expression from CD8+ T cells was induced by coculture with isolated autologous CD14+ cells, not CD4+ T cells as previously reported.38 In the previous study, CD4+ cells was positively isolated from HTLV-I–infected patients, that might result in contamination of MPs into these isolated CD4+ cells because a subset of human MPs expresses CD4.59,60 However, as the addition of CD14+ cells to isolated CD8+ T cells did not fully restore the response seen in total PBMCs (Figure 4), involvement of other cell types in addition to CD14+ cells, such as CD14− DC, or stimulation by virus-producing CD4+ T cells, might also be associated with CD107a/IFN-γ expression in CD8+ T cells. More surprising was the observation that CD14+ cells effectively induced CD107a/IFN-γ expression in CD8+ T cells of HAM/TSP patients despite lower levels of endogenous HTLV-I proviral DNA and viral expression than in CD4+ T cells. Several viruses that induce chronic infections encode proteins to downmodulate immune systems by targeting of MHC class I.61 HTLV-I also encodes p12 protein which binds MHC class I and interferes with the trafficking to the cell surface,62 suggesting that high viral production in HTLV-I–infected cells may interfere with effective antigen presentation on APCs.
Our results also provided evidence that enhanced IL-15 on the surface of CD14+ cells mediated spontaneous degranulation and IFN-γ production from CD8+ T cells of HAM/TSP patients. Stimulation with LPS and several infectious agents, including bacteria and viruses, can induced IL-15 mRNA in MPs,63-65 which present IL-15 protein in trans to neighboring cells, such as CD8+ T cells and NK cells, to stimulate development of NK cell and proliferation and survival of CD8+ T cells with naive and memory phenotypes.32,53,66-68 After simian immunodeficiency virus infection, the accumulation, persistence, and maintenance of CTL in the brain are closely linked to the increased levels of IL-15 in the CNS of infected monkeys.69 In HTLV-I, IL-15 mRNA level are up-regulated in non-T cells isolated from HAM/TSP patients and DC treated with HTLV-I Tax protein in vitro.70,71 We showed that IL-15 expression was rapidly enhanced on the surface of CD14+ cells in HAM/TSP patients more than those in ACs. In addition, rhIL-15 induced CD107a/IFN-γ expression from CD8+ T cells of HTLV-I–infected patients, especially HAM/TSP patients, but not NDs that needed much higher concentrations of IL-15 to induce CD107a/IFN-γ expression. IL-15 was stored intracellularly, and its surface expression on monocytes rapidly occurred after stimulation without requirement of de novo protein synthesis.56 These results suggested that membrane retention of IL-15 on MPs provided the initiating signal associated with IL-15–induced bystander activation of antigen-specific T cells by cell-cell interaction. Interestingly, CD107a expression was detected in Tax11-19 tetramer+ cells but not CMV pp65 tetramer+ cells after the culture with IL-15. It was reported that high expression of IL-15Rα in HTLV-I Tax tetramer+ cells, compared with CMV pp65 tetramer+ cells, might be involved in increased HTLV-I–specific T-cell proliferation in HAM/TSP patients.72 Thus, the increase of IL-15 presentation by CD14+ cells in HAM/TSP patients might enhance cytolytic activity and inflammatory cytokine production from HTLV-I–specific CD8+ T cells.
In conclusion, we have demonstrated that HTLV-I–specific CD8+ T-cell dysregulation was mediated by virus infection and enhanced IL-15 on CD14+ cells in HAM/TSP patients. Although relationship of activated macrophage and microglia to the amount of HTLV-I proviral DNA in brain of HAM/TSP patients was demonstrated,73 there are little information on the extent and frequency of HTLV-I–infected MPs in vivo and what role they play in the immune response to HTLV-I. Therefore, virus-infected or activated MPs may be a heretofore important but under recognized reservoir for HTLV-I particularly in HAM/TSP patients who could induce bystander CD8+ T-cell responses by continuous stimulation through viral antigen presentation and surface-bound IL-15 in chronic HTLV-I–infected patients. Our results have important implications for the pathogenesis of the inflammatory neurologic disease associated with retroviral infection.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Y. Tanaka (University of the Ryukyus, Okinawa, Japan) for kindly providing us with anti-Tax monoclonal antibody (Lt-4).
This research was supported by the Intramural Research Program of the NINDS, NIH.
National Institutes of Health
Authorship
Contribution: Y.E-A. performed most of the experimental work and contributed to paper writing; U.O. coordinated clinical work and contributed to discussion and paper writing; C.G. supported molecular experiments and contributed to discussion and paper writing; and S.J. supervised the project and contributed to discussion and writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Jacobson, PhD, Viral Immunology Section, Neuroimmunology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health, 9000 Rockville Pike, Building 10 Room 5C-103, Bethesda, MD 20892; e-mail: jacobsons@ninds.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal