Abstract
Mechanisms of protection against autoimmune diseases by transplantation of autologous hematopoietic progenitors remain poorly defined. We recently demonstrated that, unlike medullary hematopoietic stem cells (HSCs), mobilized hematopoietic progenitors (HPCs) stimulate peripheral Foxp3+ regulatory T cell (Treg)–expansion through cell-contact activation of Notch signaling and through as yet undetermined soluble factor(s), distinct from TGF-β1. Herein we identified one such soluble factor as granulocyte macrophage–colony stimulating factor (GM-CSF), which is produced at higher levels by HPCs than HSCs and whose neutralization significantly reduces the growth-promoting effect of HPCs on Treg. Treg express a functional GM-CSF receptor α-chain CD116 and proliferate in response to this cytokine independently from IL2. GM-CSF–expanded Treg—like HPC-expanded Treg—display enhanced suppressive capacity relative to control Treg. Hence, mobilized progenitors stimulate Treg expansion both by cell-contact dependent mechanisms and by their production of GM-CSF.
Introduction
Mobilized peripheral blood stem cells are increasingly used as an alternative to bone marrow (BM) cells for allogeneic transplantation in cancer patients and, more recently, for autologous transplantation in patients with severe autoimmune diseases. Although in the allogeneic setting regulatory T cell (Treg) accumulation triggered by myeloid Gr1+CD11b+ suppressor cells (MSC) reduces acute graft-versus-host disease (GVHD)1 but instead worsens chronic GVHD,2 in autoimmune diseases the expansion of Treg is expected to be beneficial. In the experimental model of spontaneous autoimmune diabetes in nonobese diabetic (NOD) mice, we have recently demonstrated3 that Lin−Sca1hic-kithiFlt3+CD34+CD106+CD127− multipotent hematopoietic progenitor cells (HPCs) mobilized to the spleen by a combination of granulocyte-colony stimulating factor (G-CSF) and FMS-like tyrosine kinase 3 ligand (Flt3L), have a tolerogenic potential and that their transplantation halts autoimmune diabetes. In contrast, nonmobilized Lin−Sca1hic-kithiFlt3−CD34−CD106loCD127− medullary hematopoietic stem cells (HSCs) have no such effect. This difference was linked to the capacity of HPCs, but not HSCs, to drive the expansion of host-derived CD4+CD25+Foxp3+ Treg. Furthermore, restoration of Treg numbers to normal values took place after transplantation with hematopoietic progenitors in patients with juvenile rheumatoid arthritis.4 In view of all these data, the elucidation of the mechanisms underlying Treg expansion by hematopoietic progenitors became essential. We have demonstrated that a cell-to-cell interaction between HPCs and Treg triggers the stimulation of Notch signaling in Treg and their subsequent proliferation. However, separating HPCs from Treg3 in transwell experiments only partially blocked Treg expansion by HPCs, suggesting that soluble factor(s) were implicated as well.
We now report the identification of one such soluble factor as granulocyte macrophage–colony stimulating factor (GM-CSF), which is produced at higher levels by HPCs than HSCs, and promotes expansion of functional Treg through its specific α-chain receptor CD116.
Methods
Mice
Wild-type and Rag2−/−-NOD mice were bred in our animal facility under specific pathogen-free conditions. Live animal experiments were approved by the Ministère de l'Agriculture, de la Pêche et de l'Alimentation (France).
Cell purification
HSCs or HPCs were electronically sorted as Lin−c-kit+Sca-1+ cells from the BM of untreated mice or from the spleen of mice that were injected subcutaneously with human recombinant G-CSF (Neupogen; Amgen, Neuilly sur Seine, France) at 200 μg/kg per day and mouse Flt3L (Immunotools, Friesoythe, Germany) at 10 μg/kg per day for 4 consecutive days.3
CD4+CD25hi and irradiated CD25− APCs were prepared from spleen and Treg proliferation assays conducted as described in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). In vitro expanded CD4+CD25+ Treg were assayed in adoptive cotransfer experiments of diabetes as described.3
Quantitative reverse transcriptase–polymerase chain reaction (PCR) and staining of cells for flow cytometric analysis are described in Document S1.
Statistical analysis
Statistical differences among groups were analyzed using Mann-Whitney and ANOVA tests. Diabetes incidence curves were plotted using Kaplan-Meier estimates and compared by logrank analysis. P values less than .05 were considered statistically significant.
Results and discussion
Role of GM-CSF in the selective capacity of HPCs versus HSCs to stimulate Treg proliferation
G-CSF plus Flt3L mobilization enhanced GM-CSF both at the mRNA (Figure 1A) and the protein (Figure 1B) levels within sorted spleen HPCs relative to BM HSCs. A neutralizing anti–GM-CSF monoclonal antibody (mAb) decreased the ability of HPCs to promote Treg proliferation by 60% (Figure 1C). In contrast, no TGF-β1 and only low IL10 levels were produced by mobilized progenitors (data not shown), and anti-TGF-β1, anti-IL10R or anti-IL2 mAbs did not reverse HPC-induced Treg expansion.
Both GM-CSF produced by mobilized progenitors and recombinant GM-CSF promote Treg expansion. (A) The expression of GM-CSF mRNA was measured in freshly sorted Lin−c-kit+Sca-1+ double positive HSCs and HPCs by real-time PCR. All data were normalized to GAPDH mRNA levels and expressed as fold increases relative to control values obtained from HSCs. Data are shown as the means (± SEM) of 3 experiments performed in triplicate. (B) For FACS analysis of the intracellular content of GM-CSF, sorted HSCs and HPCs were immediately fixed and permeabilized and subsequently stained with specific PE-labeled anti–GM-CSF antibody. Values represent the percentages of GM-CSF–producing cells. Data are from 1 representative experiment of 3. (C) Enhanced thymidine incorporation by Treg resulting from the coculture of CD4+CD25+-sorted Treg with HPCs and APCs at a 1:1:1 ratio, calculated as stimulation index. Cells at a 1:1:1 HPC:CD4+CD25+:APC cell ratio, 25 000 cells of each subset per well, were stimulated with the anti-CD3 mAb (clone 145-2C11) at 10 μg/mL for 96 hours, in the presence or absence of neutralizing anti-IL10R (clone 1B1.2), anti-TGFβ1 (clone 2G7), anti-IL2, (clone 1A12), and anti–GM-CSF (2 μg/mL, clone MP122E9; R&D Systems Europe, Lille, France) antibodies or control isotypes. Proliferation was measured by 3H-thymidine incorporation, 1 μCi/well, during the last 8 hours of culture.3 The stimulation index was calculated as the ratio of cpm in triplicate wells [a-b/b] where a and b stand for (CD25+ + HPCs + APCs) and (CD25+ + APCs), respectively. Results are from 1 representative experiment of 3. *P = .005, using nonparametric Mann-Whitney test. (D) CD4+CD25+ cells (2.5 × 104 per well) were cultured with APCs at a 1:1 ratio in the presence of anti-CD3 mAb (clone 145-2C11) at 10 μg/mL for 96 hours and of increasing concentrations of recombinant GM-CSF. Proliferation was measured and stimulation index calculated as in panel C. Results are expressed as means (± SEM) of quadruplicate determinations in 1 representative experiment of 4. P < .02, 1-way ANOVA. (E) Stimulation of Treg expansion by 1 ng/mL GM-CSF, performed as in panel D, in the presence or absence of neutralizing anti-IL2 antibody (2 μg/mL). Results are expressed as means (± SEM) of triplicate determinations in 1 representative experiment of 3. N.S. (F) CD4+CD25+Foxp3hi cells (1.25 × 105 cells at day 0) cocultured with HPCs (ratio 1:1) together with anti-CD3/CD28–coated microbeads (8 beads per cell; Invitrogen, Cergy Pontoise, France) in complete medium supplemented with 100 U/mL IL2 (R&D Systems) during 5 days in presence (■) or absence (□) of 2 ng/mL GM-CSF were numerated, after magnetic bead removal, by measuring CD4, CD25, and Foxp3 cell expression by flow cytometry. Results are expressed as means (± SEM) of 4 experiments. P = .028, using nonparametric Mann-Whitney test. (G) GM-CSF Rα chain expression was measured by FACS in purified CD4+CD25+ Treg at 0 hour (dotted line), and after 65 hours of activation with coated anti-CD3 (10 μg/mL) and soluble anti-CD28 (5 μg/mL) in the presence (thick line) or absence (thin line) of 2 ng/mL GM-CSF, with or without (shadowed histogram) anti-CD116 primary antibody and FITC-anti–rabbit Fab'2. Results shown are from 1 representative experiment of 3.
