Abstract
Cytokine-induced killer (CIK) cells are ex vivo–expanded T lymphocytes expressing both natural killer (NK)– and T-cell markers. CIK cells are cytotoxic against autologous and allogeneic tumors. We previously showed that adoptive transfer of allogeneic CIK cells in a murine model caused minimal graft-versus-host disease (GVHD). However, the precise mechanism of reduced GVHD is not fully understood. Therefore, we evaluated the trafficking and survival of luciferase-expressing CIK cells in an allogeneic bone marrow transplant model. The initial trafficking patterns of CIK cells were similar to conventional T cells that induced GVHD; however, CIK cells infiltrated GVHD target tissues much less and transiently. CIK cells accumulated and persisted in tumor sites, resulting in tumor eradication. We evaluated different properties of CIK cells compared with conventional T cells, demonstrating a slower division rate of CIK cells, higher susceptibility to apoptosis, persistent increased expression of interferon gamma (IFN-γ), and reduced acquisition of homing molecules required for entry of cells into inflamed GVHD target organs that lack expression of NKG2D ligands recognized by CIK cells. Due to these properties, allogeneic CIK cells had reduced expansion and caused less tissue damage. We conclude that CIK cells have the potential to separate graft-versus-tumor effects from GVHD.
Introduction
Cytokine-induced killer (CIK) cells are generated from splenocytes in mice and peripheral blood mononuclear cells in humans by culturing cells in the presence of interferon gamma (IFN-γ), anti-CD3 monoclonal antibody, and IL-2.1 After 14 to 21 days of culture, the cells expressing both natural killer (NK) and T-cell markers dominate, ranging from 30% to 70% of the cells in the culture.2 CIK cells are capable of recognizing autologous malignant cells3,4 and mediate MHC-unrestricted cytotoxicity.5 During CIK cell expansion NKG2D is up-regulated, which is an activating receptor expressed on NK cells and plays a crucial role in tumor cell killing.6 Further, DAP10 is also up-regulated, which effectively couples NKG2D mediated signaling to perforin-based cytotoxicity mechanisms.7
We previously demonstrated that CIK cells exert a graft-versus-tumor (GVT) effect after both syngeneic and allogeneic bone marrow transplantation (BMT) in rodent models.8 Interestingly, mice receiving allogeneic CIK cells showed much less graft-versus-host disease (GVHD) compared with those receiving fresh splenocytes. CIK cells produce large amounts of IFN-γ, which has protective effects against GVHD.9 In order to learn more about the potential GVT and less GVHD-inducing effects of CIK cells, we visualized the trafficking of CIK cells in both syngeneic and allogeneic BM transplant recipients using in vivo bioluminescent imaging (BLI). We compared CIK cells to naive T cells, which cause severe GVHD, focusing on cell division rate, susceptibility to apoptosis, cytokine secretion, and acquisition of homing molecules required for entry into inflamed GVHD target tissues. Furthermore, we evaluated CIK cell accumulation into tumor sites and GVT activity.
Methods
Mice
Balb/c mice (H-2d, Thy1.2), Balb/b (H-2b, Thy1.2), FVB/N (H-2q, Thy1.1), C57BL/6 (H-2b, Thy1.2), C57BL/6 (H-2b, Thy1.1) congenic mice, and GFP+C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The luciferase-expressing (luc+) transgenic FVB/N line named FVB-L2G85 was generated as previously described.10 These animals were backcrossed onto the C57BL/6 background and used after 10 generations (termed B6-L2G85). Mice were used between 8 and 12 weeks of age for all transplantation experiments. All animal studies were performed under approved protocols for the humane treatment of animals regulated by Stanford University.
Generation of CIK cells
CIK cells were generated from splenocytes as previously described.9 Briefly, splenocytes were stimulated in complete RPMI media (3 × 106 cells/mL) with 1000 U/mL recombinant mouse IFN-γ (R&D Systems, Minneapolis, MN) for 24 hours, transferred to anti-CD3 (145-2C11; BD Pharmingen, San Diego, CA) antibody-coated tissue-culture flasks, and stimulated with 300 IU/mL rhIL-2 (Chiron, San Francisco, CA) every 3 days until day 14 when the cells were harvested.
