Abstract
Despite abundant activated virus-specific cytotoxic T lymphocytes (CTLs), patients with human T-lymphotropic virus type 1 (HTLV-1)–associated myelopathy/tropical spastic paraparesis (HAM/TSP) showed a significantly higher frequency of infected T cells than did healthy virus carriers (HVCs). Here, we demonstrate that at a given proviral load, the frequency of CD8+ T cells that are negative for specific costimulatory molecules was significantly higher in HAM/TSP than in age-matched HVCs and uninfected healthy controls (HCs), whereas the frequency of intracellular perforin-positive CD8+ T cells was significantly lower in both HAM/TSP and HVCs than in HCs. An inverse correlation between HTLV-1 proviral load (PVL) and percent perforin-positive CD8+ T cells were observed only in disease-protective allele HLA-A*02–positive HVCs, but not in HAM/TSP patients, whether HLA-A*02 positive or negative, nor in HLA-A*02–negative HVCs. Significantly lower perforin expression was observed in HTLV-1–specific than in cytomegalovirus-specific CD8+ T cells. Majority of HTLV-1–specific CD8+ T cells in HVCs showed a CD28−CD27+ phenotype, whereas HAM/TSP showed a CD28−CD27− phenotype. HTLV-1–specific CD8+ T cells from HAM/TSP patients showed significantly lower degranulation than HVCs by CD107a mobilization assay. These findings suggest that an impaired function of HTLV-1–specific CTLs is associated with failing antiviral control and disease HAM/TSP.
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1) is a replication-competent human retrovirus1,2 associated with adult T-cell leukemia (ATL)3,4 and a slowly progressive neurologic disorder, HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP).5,6 The main pathologic features of HAM/TSP are chronic inflammation in the spinal cord, characterized by perivascular lymphocytic cuffing and parenchymal lymphocytic infiltration including HTLV-1–infected CD4+ T cells.7 Unlike human immunodeficiency virus (HIV), HTLV-1 causes no disease in a majority of infected subjects (healthy asymptomatic virus carriers, HVCs). However, approximately 2% to 3% develop ATL and another 2% to 3% develop a disabling chronic inflammatory disease involving the central nervous system (HAM/TSP), eyes, lungs, or skeletal muscles.8 Our previous studies in HTLV-1 endemic to southern Japan indicated that the median proviral load (PVL) in peripheral blood mononuclear cells (PBMCs) of patients with HAM/TSP was more than 10 times higher than that in HVCs, and a high PVL was also associated with an increased risk of progression to disease.9 In the same population, HLA-A*02 and HLA-Cw*08 genes were independently and significantly associated with a lower PVL and a lower risk of HAM/TSP.10,11 Namely, possession of the HLA-A*02 allele, which efficiently presents several epitopes from HTLV-1 proteins to specific CTLs, was associated with protection against HAM/TSP as well as a lower PVL.11 These data suggest that class I–restricted CD8+ CTLs play an important part in controlling HTLV-1 PVL and reducing the risk of disease. Indeed, CD8+ T cells in fresh PBMCs of HTLV-1–infected patients rapidly kill autologous CD4+ cells naturally infected with HTLV-1 by a perforin-dependent mechanism.12,13 However, because HTLV-1–specific CD8+ T cells have the potential to produce proinflammatory cytokines,14 there is a debate on the role of HTLV-1–specific CD8+ T cells; that is, whether these cells contribute to the inflammatory and demyelinating processes of HAM/TSP, or whether the dominant effect of such cells in vivo is protective against disease, although these 2 mechanisms are not mutually exclusive.
Antigen-specific CTLs are crucial components of the immune response against viruses, and they have been shown to have an important role in limiting viral replication and controlling virus-associated diseases.15 A decisive role of HTLV-1–specific CTLs has also been suggested by host genetics,10,11 in vitro T-cell function,12,13 and DNA expression microarray analysis.16 However, in HTLV-1 infection, active and abundant HTLV-1–specific CTLs do not completely eliminate infected cells, especially in patients with HAM/TSP. We have therefore hypothesized that the differences in HTLV-1 PVL and associated differences in the risk of HAM/TSP should be associated with differences between patients in the efficiency of CTL-mediated lysis of infected cells. Because both positive and zero correlations have been found between the frequency of HTLV-1–specific CD8+ T cells and PVL,17-19 the frequency of HTLV-1–specific CTL itself is not an accurate indicator of CTL strength or effectiveness. Meanwhile, DNA expression microarray analysis suggested that a high level of expression of lymphocyte lysis-related genes, including granzymes, perforin, granulysin, and NKG2D, was associated with lower PVL both in patients with HAM/TSP and in HVCs.16 Moreover, differentiation of CD8+ T cells and their functional profile, such as secretion of cytotoxic molecules, cytokines, and degranulation markers, seems to play a key role in controlling several other chronic viral infections such as herpes simplex virus (HSV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), and HIV.20-22 In this study, based on these observations, we have evaluated the expression of costimulatory markers, cytotoxic granules (perforin and GzmB), and degranulation marker CD107a in CD8+ T cells from HTLV-1–infected patients (HAM/TSP and HVCs) and age-matched healthy controls (HCs), and compared them with HTLV-1 PVL.
Methods
Patients and cells
Peripheral blood was studied from 72 patients with a clinical diagnosis of HAM/TSP, 96 HVCs, and 20 HCs in total. Characteristics of these patients are shown in Table 1. Fresh PBMCs were isolated on a Histopaque-1077 (Sigma-Aldrich, St Louis, MO) density gradient centrifugation, washed twice in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS), and stored in liquid nitrogen as stocked lymphocytes until use. All experiments were performed by using age-matched samples selected from stocked lymphocytes. The diagnosis of HAM/TSP was made according to the World Health Organization diagnostic criteria.23 All patients and control subjects were Japanese and resided in Kagoshima Prefecture, an HTLV-1 endemic region in southern Japan. Samples from patients with HAM/TSP were studied only if the patients had never received any immunomodulators (oral steroid or interferon-α injection) or more than 10 years had passed since the last immune-modulating therapies. This research was approved by the institutional review boards of the authors' institutions and informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Lymphocyte phenotyping and perforin detection by flow cytometry
After thawing, cells were washed 3 times with phosphate-buffered saline (PBS) and fixed in PBS containing 2% paraformaldehyde (Sigma-Aldrich) for 20 minutes and resuspended in PBS at 4°C. Fixed cells were washed with PBS containing 7% of normal goat serum (Sigma-Aldrich) and then incubated for 15 minutes at room temperature with various combinations of fluorescence-conjugated monoclonal antibodies (mAbs) as follows: phycoerythrin-cyanin 5.1 (PC5)–labeled anti-CD27, PC5-labeled anti-CD28, PC5-labeled anti-CD8, energy-coupled dye (ECD)–labeled anti-CD8 (Beckman Coulter, Fullerton, CA), phycoerythrin (PE)–labeled anti-CD28, fluorescein isothiocyanate (FITC)–labeled antiperforin and GzmB (BD PharMingen, San Diego, CA). Isotype-matched mouse immunoglobulins were used as a control. The phenotype was determined by flow cytometry (EPICS XL; Beckman Coulter) in the lymphocyte gate based on forward versus side scatter. For intracellular staining of perforin and GzmB, PBMCs were first surface stained with the ECD-labeled anti-CD8 mAb for 15 minutes at room temperature. After cell-surface labeling, cells were washed and permeabilized with PBS/7% normal goat serum containing 0.2% saponin (PBS-SAPO; Sigma-Aldrich) for 10 minutes at room temperature. The cells were then washed twice and resuspended for 20 minutes at room temperature in PBS-SAPO containing FITC-labeled anticytotoxin mAbs (perforin, GzmB; BD PharMingen) or an isotype control mAb. Finally, the cells were washed twice and analyzed by an EPICS XL flow cytometer and EXPO32 analysis software (Beckman Coulter) in the lymphocyte gate based on forward versus side scatter.