Both GM-CSF produced by mobilized progenitors and recombinant GM-CSF promote Treg expansion. (A) The expression of GM-CSF mRNA was measured in freshly sorted Lin−c-kit+Sca-1+ double positive HSCs and HPCs by real-time PCR. All data were normalized to GAPDH mRNA levels and expressed as fold increases relative to control values obtained from HSCs. Data are shown as the means (± SEM) of 3 experiments performed in triplicate. (B) For FACS analysis of the intracellular content of GM-CSF, sorted HSCs and HPCs were immediately fixed and permeabilized and subsequently stained with specific PE-labeled anti–GM-CSF antibody. Values represent the percentages of GM-CSF–producing cells. Data are from 1 representative experiment of 3. (C) Enhanced thymidine incorporation by Treg resulting from the coculture of CD4+CD25+-sorted Treg with HPCs and APCs at a 1:1:1 ratio, calculated as stimulation index. Cells at a 1:1:1 HPC:CD4+CD25+:APC cell ratio, 25 000 cells of each subset per well, were stimulated with the anti-CD3 mAb (clone 145-2C11) at 10 μg/mL for 96 hours, in the presence or absence of neutralizing anti-IL10R (clone 1B1.2), anti-TGFβ1 (clone 2G7), anti-IL2, (clone 1A12), and anti–GM-CSF (2 μg/mL, clone MP122E9; R&D Systems Europe, Lille, France) antibodies or control isotypes. Proliferation was measured by 3H-thymidine incorporation, 1 μCi/well, during the last 8 hours of culture.3 The stimulation index was calculated as the ratio of cpm in triplicate wells [a-b/b] where a and b stand for (CD25+ + HPCs + APCs) and (CD25+ + APCs), respectively. Results are from 1 representative experiment of 3. *P = .005, using nonparametric Mann-Whitney test. (D) CD4+CD25+ cells (2.5 × 104 per well) were cultured with APCs at a 1:1 ratio in the presence of anti-CD3 mAb (clone 145-2C11) at 10 μg/mL for 96 hours and of increasing concentrations of recombinant GM-CSF. Proliferation was measured and stimulation index calculated as in panel C. Results are expressed as means (± SEM) of quadruplicate determinations in 1 representative experiment of 4. P < .02, 1-way ANOVA. (E) Stimulation of Treg expansion by 1 ng/mL GM-CSF, performed as in panel D, in the presence or absence of neutralizing anti-IL2 antibody (2 μg/mL). Results are expressed as means (± SEM) of triplicate determinations in 1 representative experiment of 3. N.S. (F) CD4+CD25+Foxp3hi cells (1.25 × 105 cells at day 0) cocultured with HPCs (ratio 1:1) together with anti-CD3/CD28–coated microbeads (8 beads per cell; Invitrogen, Cergy Pontoise, France) in complete medium supplemented with 100 U/mL IL2 (R&D Systems) during 5 days in presence (■) or absence (□) of 2 ng/mL GM-CSF were numerated, after magnetic bead removal, by measuring CD4, CD25, and Foxp3 cell expression by flow cytometry. Results are expressed as means (± SEM) of 4 experiments. P = .028, using nonparametric Mann-Whitney test. (G) GM-CSF Rα chain expression was measured by FACS in purified CD4+CD25+ Treg at 0 hour (dotted line), and after 65 hours of activation with coated anti-CD3 (10 μg/mL) and soluble anti-CD28 (5 μg/mL) in the presence (thick line) or absence (thin line) of 2 ng/mL GM-CSF, with or without (shadowed histogram) anti-CD116 primary antibody and FITC-anti–rabbit Fab'2. Results shown are from 1 representative experiment of 3.
The addition of recombinant GM-CSF enhanced anti-CD3–induced Treg proliferation in the presence of APCs in a dose-dependent manner within the range of 0.005 to 2 ng/mL (Figure 1D). The stimulatory effect of GM-CSF persisted even in the presence of neutralizing anti-IL2 antibody (Figure 1E), suggesting a mechanism independent from secondary IL2 production. GM-CSF triggered Treg expansion in the absence of APCs as well, using instead anti-CD3/CD28–coated microbeads (Figure 1F).