Transplantation model
To reproduce allogeneic major and minor histocompatibility mismatched, minor histocompatibility antigen mismatched, and syngeneic BM transplants, we used several donor-recipient combinations as indicated in each experiment. Female 8- to 12-week-old recipient mice received 2 split doses of either 400 cGy (Balb/c or Balb/b) or 450 cGy total body irradiation (FVB/N or C57BL/6) 4 hours apart, followed by the infusion of either 5 × 106 bone marrow (BM) or T cell–depleted BM (TCD-BM) cells. In all experiments, except for the CIK dose escalation study, 10 × 106 CIK cells were coinjected with the matched strain of either BM or TCD-BM cells. To compare GVHD progression, acute GVHD was induced by administering 10 × 106 splenocytes. To distinguish CIK cells or splenocytes from BM-derived T cells, GFP+ or Thy1.1 was used as a donor specific marker. Exogenous IL-2 (50 000 U/mouse) was administered intraperitoneally from day 0 to day 15 after BMT in indicated experiments.
In vivo and ex vivo bioluminescent imaging
Bioluminescent imaging (BLI) was performed as previously described.11 Briefly, mice were injected intraperitoneally with luciferin (10 μg/g body weight). Ten minutes later, mice were imaged using an IVIS100 charge-coupled device imaging system (Xenogen, Alameda, CA) for 5 minutes. Imaging data were analyzed and quantified with Living Image Software (Xenogen) and IgorProCarbon (WaveMetrics, Lake Oswego, OR). Ex vivo BLI was performed according to previously described methods.12
Flow cytometry
The following antibodies were purchased from BD Pharmingen, eBioscience (San Diego, CA), and R&D Systems: CD3 (145-2c11), CD4 (GK1.5), CD8α (53-6.7), CD25 (PC61), CD44 (IM7), CD45R/B220 (RA3-6B2), CD69 (H1.2F3), Thy1.1 (HIS51), LPAM-1/α4β7 (DATK32), CCR5 (C34-3448), CCR9 (242503), CXCR3 (220803), Fas (DX2), Fas Ligand (MFL3), NKG2D (CX5), PD-1 (RMP1-30), CTLA-4 (UC10-4B9), Annexin V, Foxp3 (FJK-16s), IFN-γ (XMG1.2), Rae1 (199215), H60 (205326), MULT1 (237104), and appropriate isotype controls. To detect E-selectin ligand and P-selectin ligand, recombinant mouse E-selectin/Fc chimera (R&D Systems) and P-selectin–IgG fusion protein (BD Pharmingen) were used, respectively. All staining was performed in phosphate-buffered saline (PBS)/2% fetal calf serum after blocking unspecific staining via FcRII/III. Propidium iodide was added before analysis to exclude dead cells. Intracellular staining of FoxP3 was performed using an antimouse/rat Foxp3 staining kit (eBioscience). Intracellular IFN-γ was determined using Leukocyte Activation Cocktail with GolgiPlug (BD Pharmingen). All assays were performed according to the manufacturer's instructions. Flow cytometric data were collected on a LSR flow cytometer (Becton Dickinson, Mountain View, CA) and analyzed with FlowJo Software (TreeStar, Ashland, OR).
CFSE-based cell proliferation assay
For analysis of cell proliferation, splenocytes and CIK cells (1 × 107/mL) were resuspended in PBS and stained with Vybrant CDDA SE (carboxyfluorescein diacetate, succinimidyl ester) Tracer kit (Invitrogen, Carlsbad, CA) at a final concentration of 5 μM for exactly 6 minutes at 37°C. Immediately after staining, cells were washed in 5 volumes ice-cold RPMI plus 10% fetal bovine serum (Invitrogen) twice and resuspended in PBS. Ten million CFSE (carboxyfluorescein succinimidyl ester)–labeled splenocytes or CIK cells (C56BL/6, Thy1.1), with 5 × 106 BM (C57BL/6, Thy1.2) were injected into lethally irradiated Balb/c mice. On day 3 after BMT, the cell division rates of infused splenocytes and CIK cells from the spleen were analyzed by fluorescence-activated cell sorting (FACS).