Detection of HTLV-1 Tax– and CMV pp65–specific CTLs by HLA class I tetramer
To evaluate the expression of perforin in antigen-specific CD8+ T cells, CD8+ T cells were stained with fluorescent-labeled tetramers of HLA-A*02 + β2 microglobulin + Tax11-19 (LLFGYPVYV) or CMV pp65 (NLVPMVATV) peptides, which were purchased from Medical Biological Laboratories (Nagoya, Japan). PBMCs were incubated with each tetramer and ECD-labeled anti-CD8 mAb at 37°C for 25 minutes then washed 3 times in ice-cold PBS, fixed in 2% paraformaldehyde for 20 minutes at 4°C. Fixed PBMCs were then permeabilized with PBS/7% normal goat serum (NGS) containing 0.2% PBS-SAPO for 10 minutes at room temperature for intracellular staining of perforin and GzmB. The cells were washed again and resuspended for 20 minutes at room temperature in PBS-SAPO containing anticytotoxin mAbs (perforin, GzmB) or an isotype control mAb. Finally, the cells were washed and analyzed by flow cytometry. At least 5 × 104 events were collected in each assay.
Quantification of HTLV-1 proviral load and anti–HTLV-1 antibody titers
To examine the HTLV-1 PVL, we carried out a quantitative polymerase chain reaction (PCR) method using ABI Prism 7700 (Applied Biosystems, Foster City, CA) with 100 ng genomic DNA (roughly equivalent to 104 cells) from PBMC samples, as reported previously.9 Based on the standard curve created by 4 known concentrations of template, the concentrations of unknown samples were determined. Using β-actin as an internal control, the amount of HTLV-1 proviral DNA was calculated by the following formula: copy number of HTLV-1 tax per 1 × 104 PBMC = [(copy number of tax) / (copy number of β-actin/2)] × 104. All samples were performed in triplicate. Serum antibody titers to HTLV-1 were determined by a particle agglutination method.
CD107a mobilization assay
To evaluate cell-mediated cytotoxicity of HTLV-1–specific CD8+ T cells in patients with HAM/TSP and HVCs, we performed a CD107a mobilization assay.24,25 This assay enables rapid assessment of cell-mediated cytotoxicity via sensitive detection of CD107a exposed on the cell surface after antigen stimulation and subsequent secretion of the lytic granule contents such as perforin and granzymes. PBMCs (2 × 106) were cultured for 4 hours with or without 1 μg/mL HTLV-1 Tax peptide in combination with FITC-labeled anti-CD107a mAb and the secretion inhibitor monensin in RPMI 1640 complete medium with 50 IU/mL IL-2, according to the manufacturer's instruction (IMMUNOCYTO CD107a detection kit; MBL, Nagoya, Japan). After incubation, cell suspensions were washed with PBS and the cells were further stained with Tax-tetramer–PE and PC5-labeled anti-CD8 mAb (Beckman Coulter). A minimum of 3 × 104 CD8+ T lymphocytes per sample were acquired by flow cytometry.
Statistical analysis
To test for significant differences among the cell populations between 3 different groups of subjects (HAM/TSP, HVCs, and HCs), one-factor analysis of variance (ANOVA) was done when the variance of each group was equal by Levene test. If the variance of each group was different, the Kruskal-Wallis test was used. For multiple comparisons, we used Sheffe F to analyze statistical difference. The Mann-Whitney U test was used for comparing the differences in the frequencies of cell populations or PVL between patients with HAM/TSP and HVCs. The results represent the mean plus or minus the standard error (SE) where applicable. Correlations between variables were examined by Spearman rank correlation analysis. All statistical analyses were performed using SPSS version 13.5 software (SPSS, Chicago, IL). Values of P less than .05 were considered statistically significant.
Results
The expression of costimulatory molecules on T cells of HTLV-1–infected patients and uninfected controls
To determine whether the frequency of costimulatory molecules differed between patients with HAM/TSP and HVCs as well as HCs, we stained PBMCs with monoclonal antibodies to CD27, CD28, CD80, CD86, and CD152 (cytotoxic T-cell lymphocyte–associated antigen-4 [CTLA-4]) in 3 groups of age-matched patients with HAM/TSP, HVCs, and HCs (Table 2). The percentages of CD8+ cells that were negative for costimulatory molecules were significantly higher in patients with HAM/TSP than in HCs; however, these differences were not observed between HVCs and HCs. Because the mean HTLV-1 PVL of the HAM/TSP group was significantly higher than that of the HVCs (HAM/TSP 805.1 ± 461.0 vs HVCs 354.5 ± 106.2, P = .001, Mann-Whitney), we further compared the frequencies of each costimulatory molecule between patients with HAM/TSP and the HVCs with a similar PVL (HAM/TSP 529.8 ± 79.0 vs HVCs 486.0 ± 104.5, P = .79, Mann-Whitney). The mean frequency of each costimulatory molecule was still significantly higher in patients with HAM/TSP than HCs except for CD8+ CD152− (Table 2), and there was no correlation between the mean frequency of each costimulatory molecule and PVL (data not shown). These data indicate that the observed differences do not simply reflect differences in PVL (ie, in the frequency of HTLV-1–infected cells). Furthermore, although HTLV-1 mainly infects CD4+ cells, the percentages of CD4+ cells that were negative for costimulatory molecules were not significantly different between patients with HAM/TSP and HVCs or HCs (Table S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), indicating that the mean frequency of each costimulatory molecule on CD4+ cells was not influenced by the PVL.
Expression of perforin and granzyme B in CD8+CD28− and CD8+CD27− T-cell populations of patients with HAM/TSP and healthy virus carriers
We next focused on the CD8+CD28− T cells and CD8+CD27− T cells, because these subsets include CTLs and because abnormal expansion of these subsets was also described in chronic CMV,26 EBV,27 and HIV20 infection. As shown in Table 2, both percent CD8+CD28− and percent CD8+CD27− T-cell populations in PBMCs were significantly higher in patients with HAM/TSP than in HVCs and HCs, whereas there were no significant differences of percent CD8+CD28− and percent CD8+CD27− T-cell populations between HVCs and HCs. Interestingly, as typical examples show in Figure 1A, the percentages of perforin+CD8+, perforin+CD8+CD28−, and perforin+CD8+CD27− T cells were significantly lower in HTLV-1–infected patients (patients with HAM/TSP and HVCs) than in HCs, irrespective of PVL (Figure 1B,D). The differences between HVCs and patients with HAM/TSP were smaller than the differences between HCs and HVCs or patients with HAM/TSP alone, indicating that a selective decrease of perforin expression in CD8+ T cells was associated with persistent HTLV-1 infection. The mean fluorescence intensity (MFI) of the perforin+CD8+, perforin+CD8+CD28−, and perforin+CD8+CD27− T-cell populations were significantly lower in patients with HAM/TSP than in HCs, irrespective of PVL (Figure 1B,D). In contrast to the perforin expression, both the percentages and MFI of the GzmB+CD8+, GzmB +CD8+CD28−, and GzmB +CD8+CD27− T-cell populations were not significantly different among the 3 subject groups (patients with HAM/TSP, HVCs, and HCs) when HTLV-1–infected patients were compared with a similar proviral load (Figure 1C,E).