The GM-CSF receptor is constitutively expressed by myelomonocytic cells,5 and has been demonstrated on both leukemic5 and nonleukemic T cells.6,7 It is composed of a specific α chain (CD116) associated with a β chain shared with the IL3 and IL5 receptors.8 We found that CD116 was constitutively expressed by freshly isolated resting Treg. The level of CD116 expression decreased in approximately 30% of Treg upon anti-CD3/CD28 activation, both in the presence and absence of GM-CSF (Figure 1G), suggesting heterogeneous response of Treg to activation.
Treg expanded with GM-CSF display enhanced suppressive function
Polyclonal Treg expanded in the presence of GM-CSF over 3 days, like those expanded in the presence of HPCs,3 displayed significantly enhanced suppressive capacity in vivo in cotransfer experiments (Figure 2) relative to those expanded with CD3/CD28 beads only.
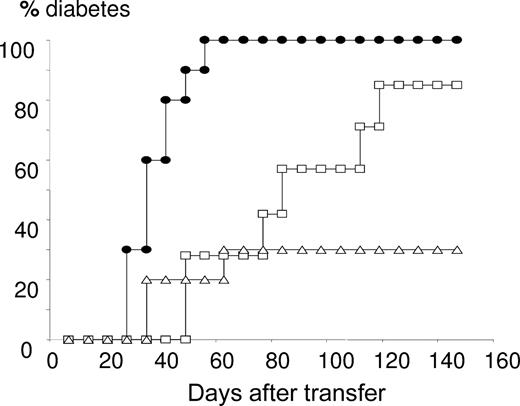
Functional properties of Treg after expansion with GM-CSF. After 3 days of culture of CD4+CD25+ T cells with CD3/CD28-coated beads and 2 ng/mL GM-CSF (Δ) or PBS (□), cells were washed and cotransferred (106) with diabetogenic NOD splenocytes (3 × 106) into 4-week-old NOD-Rag2−/− recipients. A control transfer group was injected with diabetogenic NOD splenocytes (3 × 106) alone (●). Mice (n = 8-10 per group) were screened for glycosuria (Glucotest, Boehringer-Mannheim, Mannheim, Germany) twice a week and/or glycemia (Haemoglukotest and Reflolux F, Boehringer-Mannheim) and considered diabetic when nonfasting blood glucose levels were more than 250 mg/dL on 2 consecutive readings. P < .001, using Kaplan-Meier estimates and log-rank analysis, comparing diabetes incidence measured in mice cotransferred with Treg and diabetogenic splenocytes versus injected with diabetogenic NOD splenocytes (3 × 106) alone. P = .047, comparing diabetes incidence in mice cotransferred with GM-CSF– versus PBS-expanded CD4+CD25+ cells. One representative experiment of 2 is shown.
Functional properties of Treg after expansion with GM-CSF. After 3 days of culture of CD4+CD25+ T cells with CD3/CD28-coated beads and 2 ng/mL GM-CSF (Δ) or PBS (□), cells were washed and cotransferred (106) with diabetogenic NOD splenocytes (3 × 106) into 4-week-old NOD-Rag2−/− recipients. A control transfer group was injected with diabetogenic NOD splenocytes (3 × 106) alone (●). Mice (n = 8-10 per group) were screened for glycosuria (Glucotest, Boehringer-Mannheim, Mannheim, Germany) twice a week and/or glycemia (Haemoglukotest and Reflolux F, Boehringer-Mannheim) and considered diabetic when nonfasting blood glucose levels were more than 250 mg/dL on 2 consecutive readings. P < .001, using Kaplan-Meier estimates and log-rank analysis, comparing diabetes incidence measured in mice cotransferred with Treg and diabetogenic splenocytes versus injected with diabetogenic NOD splenocytes (3 × 106) alone. P = .047, comparing diabetes incidence in mice cotransferred with GM-CSF– versus PBS-expanded CD4+CD25+ cells. One representative experiment of 2 is shown.