Tumor model
To investigate the GVT effect of CIK cells in vivo, 106 luciferase-positive A20 leukemia/lymphoma B cells were injected subcutaneously into the right flank of lethally irradiated Balb/c recipient mice. Three hours later, TCD-BM and CIK cells from FVB wild-type animals were coinjected intravenously. Similarly, to visualize how CIK cells home to the tumor site, luc+ L2G85 CIK cells with FVB TCD-BM cells were injected into lethally irradiated Balb/c recipients bearing wild-type A20 tumor cells. Tumor growth, regression, and homing of CIK cells to the tumor site were visualized and quantified by BLI.
51Cr release cytotoxicity assay
A20 target cells (106) were labeled for 2 hours at 37°C with 300 μCi (11.1 megabecquerels) 51Cr (DuPont-NEN, Boston, MA). The labeled cells were washed with PBS twice and distributed in 96-well plates at 2 × 104 cells/well. Effector cells were added at the indicated ratio of target to effector cells and incubated for 4 hours at 37°C. The cells were sedimented by centrifugation and aliquots of supernatant were counted in a gamma counter. The percentage of specific 51Cr release was calculated as % specific lysis = (test release) − (spontaneous release) × 100 / (maximal release) − (spontaneous release).
Histologic evaluation
For histology, mice receiving splenocytes or CIK cells were killed on day 7 after BMT. Histologic GVHD scoring was performed according to a previously published system,13 which was calculated based on tissue damaged such as liver and small and large bowel.
Immunofluorescence microscopy
Immunoflluorescence microscopy was performed according to previously described methods.12 Briefly, fresh frozen sections were stained with purified antibody and then with Alexa Fluor-546 (S-11 225; Invitrogen, Frederick, MD) as a secondary antibody. Nuclei were stained with DAPI (4′, 6-diamidino-2-phenylindole). Fluorescence microscopic evaluation was performed on a Nikon microscope (Eclipse, TE 300; Melville, NY).
Statistical analysis
Statistical differences were analyzed using the 2-tailed Student t test. A P value of less than .05 was considered to be significant.
Results
Mice receiving allogeneic CIK cells survive with minimal GVHD
CIK cells were expanded from splenocytes in the presence of IFN-γ, anti-CD3 MAbs, and IL-2. By day 14 of culture, CIK cells mainly consisted of CD8+ cells (80% to 95% when generated from FVB mice and more than 95% from C57BL/6 mice). Expanded cells coexpressed NK-cell markers such as DX5, NK1.1 (30%-50%), and NKG2D (40%-60%) along with T-cell activation markers, including CD25, CD44, and CD69 (> 95%). The remaining CIK cells consisted of CD4+ cells expressing these activation markers with few B cells, CD4+CD25+FoxP3+ Treg or granulocytes.
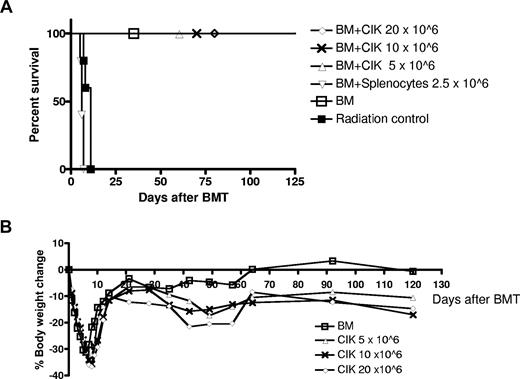
CIK cells generated from FVB mice were injected into lethally irradiated allogeneic Balb/c mice with 5 × 106 FVB BM cells. As shown in Figure 1A, all mice receiving 2.5 × 106 splenocytes developed severe acute GVHD and died within 12 days. In contrast, mice receiving 5 × 106, 10 × 106, and even 20 × 106 allogeneic CIK cells showed minimal signs of GVHD and all animals survived. These mice experienced mild weight loss compared with the BM group (Figure 1B), demonstrating the possibility of mild subclinical chronic GVHD.