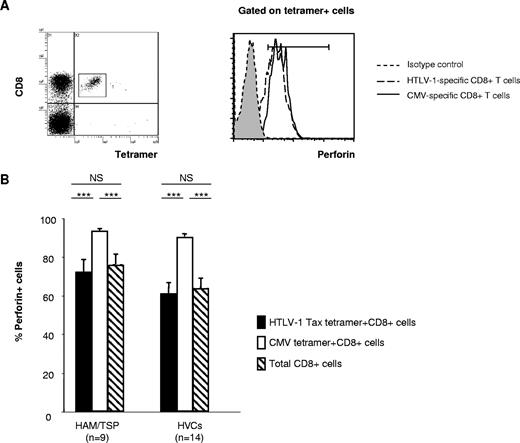
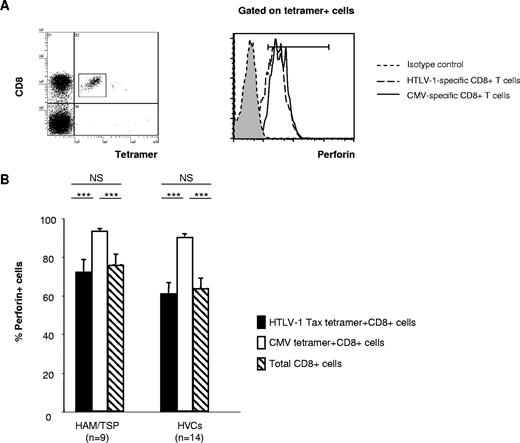
Frequencies and mean fluorescence intensities of perforin- and granzyme B–positive CD8+ T-cell populations in HTLV-1–infected patients. (A) Representative dot plots of PBMCs stained for cytotoxic function markers and costimulatory molecules. PBMCs from patients with HAM/TSP, asymptomatic healthy virus carriers (HVCs), and uninfected healthy controls (HCs) were costained for CD8, CD27, or CD28, and markers associated with cytotoxic function (ie, perforin or granzyme B [GzmB]). Cells in a CD8+ lymphocyte gate were analyzed. Representative dot plots from 1 uninfected control and 1 HAM/TSP patient are shown. (B) The frequency and mean fluorescence intensity (MFI) of the perforin-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in patients with HAM/TSP, HVCs, and HCs. (C) Frequency and MFI of the GzmB-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in patients with HAM/TSP, HVCs, and HCs. (D) Frequency and MFI of the perforin-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in proviral load (PVL)–matched patients with HAM/TSP, HVCs, and HCs. (E) The frequency and MFI of the GzmB-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in PVL-matched patients with HAM/TSP, HVCs, and HCs. The frequency of perforin- or GzmB-positive cells is shown as a percentage within each cell subset (CD8+, CD8+CD28−, CD8+CD27−). Values represent the means plus or minus the standard error (SE). To test for significant differences among the cell populations between 3 different groups of subjects (HAM/TSP, HVCs, and HCs), one-factor ANOVA was done when the variance of each group was equal by Levene test. If the variance of each group was different, the Kruskal-Wallis test was used. For multiple comparisons, we used Sheffe F to analyze statistical difference. Values of P < .05 were considered statistically significant. ***P < .001; **P < .01; *P < .05.
Frequencies and mean fluorescence intensities of perforin- and granzyme B–positive CD8+ T-cell populations in HTLV-1–infected patients. (A) Representative dot plots of PBMCs stained for cytotoxic function markers and costimulatory molecules. PBMCs from patients with HAM/TSP, asymptomatic healthy virus carriers (HVCs), and uninfected healthy controls (HCs) were costained for CD8, CD27, or CD28, and markers associated with cytotoxic function (ie, perforin or granzyme B [GzmB]). Cells in a CD8+ lymphocyte gate were analyzed. Representative dot plots from 1 uninfected control and 1 HAM/TSP patient are shown. (B) The frequency and mean fluorescence intensity (MFI) of the perforin-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in patients with HAM/TSP, HVCs, and HCs. (C) Frequency and MFI of the GzmB-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in patients with HAM/TSP, HVCs, and HCs. (D) Frequency and MFI of the perforin-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in proviral load (PVL)–matched patients with HAM/TSP, HVCs, and HCs. (E) The frequency and MFI of the GzmB-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in PVL-matched patients with HAM/TSP, HVCs, and HCs. The frequency of perforin- or GzmB-positive cells is shown as a percentage within each cell subset (CD8+, CD8+CD28−, CD8+CD27−). Values represent the means plus or minus the standard error (SE). To test for significant differences among the cell populations between 3 different groups of subjects (HAM/TSP, HVCs, and HCs), one-factor ANOVA was done when the variance of each group was equal by Levene test. If the variance of each group was different, the Kruskal-Wallis test was used. For multiple comparisons, we used Sheffe F to analyze statistical difference. Values of P < .05 were considered statistically significant. ***P < .001; **P < .01; *P < .05.
There was a marginal negative correlation between percent perforin+CD8+ T cells and HTLV-1 PVL in all HTLV-1–infected patients (patients with HAM/TSP + HVCs; P = .046, r = −0.29 by Spearman rank correlation analysis) but not in patients with HAM/TSP or HVCs alone (Figure 2 left panels). Meanwhile, a significant negative correlation between percent perforin+CD8+ T cells and HTLV-1 PVL was observed in all HLA-A*02–positive HTLV-1–infected patients (patients with HAM/TSP + HVCs; P = .003, r = −0.58) and HVCs alone (P = .024, r = −0.64), but not in patients with HAM/TSP (P = .15, r = −0.35; Figure 2 right panels). No correlation was observed between expression of GrzB and HTLV-1 PVL in patients with HAM/TSP, HVCs, or both groups combined, irrespective of HLA status (data not shown).