In the thymus, TCR signals in the first place enhance cytokine, chiefly IL2, responsiveness enabling later on Stat5-inducing cytokines to complete the Treg cell differentiation program.9-12 Although Treg expansion in the periphery has been mainly attributed to TGF-β1,13 we have demonstrated that the growth-promoting effect of HPCs is TGFβ-independent3 but GM-CSF-dependent (Figure 1A-F) and proceeds through a functional GM-CSF receptor on Treg. Binding of GM-CSF to its receptor is known to activate JAK2 kinases leading to Stat5 phosphorylation.14,15 Treg suppressive activity correlates with Foxp3 gene expression. The latter is up-regulated by Stat5 binding to its promoter, but instead down-modulated by IL6-activated Stat3.16 Although we observed that Treg activated with anti-CD3/CD28 and cultured in the presence of HPCs exhibited enhanced Foxp33 and phospho-Stat5 levels (data not shown), culture with recombinant GM-CSF induced only late (> 60 hours) and moderate modulation of these parameters, raising the possibility that a second wave of cytokines participates in full Treg activation by HPCs.
The fact that GM-CSF did not enhance Notch3 expression on Treg (not shown) suggests that the 2 stimulatory pathways by which HPCs promote Treg expansion, namely cell-contact and Notch-mediated and secretory GM-CSF–dependent pathways, play additive yet independent roles.
GM-CSF can be produced by a variety of cell types besides HPCs, including activated CD4+, CD8+ T cells,17 NK,18 NKT19 and dendritic cells (DCs).20 High doses of GM-CSF are known to recruit myeloid-restricted CD11b+Gr1+ precursors (MSCs), that in turn can recruit Treg. MSCs are unlikely to contaminate our electronically sorted HPCs which exhibit either low or negative expression of the lineage markers CD11b and Gr1. Lin−Sca-1+c-kit+ HPCs displayed both myeloid and lymphoid clonogenic potential in vitro on methylcellulose and multilineage differentiation in vivo.3 In addition to a predominant multipotent progenitor population (MPP), sorted HPCs may still include ST-HSCs as well as LT-HSCs. However, BM HSCs, enriched in the 2 latter populations, hardly produced GM-CSF and did not trigger Treg expansion. A unique G-CSF–induced type 2 DC precursor has been reported to restrain human T-cell response.21 The possibility remains that sorted HPCs include early DC precursors that share Flt3 expression with HPCs. However, Flt3+ CMPs and GMPs, which are Sca1−, should not be included.
GM-CSF either stimulates immune response, harnessing DCs with improved antigen presentation capacity,22 or instead favors the development of semimature DCs that recruit Tr1 and Th2 cells23 and prevents autoimmune thyroiditis as well as type 1 diabetes in the NOD mouse.24 Data presented herein show that the interaction of GM-CSF with CD116 on Treg may promote immune tolerance. Interestingly, another regulatory T-cell subset, namely invariant natural killer T (iNKT) cells, expresses CD116 through which GM-CSF regulates their effector differentiation.6
Finally, the recent report that adaptive secretion of GM-CSF occurs in human progenitors25 in BCR/ABL+ patients opens the possibility that GM-CSF may play a role in human peripheral Treg amplification upon transplantation with mobilized hematopoietic progenitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Corinne Garcia-Cordier and Jérôme Mégret, Institut Fédératif de Recherche Necker-Enfants Malades, for expert cell sorting, and Amgen, Neuilly sur Seine, France, for the kind gift of Neupogen.
This work was supported by the Juvenile Diabetes Research Foundation, New York, NY, and by a doctoral grant to H.K. by Fondation de la Recherche Médicale, Paris, France.
Authorship
Contribution: H.K., B.L., and R.M. performed research and analyzed data; A.R. performed research; L.C., E.L.E., Y.R., E.S., and M.D. discussed data, provided materials, and critically read the manuscript; and F.Z. was responsible for the overall study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Flora Zavala, Université Paris Descartes, Faculté de Médecine, Centre National de la Recherche Scientifique UMR 8147, 161 rue de Sèvres, 75015 Paris, France; e-mail: zavala@necker.fr.
References
Author notes
*H.K. and B.L. contributed equally to this work.