Perforin-positive CD8+ T cells and HTLV-1 proviral load in HTLV-1 infection. (Left panels) There was a marginal negative correlation between the percentages of perforin-positive CD8+ T cells and HTLV-1 proviral load (PVL) in whole HTLV-1–infected patients (HAM/TSP + HVCs; P = .046, r = −0.29 by Spearman rank correlation analysis), but not in patients with HAM/TSP or in HVCs alone. (Right panels) There was a significant negative correlation between the percentages of perforin-positive CD8+ T cells and HTLV-1 PVL in whole HLA-A*02–positive HTLV-1–infected patients (HAM/TSP + HVCs; P = .003, r = −0.58) and in HVCs alone (P = .024, r = −0.64), but not in patients with HAM/TSP (P = .15, r = −0.35). The x axis denotes percentage of perforin-positive CD8+ T cells, and the y axis denotes HTLV-1 PVL (copy number of HTLV-1 tax per 104 PBMCs = [(copy number of tax)/(copy number of β-actin/2)] × 104). Data were analyzed by Spearman rank correlation. The solid line represents a least-squares regression line.
Perforin-positive CD8+ T cells and HTLV-1 proviral load in HTLV-1 infection. (Left panels) There was a marginal negative correlation between the percentages of perforin-positive CD8+ T cells and HTLV-1 proviral load (PVL) in whole HTLV-1–infected patients (HAM/TSP + HVCs; P = .046, r = −0.29 by Spearman rank correlation analysis), but not in patients with HAM/TSP or in HVCs alone. (Right panels) There was a significant negative correlation between the percentages of perforin-positive CD8+ T cells and HTLV-1 PVL in whole HLA-A*02–positive HTLV-1–infected patients (HAM/TSP + HVCs; P = .003, r = −0.58) and in HVCs alone (P = .024, r = −0.64), but not in patients with HAM/TSP (P = .15, r = −0.35). The x axis denotes percentage of perforin-positive CD8+ T cells, and the y axis denotes HTLV-1 PVL (copy number of HTLV-1 tax per 104 PBMCs = [(copy number of tax)/(copy number of β-actin/2)] × 104). Data were analyzed by Spearman rank correlation. The solid line represents a least-squares regression line.
Lower perforin expression in HTLV-1–specific CD8+ T cells than in CMV-specific CD8+ T cells within HTLV-1–infected patients
Perforin is essential for killing targets of CTLs by means of the granule exocytosis pathway, and its expression is more restricted than that of serine proteases. As we observed significant differences in perforin expression in CD8+ T cells lacking the costimulatory molecules in PBMCs between HTLV-1–infected patients and healthy controls, and a significant negative correlation between percent perforin+CD8+ T cells and HTLV-1 PVL in HLA-A*02–positive HTLV-1–infected patients, we next focused on the perforin expression in HTLV-1–specific CD8+ T cells and CMV-specific CD8+ T cells in the same group of HTLV-1–infected patients (Figure 3A). Although there was no significant difference in percent perforin expression in virus-specific (both HTLV-1 Tax–specific and CMV-specific) CD8+ T cells between patients with HAM/TSP and HVCs, the percentage perforin expression in HTLV-1 Tax–specific CD8+ T cells was significantly lower than CMV-specific CD8+ T cells in HTLV-1–infected patients (both patients with HAM/TSP and HVCs) (Figure 3B). The expression of perforin in CMV-specific CD8+ T cells was always higher than the expression of perforin in Tax-specific CD8+ T cells in the same patients in both patients with HAM/TSP and HVCs.
Perforin expression in HTLV-1 Tax– and CMV pp65–specific CD8+ T cells in HTLV-1 infection. (A) Left panel: Dot plots of HLA-A*02/Tax11-19 tetramer-PE (x axis) versus CD8-ECD fluorescence within the lymphocyte gate based on forward versus side scatter. Right panel: Histogram of perforin expression (x axis, arbitrary units, log scale) versus cell number (y axis) in gated virus-specific (tetramer-positive) cells. Representative dot plot from one patient with HAM/TSP is shown. (B) The perforin expression in HTLV-1 Tax– and CMV pp65–specific CD8+ T cells in HTLV-1–infected patients. Statistical analysis was done as described in the legend for Figure 1. ***P < .001
Perforin expression in HTLV-1 Tax– and CMV pp65–specific CD8+ T cells in HTLV-1 infection. (A) Left panel: Dot plots of HLA-A*02/Tax11-19 tetramer-PE (x axis) versus CD8-ECD fluorescence within the lymphocyte gate based on forward versus side scatter. Right panel: Histogram of perforin expression (x axis, arbitrary units, log scale) versus cell number (y axis) in gated virus-specific (tetramer-positive) cells. Representative dot plot from one patient with HAM/TSP is shown. (B) The perforin expression in HTLV-1 Tax– and CMV pp65–specific CD8+ T cells in HTLV-1–infected patients. Statistical analysis was done as described in the legend for Figure 1. ***P < .001
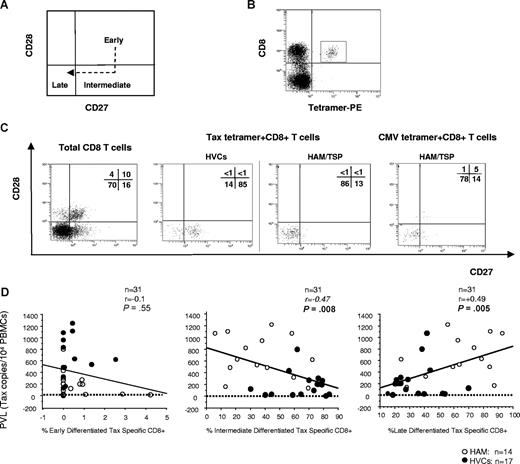
HTLV-1 Tax–specific CD8+ T cells in patients with HAM/TSP and HVCs appear to be at different expression patterns of costimulatory molecule
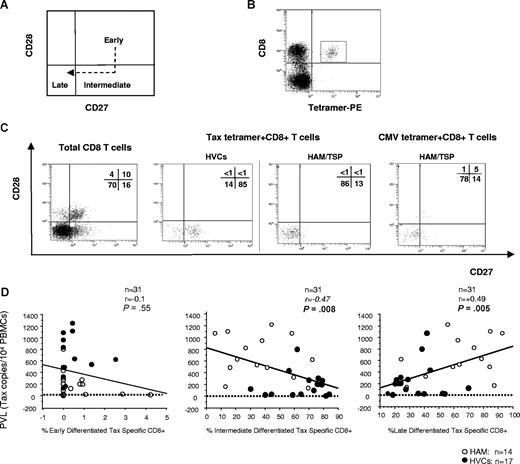
Because it has been reported that the cell-surface phenotype of virus-specific cells was affected by the stage of infection (ie, acute or chronic) as well as by viral specificity,20 we further investigated whether the cell-surface phenotype of HTLV-1–specific CD8+ cells differed between patients with HAM/TSP and HVCs. According to a simple classification of 3 functional subsets based on CD28 and CD27 expression (ie, early [CD28+CD27+], intermediate [CD28−CD27+], and late [CD28−CD27−] differentiated T cells; Figure 4A),20 more than 60% of HTLV-1 Tax–specific CD8+ T cells from patients with HAM/TSP showed late differentiated phenotype, whereas more than 60% of HTLV-1 Tax–specific CD8+ T cells from HVCs showed intermediate differentiated phenotype (Table 3 and Figure 4B,C). These phenotypes did not change even when we compared the frequencies between patients with HAM/TSP and the HVCs with a similar PVL (P = .11, Mann-Whitney U test, Table 3). As previously reported, more than 80% of CMV-tetramer–specific CD8+ T cells both from patients with HAM/TSP and HVCs showed a late differentiated phenotype (Table 3 and Figure 4B,C).20 As shown in Figure 4D, there was a negative correlation between percent Tax-specific intermediate differentiated T cells and HTLV-1 PVL in the whole cohort (n = 31; P = .008, r = −0.47 by Spearman rank correlation analysis) whereas there was a positive correlation between percent Tax-specific late differentiated T cells and HTLV-1 PVL in the whole cohort (n = 31; P = .005, r = 0.49 by Spearman rank correlation analysis). There was no correlation between percent Tax-specific early differentiated T cells and HTLV-1 PVL in the whole cohort (n = 31; P = .55, r = −0.1 by Spearman rank correlation analysis).
CD27 and CD28 coexpression on virus-specific CD8+ T cells in HTLV-1 infection. Flow cytometric analysis of CD27 and CD28 coexpression gated on HLA-A*02/Tax11-19 tetramer–positive cells. (A) The model of antigen-specific CD8+ T-cell differentiation based on the expression of CD27 and CD28. In the CD8+ T-cell population, early differentiated cells differentiate into late-differentiated cells, following a stage of intermediate cells that have down-regulated CD28, but not yet CD27. (B) Dot plots of HLA-A*02/Tax11-19 tetramer-PE (x axis) versus CD8-ECD fluorescence within the lymphocyte gate based on forward versus side scatter. (C) CD28/CD27 expression on total CD8+ T-cell– and HTLV-1– or CMV-specific T cells detected by tetramers were shown. The HTLV-1 Tax–specific CD8 T cells were enriched in intermediate-differentiated stage in HVCs and in late-differentiated stage in patients with HAM/TSP, whereas there was no difference in distribution of CMV pp65–specific CD8 T cells between patients with HAM/TSP and HVCs. Representative dot plots from 1 HVC and 1 patient with HAM/TSP for HTLV-1–specific cells, and 1 HAM/TSP patient for CMV-specific cells are shown. (D) Correlation between PVL (y axis; copy number of HTLV-1 tax per 104 PBMCs) and “early,” “intermediate,” or “late” phenotype of HTLV-1 Tax–specific cells (x axis; percentage of Tax-tetramer–positive cells in each subset) in PBMCs from HTLV-1–infected patients. Data were analyzed by Spearman rank correlation.
CD27 and CD28 coexpression on virus-specific CD8+ T cells in HTLV-1 infection. Flow cytometric analysis of CD27 and CD28 coexpression gated on HLA-A*02/Tax11-19 tetramer–positive cells. (A) The model of antigen-specific CD8+ T-cell differentiation based on the expression of CD27 and CD28. In the CD8+ T-cell population, early differentiated cells differentiate into late-differentiated cells, following a stage of intermediate cells that have down-regulated CD28, but not yet CD27. (B) Dot plots of HLA-A*02/Tax11-19 tetramer-PE (x axis) versus CD8-ECD fluorescence within the lymphocyte gate based on forward versus side scatter. (C) CD28/CD27 expression on total CD8+ T-cell– and HTLV-1– or CMV-specific T cells detected by tetramers were shown. The HTLV-1 Tax–specific CD8 T cells were enriched in intermediate-differentiated stage in HVCs and in late-differentiated stage in patients with HAM/TSP, whereas there was no difference in distribution of CMV pp65–specific CD8 T cells between patients with HAM/TSP and HVCs. Representative dot plots from 1 HVC and 1 patient with HAM/TSP for HTLV-1–specific cells, and 1 HAM/TSP patient for CMV-specific cells are shown. (D) Correlation between PVL (y axis; copy number of HTLV-1 tax per 104 PBMCs) and “early,” “intermediate,” or “late” phenotype of HTLV-1 Tax–specific cells (x axis; percentage of Tax-tetramer–positive cells in each subset) in PBMCs from HTLV-1–infected patients. Data were analyzed by Spearman rank correlation.
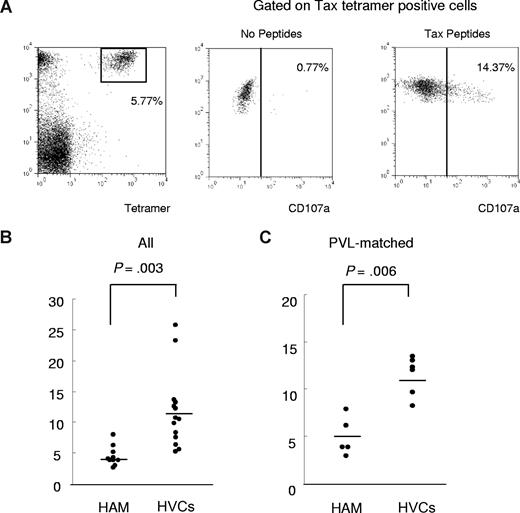
CD107a mobilization assays
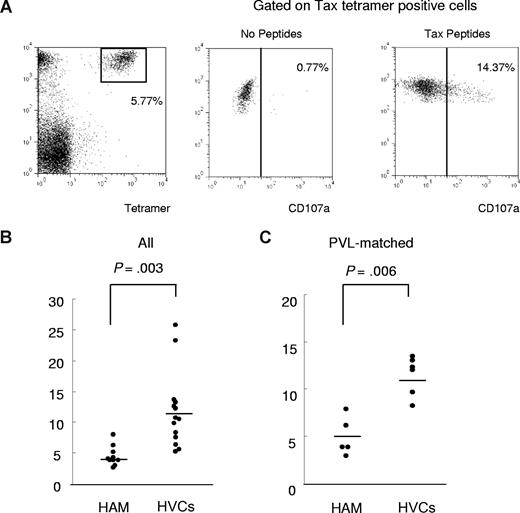
To determine the functional reactivity of HTLV-1 Tax-specific CD8+ T cells, we performed a CD107a mobilization assay (Figure 5). PBMCs derived from HLA-A*02–positive HTLV-1–infected patients (patients with HAM/TSP and HVCs) were stained with HLA-A*02/ Tax11-19 tetramer after incubation with Tax11-19 peptide and anti-CD107a monoclonal antibody for 4 hours. Significant anti-CD107a staining was observed on the Tax-tetramer–positive population after coculture with Tax11-19 peptide, whereas only background staining was seen without peptide (Figure 5A). We compared the frequency of expression of the degranulation marker CD107a in Tax-tetramer–positive cells, which allows measurement of cytolytic cell activation, between 10 patients with HAM/TSP and 14 HVCs. The percentage of CD107a+ HLA-A*02/Tax11-19 tetramer–positive cells within the HLA-A*02/Tax11-19 tetramer–positive gate (percent Tet+ CD107a+/ Tet+) was significantly lower in patients with HAM/TSP than in HVCs (Figure 5B). The same results were obtained in a comparison between 5 patients with HAM/TSP and 6 HVCs with similar PVL (patients with HAM/TSP 307.2 ± 125.3 vs HVCs 270.0 ± 92.2, P = .57, Mann-Whitney), indicating that the observed difference did not simply reflect the higher PVL (ie, the higher frequency of HTLV-1–infected T cells) in patients with HAM/TSP (Figure 5.C).