![Figure 1. Both GM-CSF produced by mobilized progenitors and recombinant GM-CSF promote Treg expansion. (A) The expression of GM-CSF mRNA was measured in freshly sorted Lin−c-kit+Sca-1+ double positive HSCs and HPCs by real-time PCR. All data were normalized to GAPDH mRNA levels and expressed as fold increases relative to control values obtained from HSCs. Data are shown as the means (± SEM) of 3 experiments performed in triplicate. (B) For FACS analysis of the intracellular content of GM-CSF, sorted HSCs and HPCs were immediately fixed and permeabilized and subsequently stained with specific PE-labeled anti–GM-CSF antibody. Values represent the percentages of GM-CSF–producing cells. Data are from 1 representative experiment of 3. (C) Enhanced thymidine incorporation by Treg resulting from the coculture of CD4+CD25+-sorted Treg with HPCs and APCs at a 1:1:1 ratio, calculated as stimulation index. Cells at a 1:1:1 HPC:CD4+CD25+:APC cell ratio, 25 000 cells of each subset per well, were stimulated with the anti-CD3 mAb (clone 145-2C11) at 10 μg/mL for 96 hours, in the presence or absence of neutralizing anti-IL10R (clone 1B1.2), anti-TGFβ1 (clone 2G7), anti-IL2, (clone 1A12), and anti–GM-CSF (2 μg/mL, clone MP122E9; R&D Systems Europe, Lille, France) antibodies or control isotypes. Proliferation was measured by 3H-thymidine incorporation, 1 μCi/well, during the last 8 hours of culture.3 The stimulation index was calculated as the ratio of cpm in triplicate wells [a-b/b] where a and b stand for (CD25+ + HPCs + APCs) and (CD25+ + APCs), respectively. Results are from 1 representative experiment of 3. *P = .005, using nonparametric Mann-Whitney test. (D) CD4+CD25+ cells (2.5 × 104 per well) were cultured with APCs at a 1:1 ratio in the presence of anti-CD3 mAb (clone 145-2C11) at 10 μg/mL for 96 hours and of increasing concentrations of recombinant GM-CSF. Proliferation was measured and stimulation index calculated as in panel C. Results are expressed as means (± SEM) of quadruplicate determinations in 1 representative experiment of 4. P < .02, 1-way ANOVA. (E) Stimulation of Treg expansion by 1 ng/mL GM-CSF, performed as in panel D, in the presence or absence of neutralizing anti-IL2 antibody (2 μg/mL). Results are expressed as means (± SEM) of triplicate determinations in 1 representative experiment of 3. N.S. (F) CD4+CD25+Foxp3hi cells (1.25 × 105 cells at day 0) cocultured with HPCs (ratio 1:1) together with anti-CD3/CD28–coated microbeads (8 beads per cell; Invitrogen, Cergy Pontoise, France) in complete medium supplemented with 100 U/mL IL2 (R&D Systems) during 5 days in presence (■) or absence (□) of 2 ng/mL GM-CSF were numerated, after magnetic bead removal, by measuring CD4, CD25, and Foxp3 cell expression by flow cytometry. Results are expressed as means (± SEM) of 4 experiments. P = .028, using nonparametric Mann-Whitney test. (G) GM-CSF Rα chain expression was measured by FACS in purified CD4+CD25+ Treg at 0 hour (dotted line), and after 65 hours of activation with coated anti-CD3 (10 μg/mL) and soluble anti-CD28 (5 μg/mL) in the presence (thick line) or absence (thin line) of 2 ng/mL GM-CSF, with or without (shadowed histogram) anti-CD116 primary antibody and FITC-anti–rabbit Fab'2. Results shown are from 1 representative experiment of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2008-02-140681/7/m_zh80190824540001.jpeg?Expires=1769148576&Signature=VjX12UN8i8Q4tF-5G4kwdnJ~4EWAV0LGCv94bvsMbz9hXKmxFyJ0Y7hXqEu9e8rzV5Kv2Y5CGtVNlnuDwt3aj9FKfEMmqSVuoFCIr3cx~MuAauXtrv1Pf5MykvpA7~hnbKluECtw2SJ5yM2HEcakLbDl1xCBbK69gIr7cbO8YIoZTFJDzVn77RRT5-Nh-W63PN9WC-IBBEwg2rXGiHfFZQ61iYr-cibIs9kWT28ZwPlfYW60ng1zSQops2CqgS-ii0MKrubVPTp0G6ig1z-wECZhWcGUDGmhkZlZsz8UgL0ExPYyXg0wyJITR0Z~nbB~Lqz8Nmfhn3IFI-UV8nqSAQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Both