CD107a expression in patients with HAM/TSP and in HVCs after coculture with immunodominant Tax peptide. To determine the functional reactivity of HTLV-1 Tax–specific CD8+ T cells, we performed a CD107a mobilization assay. PBMCs derived from HLA-A*02–positive HTLV-1–infected patients (patients with HAM/TSP and HVCs) were stained with anti–CD8-PC5 and HLA-A*02/Tax11-19 tetramer after incubation with Tax11-19 peptide and anti-CD107a monoclonal antibody for 4 hours. (A) Left panel: Dot plots of HLA-A*02/Tax11-19 tetramer-PE (x axis) versus CD8-PC5 (y axis) fluorescence within the lymphocyte gate based on forward versus side scatter. Significant anti-CD107a staining was observed on the Tax-tetramer–positive population after coculture with Tax11-19 peptide (left panel), whereas only background staining was seen without peptide (center panel). (B) Comparison of degranulation marker CD107a expression in Tax-tetramer–positive cells between 10 patients with HAM/TSP and 14 HVCs. The percentage of CD107a+ Tax11-19 tetramer–positive cells/ Tax11-19 tetramer–positive cells was significantly decreased in patients with HAM/TSP compared with HVCs (P = .003, Mann Whitney). (C) The CD107a staining was still significantly lower in patients with HAM/TSP than in HVCs compared between PVL-matched groups (P = .006, Mann-Whitney). Horizontal bars represent the median value of percent CD107a+ Tax11-19 tetramer–positive cells/Tax11-19 tetramer–positive cells.
CD107a expression in patients with HAM/TSP and in HVCs after coculture with immunodominant Tax peptide. To determine the functional reactivity of HTLV-1 Tax–specific CD8+ T cells, we performed a CD107a mobilization assay. PBMCs derived from HLA-A*02–positive HTLV-1–infected patients (patients with HAM/TSP and HVCs) were stained with anti–CD8-PC5 and HLA-A*02/Tax11-19 tetramer after incubation with Tax11-19 peptide and anti-CD107a monoclonal antibody for 4 hours. (A) Left panel: Dot plots of HLA-A*02/Tax11-19 tetramer-PE (x axis) versus CD8-PC5 (y axis) fluorescence within the lymphocyte gate based on forward versus side scatter. Significant anti-CD107a staining was observed on the Tax-tetramer–positive population after coculture with Tax11-19 peptide (left panel), whereas only background staining was seen without peptide (center panel). (B) Comparison of degranulation marker CD107a expression in Tax-tetramer–positive cells between 10 patients with HAM/TSP and 14 HVCs. The percentage of CD107a+ Tax11-19 tetramer–positive cells/ Tax11-19 tetramer–positive cells was significantly decreased in patients with HAM/TSP compared with HVCs (P = .003, Mann Whitney). (C) The CD107a staining was still significantly lower in patients with HAM/TSP than in HVCs compared between PVL-matched groups (P = .006, Mann-Whitney). Horizontal bars represent the median value of percent CD107a+ Tax11-19 tetramer–positive cells/Tax11-19 tetramer–positive cells.
Discussion
To characterize the HTLV-1–specific CD8+ T cells in infected patients, we first examined the expression of costimulatory molecules on CD4+ and CD8+ T cells, which have a significant impact on the cytokine profile and proliferation response. Interestingly, the percentages of CD8+ T cells that were negative for costimulatory molecules CD27, CD28, CD152 (CTLA-4), CD80 (B7-1), and CD86 (B7-2) were significantly higher in patients with HAM/TSP compared with HVCs or HCs, whereas this discrepancy was not found within the CD4+ T-cell population. Namely, there was a selective decrease of costimulatory molecule expression on CD8+ T cells in patients with HAM/TSP. It is noteworthy that the same differences were observed in a comparison between patients with HAM/TSP and HVCs with a similar PVL, whereas there were no significant differences between the patients with HAM/TSP and HVCs in either the frequency of CD4+ cells negative for costimulatory molecules or the PVL in PBMCs. These findings suggest that the expression of costimulatory molecules was influenced by the disease status rather than the PVL. The emergence of high frequencies of CD8+ T cells negative for costimulatory molecules might have been caused by a greater degree of continuous or repeated antigenic stimulation in patients with HAM/TSP than in HVCs at a similar PVL; such repeated antigenic stimulation might lead immune cells into a loss of antiviral CD8+ T-cell function, as suggested in HIV infection. In fact, it has been reported that the number of naive T cells was low in HTLV-1–infected patients compared with uninfected controls, whereas the number of memory T lymphocytes was greater in HTLV-1–infected patients.28 This finding supports the rapid turnover of T cells by antigenic stimulation in HTLV-1–infected patients, which has recently been directly demonstrated by metabolic labeling of lymphocytes in vivo in human HTLV-1–infected subjects.29 We next focused on the CD8+CD28− T cells and CD8+CD27− T cells, because these subsets include CTLs and abnormal expansion of these subsets was also described in chronic CMV,26 EBV,27 and HIV20 infection. Especially, increased expression of CD8+CD28− T cells was associated with disease activity and CD8+ T-cell dysfunction in HIV infection.30,31 As expected, the percentages of CD8+ cells that were negative for CD28 or CD27 were significantly increased in patients with HAM/TSP by comparison with HVCs or HCs, irrespective of PVL.
Because impaired CTL-mediated lysis is a general feature of chronic HIV, CMV, and EBV infections,32 especially when the antigen load is high,33 it is important to examine the phenotypic and functional property of CD8+ T cells in HTLV-1 infection. Our data showed that the frequency of perforin and GzmB+CD8+, CD8+CD28−, and CD8+CD27− T cells in PBMCs was significantly lower in HTLV-1–infected patients (patients with HAM/TSP and HVCs) than in HCs. Interestingly, although both the percentage and MFI of perforin+CD8+, perforin+CD8+CD28−, and perforin+CD8+CD27− T-cell populations were significantly lower in HTLV-1–infected patients (patients with HAM/TSP and HVCs) than in HCs, irrespective of PVL, the MFIs of GzmB+CD8+, GzmB +CD8+CD28−, and GzmB+CD8+CD27− T-cell populations were not significantly different among patients with HAM/TSP, HVCs, and HCs compared with similar PVLs. Such discordant expression patterns between perforin and granzymes are a common feature of circulating virus-specific CD8+ T cells in chronic HIV, CMV, and EBV infection.32,34-37 Because perforin staining often lessens after T-cell activation, presumably because of the release of preformed perforin,34 decreased perforin expression in HTLV-1 infection, especially patients with HAM/TSP, might arise from aberrant perforin secretion or from frequent discharge of perforin caused by constant antigenic stimulation.38 In this case, the higher MFI of GzmB compared with perforin in CD8+ T cells of patients with HAM/TSP might be a result of the lack of granzyme release caused by lower perforin levels. Interestingly, there was a significant negative correlation between percent perforin+CD8+ T cells and HTLV-1 PVL in HTLV-1–infected HLA-A*02–positive HVCs, but not in HLA-A*02–negative HVCs and patients with HAM/TSP who were either HLA-A*02 positive or negative. As the possession of HLA-A*02 was associated with a significant reduction in both HTLV-1 PVL and the risk of HAM/TSP,11 these findings suggest that the HLA-A*02–restricted CTLs are particularly efficient at killing HTLV-1–infected cells.39 The absence of correlation between perforin-expressing CD8+ T cells and HTLV-1 PVL in patients with HAM/TSP also supports the hypothesis of CD8+ T-cell inefficiency in the diseased situation.
A large number of phenotypic markers have been used to define different populations of antigen-specific CD4+ and CD8+ T cells, and these markers have been proposed to identify functionally distinct T-cell populations and different stages of T-cell function. A previous study indicated that HIV-specific CD8+ T cells appear to have a different cell-surface phenotype from CMV-specific CD8+ T cells,20 and the lack of perforin in HIV-specific populations was correlated with decreased cytotoxic activity compared with CMV-specific populations.34 In this study, we have also shown that the cell-surface phenotypes of virus-specific CD8+ T cells are distinct among HVCs and patients with HAM/TSP, irrespective of PVL, indicating distinct characteristics of the anti–HTLV-1 immune response in infected patients with different disease status. The observed increase in the frequencies of CD8+, CD8+CD28−, and CD8+CD27− T-cell population in PBMCs in HTLV-1 infection without correlation with perforin or granzyme expression in the corresponding cells suggests that the variation in perforin and GzmB staining among different subjects with HTLV-1 infection results from different levels of antigenic stimulation among these hosts. In addition, the costimulatory molecule expression, perforin content, and GzmB content might represent the state of activation of these cells when viral antigen expression reaches equilibrium with the CTL population. It is highly likely that this point of equilibrium will differ not only between different viral infections because of different kinetics of viral expression, but also between patients with a different “efficiency” of the HTLV-1–specific CTL response.
Finally, we performed a CD107a mobilization assay to compare the functional reactivity of HTLV-1–specific CD8+ T cells between patients with HAM/TSP and HVCs, because the CD107a is much closer to the actual effector function than the equilibrium content of perforin and GzmB. After cultivation with Tax11-19 peptide, significantly higher anti-CD107a staining was observed in Tax-tetramer–positive CD8+ T cells in HVCs than in patients with HAM/TSP. This was also the case when we compared patients with HAM/TSP and HVCs with a similar PVL, indicating that the observed differences were not attributable to the difference in PVL between patients with HAM/TSP and HVCs. Rather, the data suggest that the CTLs in PBMCs from patients with HAM/TSP have discharged their granules much more recently because of the greater antigenic stimulus, and so have fewer lytic granules and less CD107a to discharge in the short-term in vitro assay. Because there is evidence that the concentration of antigen required to stimulate CD8+ T cells to produce cytokines is greater than the concentration required to induce CTL killing of target cells,40 higher HTLV-1 PVL as well as higher antigen load in patients with HAM/TSP may deteriorate the function of CD8+ T cells. In this case, the abundance of antigen in patients with HAM/TSP might exceed the threshold required to stimulate the CD8+ T cells to produce inflammatory cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α); therefore, the effect of CTLs is not protective but harmful for infected patients.
In conclusion, our present data indicate that CTL efficiency appears to be lower in patients with HAM/TSP than in HVCs. The different costimulatory molecule expression, perforin content, and GzmB content between patients with HAM/TSP and HVCs may reflect the different state of activation of the cells when viral antigen expression reaches equilibrium with the CTL population within infected patients. This point of equilibrium may differ not only between different viral infections (ie, different kinetics of viral expression), but also between HTLV-1–infected patients with a different “efficiency” of CTL response. Future studies investigating the nature of the defects in these T cells may provide opportunities to overcome such inefficient functions, and may hold the potential for reducing disease or eradicating persisting infection.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the staff and blood donors of Kagoshima University Hospital. The authors thank Ms Fumiko Inoue and Yoko Nishino of Kagoshima University and Ms Sumie Saito of Kanazawa Medical University for their excellent technical assistance.
This work was supported by the Ministry of Health, Labor and Welfare, Japan (Neuroimmunological Disease Research Committee Grant; S.I., M.O., and Y.O.); the Japan Society for the Promotion of Science (JSPS; grant 17590886; M.S.); the Japan Brain Foundation (M.S.); the Takeda Science Foundation (M.S.); Kanazawa Medical University (grants S2006-1, H2007-11, and C2007-5; M.S.). A.H.S. was supported by a Postdoctoral Fellowship for Foreign Researchers from JSPS.
Authorship
Contribution: A.H.S. designed and performed the experiments, analyzed the data, and wrote the paper; K.U. provided input into the data analyses and interpretation; M.O. and D.H. provided samples, clinical data, and advice; S.I., M.O., and Y.O. contributed to obtaining funding and gave advice; M.S. designed and supervised the research, performed experiments, and wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mineki Saito, Department of Microbiology, Kanazawa Medical University, 1-1 Daigaku, Uchinada-machi, Ishikawa 920-0293, Japan; e-mail: mineki@kanazawa-med.ac.jp.