GM-CSF produced by mobilized progenitors and recombinant GM-CSF promote Treg expansion. (A) The expression of GM-CSF mRNA was measured in freshly sorted Lin−c-kit+Sca-1+ double positive HSCs and HPCs by real-time PCR. All data were normalized to GAPDH mRNA levels and expressed as fold increases relative to control values obtained from HSCs. Data are shown as the means (± SEM) of 3 experiments performed in triplicate. (B) For FACS analysis of the intracellular content of GM-CSF, sorted HSCs and HPCs were immediately fixed and permeabilized and subsequently stained with specific PE-labeled anti–GM-CSF antibody. Values represent the percentages of GM-CSF–producing cells. Data are from 1 representative experiment of 3. (C) Enhanced thymidine incorporation by Treg resulting from the coculture of CD4+CD25+-sorted Treg with HPCs and APCs at a 1:1:1 ratio, calculated as stimulation index. Cells at a 1:1:1 HPC:CD4+CD25+:APC cell ratio, 25 000 cells of each subset per well, were stimulated with the anti-CD3 mAb (clone 145-2C11) at 10 μg/mL for 96 hours, in the presence or absence of neutralizing anti-IL10R (clone 1B1.2), anti-TGFβ1 (clone 2G7), anti-IL2, (clone 1A12), and anti–GM-CSF (2 μg/mL, clone MP122E9; R&D Systems Europe, Lille, France) antibodies or control isotypes. Proliferation was measured by 3H-thymidine incorporation, 1 μCi/well, during the last 8 hours of culture.3 The stimulation index was calculated as the ratio of cpm in triplicate wells [a-b/b] where a and b stand for (CD25+ + HPCs + APCs) and (CD25+ + APCs), respectively. Results are from 1 representative experiment of 3. *P = .005, using nonparametric Mann-Whitney test. (D) CD4+CD25+ cells (2.5 × 104 per well) were cultured with APCs at a 1:1 ratio in the presence of anti-CD3 mAb (clone 145-2C11) at 10 μg/mL for 96 hours and of increasing concentrations of recombinant GM-CSF. Proliferation was measured and stimulation index calculated as in panel C. Results are expressed as means (± SEM) of quadruplicate determinations in 1 representative experiment of 4. P < .02, 1-way ANOVA. (E) Stimulation of Treg expansion by 1 ng/mL GM-CSF, performed as in panel D, in the presence or absence of neutralizing anti-IL2 antibody (2 μg/mL). Results are expressed as means (± SEM) of triplicate determinations in 1 representative experiment of 3. N.S. (F) CD4+CD25+Foxp3hi cells (1.25 × 105 cells at day 0) cocultured with HPCs (ratio 1:1) together with anti-CD3/CD28–coated microbeads (8 beads per cell; Invitrogen, Cergy Pontoise, France) in complete medium supplemented with 100 U/mL IL2 (R&D Systems) during 5 days in presence (■) or absence (□) of 2 ng/mL GM-CSF were numerated, after magnetic bead removal, by measuring CD4, CD25, and Foxp3 cell expression by flow cytometry. Results are expressed as means (± SEM) of 4 experiments. P = .028, using nonparametric Mann-Whitney test. (G) GM-CSF Rα chain expression was measured by FACS in purified CD4+CD25+ Treg at 0 hour (dotted line), and after 65 hours of activation with coated anti-CD3 (10 μg/mL) and soluble anti-CD28 (5 μg/mL) in the presence (thick line) or absence (thin line) of 2 ng/mL GM-CSF, with or without (shadowed histogram) anti-CD116 primary antibody and FITC-anti–rabbit Fab'2. Results shown are from 1 representative experiment of 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/112/6/10.1182_blood-2008-02-140681/7/m_zh80190824540001.jpeg?Expires=1769148577&Signature=DFrl~rprq2xOV0ipR7fyV5Gfw7lOfUt9EDxq5x82SHPVV9h58ku~3zPNhhLvYAevU-1Yk91J8j~uV6zfqP4qAHFpV02POEnIL8cxDd5mnfQgTExXFxbHLc0YHPYa33DY1rOH6zpAr2FtnmIvQHcjezzQPVfkER8o~XNLuf6oEzS4pF8ikhMhRwNaq9H2BAZuAkP1AAsLWOOLv8vjCT5ZTLqFPLy4AiNeyyG25mB5asq3vf4cvxdaxw9kDTKOb97DetODeT~RLxDL7K~O4QUerCWR4g-1F3o-ASKYAD88pgnQjFQOqGcmMfQy80nG53tP1iQzjREm8pnZAHskAVyArg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)