![Figure 1. Frequencies and mean fluorescence intensities of perforin- and granzyme B–positive CD8+ T-cell populations in HTLV-1–infected patients. (A) Representative dot plots of PBMCs stained for cytotoxic function markers and costimulatory molecules. PBMCs from patients with HAM/TSP, asymptomatic healthy virus carriers (HVCs), and uninfected healthy controls (HCs) were costained for CD8, CD27, or CD28, and markers associated with cytotoxic function (ie, perforin or granzyme B [GzmB]). Cells in a CD8+ lymphocyte gate were analyzed. Representative dot plots from 1 uninfected control and 1 HAM/TSP patient are shown. (B) The frequency and mean fluorescence intensity (MFI) of the perforin-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in patients with HAM/TSP, HVCs, and HCs. (C) Frequency and MFI of the GzmB-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in patients with HAM/TSP, HVCs, and HCs. (D) Frequency and MFI of the perforin-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in proviral load (PVL)–matched patients with HAM/TSP, HVCs, and HCs. (E) The frequency and MFI of the GzmB-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in PVL-matched patients with HAM/TSP, HVCs, and HCs. The frequency of perforin- or GzmB-positive cells is shown as a percentage within each cell subset (CD8+, CD8+CD28−, CD8+CD27−). Values represent the means plus or minus the standard error (SE). To test for significant differences among the cell populations between 3 different groups of subjects (HAM/TSP, HVCs, and HCs), one-factor ANOVA was done when the variance of each group was equal by Levene test. If the variance of each group was different, the Kruskal-Wallis test was used. For multiple comparisons, we used Sheffe F to analyze statistical difference. Values of P < .05 were considered statistically significant. ***P < .001; **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2008-02-140335/7/m_zh80160822150001.jpeg?Expires=1768101791&Signature=B8e~~Gr7MuYWq4mRfF91YIfIJSrFFHQCgUvhzjqEO7FNqmHA1edTRzIXiRb7kE7C4FZTJfimfzj0rrEG6qBg18rpIJe8qtfaXjbLOUIaXIQW5OvMEzk1KsrWW5pk88dnYEKEyVQbXSqTcDHLzDeUTM~dIYzVDo5o7xOzgUm9ip1TAZ1whk9zrT02W7rxywQadjOfyNEbo9zjZLpSCHRDBeIb2rrLo3jthsW589gp2j7nPPpCo7R4z7WhEocHJsKrf~ofRsrvpJwy1qQyEOx9SO1ImOe68Lqp0~hnYtZvPzgMmnBgLmMiTxrWaumfxKWceQBg2Q9~TI9zNQaYRq2BwA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Perforin-positive CD8+ T cells and HTLV-1 proviral load in HTLV-1 infection. (Left panels) There was a marginal negative correlation between the percentages of perforin-positive CD8+ T cells and HTLV-1 proviral load (PVL) in whole HTLV-1–infected patients (HAM/TSP + HVCs; P = .046, r = −0.29 by Spearman rank correlation analysis), but not in patients with HAM/TSP or in HVCs alone. (Right panels) There was a significant negative correlation between the percentages of perforin-positive CD8+ T cells and HTLV-1 PVL in whole HLA-A*02–positive HTLV-1–infected patients (HAM/TSP + HVCs; P = .003, r = −0.58) and in HVCs alone (P = .024, r = −0.64), but not in patients with HAM/TSP (P = .15, r = −0.35). The x axis denotes percentage of perforin-positive CD8+ T cells, and the y axis denotes HTLV-1 PVL (copy number of HTLV-1 tax per 104 PBMCs = [(copy number of tax)/(copy number of β-actin/2)] × 104). Data were analyzed by Spearman rank correlation. The solid line represents a least-squares regression line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2008-02-140335/7/m_zh80160822150002.jpeg?Expires=1768101791&Signature=KS1r7R4j04be1960FAATXFaWJzwI82xv9Hfp-nyyqozcyGZsNcstZz-2ChgKZlYUEO88w1bo02VdKxFOz0AbFJ~rVYi2Zeb7R-pEoHCq3AKk36wACiFlfxmKfp4juQCF~5XZ6V6fzJJWQBLeTiIocL2AopmUHMNYRITXEQ6Y15klLo2w2D2~EUYMdWRLtSMiLJyKL-XtuvVLBKDmmuEw~Rd3tDMZTo6uRlRIGEluUcvpyszw-OX4C0wbwvZbTR1CCRwkeG4keHT1R4GHBVXrdu~R9PY~PWfhDl253WsFqdBDP5b8NUY5cqiAhavHLzpv1RHbAxw2n1i9QCLEeAGSqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Frequencies and mean fluorescence intensities of perforin- and granzyme B–positive CD8+ T-cell populations in HTLV-1–infected patients. (A) Representative dot plots of PBMCs stained for cytotoxic function markers and costimulatory molecules. PBMCs from patients with HAM/TSP, asymptomatic healthy virus carriers (HVCs), and uninfected healthy controls (HCs) were costained for CD8, CD27, or CD28, and markers associated with cytotoxic function (ie, perforin or granzyme B [GzmB]). Cells in a CD8+ lymphocyte gate were analyzed. Representative dot plots from 1 uninfected control and 1 HAM/TSP patient are shown. (B) The frequency and mean fluorescence intensity (MFI) of the perforin-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in patients with HAM/TSP, HVCs, and HCs. (C) Frequency and MFI of the GzmB-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in patients with HAM/TSP, HVCs, and HCs. (D) Frequency and MFI of the perforin-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in proviral load (PVL)–matched patients with HAM/TSP, HVCs, and HCs. (E) The frequency and MFI of the GzmB-positive CD8+ T-cell population and its subpopulations (CD8+CD28− and CD8+CD27−) in PVL-matched patients with HAM/TSP, HVCs, and HCs. The frequency of perforin- or GzmB-positive cells is shown as a percentage within each cell subset (CD8+, CD8+CD28−, CD8+CD27−). Values represent the means plus or minus the standard error (SE). To test for significant differences among the cell populations between 3 different groups of subjects (HAM/TSP, HVCs, and HCs), one-factor ANOVA was done when the variance of each group was equal by Levene test. If the variance of each group was different, the Kruskal-Wallis test was used. For multiple comparisons, we used Sheffe F to analyze statistical difference. Values of P < .05 were considered statistically significant. ***P < .001; **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2008-02-140335/7/m_zh80160822150001.jpeg?Expires=1768101792&Signature=LwB24JxJ~wkCS3aC04hsjFpAaBd6B6-b7ORwqc8rUL7YVByQhjePYtsCJkEHGsmSYcWlJPD0SR9HuwCc1aXYRIK~bsPoIdUb-PKT-61gQvtOAKHn~Z9yc6yOFlUIiD8tgUX59MeeHBB~nO9wB8btzBvq7PM-gP1Xh9pbh~MmjAEr5odNQHMb0JX140xp4cDs032UhWO3bgPuDvQRDXZ7X8CW3l4Kx3Vwoj4ygTeyDlMV3u9xmme8sLO5nJ1QyLS4I2-0EM0P3CUp5OP8vLDpLxresKQeoKrW2RTP14tQ2kTsgCbsUhb334jtaX3X9sY04AOLAcKqy646Qd78fv8RSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Perforin-positive CD8+ T cells and HTLV-1 proviral load in HTLV-1 infection. (Left panels) There was a marginal negative correlation between the percentages of perforin-positive CD8+ T cells and HTLV-1 proviral load (PVL) in whole HTLV-1–infected patients (HAM/TSP + HVCs; P = .046, r = −0.29 by Spearman rank correlation analysis), but not in patients with HAM/TSP or in HVCs alone. (Right panels) There was a significant negative correlation between the percentages of perforin-positive CD8+ T cells and HTLV-1 PVL in whole HLA-A*02–positive HTLV-1–infected patients (HAM/TSP + HVCs; P = .003, r = −0.58) and in HVCs alone (P = .024, r = −0.64), but not in patients with HAM/TSP (P = .15, r = −0.35). The x axis denotes percentage of perforin-positive CD8+ T cells, and the y axis denotes HTLV-1 PVL (copy number of HTLV-1 tax per 104 PBMCs = [(copy number of tax)/(copy number of β-actin/2)] × 104). Data were analyzed by Spearman rank correlation. The solid line represents a least-squares regression line.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2008-02-140335/7/m_zh80160822150002.jpeg?Expires=1768101792&Signature=zwIRTbIA6O0t0WZAYm20nVYlrjem1jqeZGeXgrmk~bxxuull1AmP37YlE8EpYm0Rp8MxZsww4Jw05CjSQIgHNchmqr45dZGKdBUb2g7DPAixVsiW9vuoDu4atPBW5QsmKlb-nkHkf1nPvoePKotC2NvoM~A5jYSg9tp80uUA523~wd5A-mWllt2tJ5N-bmHgnUjQkIEy-RzbbO0IOl6chj5V27lK-5SVN7EiQAVRFatXlB4XfRhO8b-w70S2qdwH7RwMaEbolVWEaY~Yv5-G4PG~HdUCD7l7BikCRC28FBtn0o~DXRDLR5sSkuAJhlHY6Kb3Lyd3sL7BzlclP1WhPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)