CIK cells induce much less GVHD in a major-mismatched BMT model. Lethally irradiated Balb/c (H-2d) hosts received BM with either CIK cells or fresh splenocytes at the indicated dose from wild-type FVB (H-2q) mice. Percent survival (A) and percent body weight change (B) are presented. □ indicates BM (n = 5); ▵, CIK cells 5 × 106 (n = 5); ×, CIK cells 10 × 106 (n = 5); ◇, CIK cells 20 × 106 (n = 5); ▿, fresh splenocytes 2.5 × 106 (n = 5); ■, radiation control (n = 5). Results from one representative experiment are shown.
CIK cells induce much less GVHD in a major-mismatched BMT model. Lethally irradiated Balb/c (H-2d) hosts received BM with either CIK cells or fresh splenocytes at the indicated dose from wild-type FVB (H-2q) mice. Percent survival (A) and percent body weight change (B) are presented. □ indicates BM (n = 5); ▵, CIK cells 5 × 106 (n = 5); ×, CIK cells 10 × 106 (n = 5); ◇, CIK cells 20 × 106 (n = 5); ▿, fresh splenocytes 2.5 × 106 (n = 5); ■, radiation control (n = 5). Results from one representative experiment are shown.
Trafficking of CIK cells after major histocompatibility complex (MHC) mismatched BMT
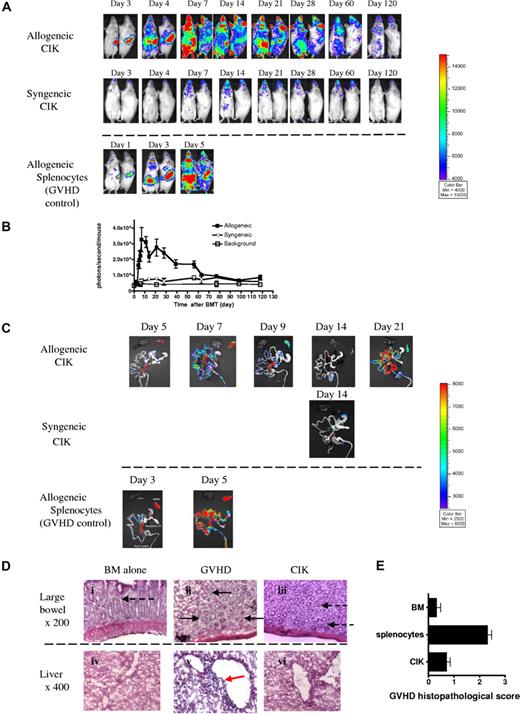
The trafficking and survival of allogeneic versus syngeneic CIK cells was evaluated by injecting FVB-L2G85 luc+ CIK cells into either allogeneic Balb/c or syngeneic FVB recipients. Allogeneic CIK cells homed to and proliferated in the spleen and cervical lymph nodes by day 3 and expanded dramatically (Figure 2A). By day 7, intense signals were observed diffusely over the abdomen and skin. The early homing and progression patterns of allogeneic CIK cells were similar to the patterns seen in GVHD progression after the injection of unmanipulated splenocytes, however, the rapidity of signal increase was greater when animals were administered allogeneic naive T cells. All GVHD control mice died within 7 to 10 days after transplantation due to severe GVHD (Figure 2A). In the syngeneic mice, there was minimal proliferation of CIK cells and only a transient signal was observed on day 14 in cervical lymph nodes and the abdomen.
Trafficking and survival of CIK cells upon adoptive transfer. Lethally irradiated host allogeneic (Balb/c, H-2d, n = 5) and syngeneic (FVB/N, H-2q, n = 5) recipients were injected with 5 × 106 FVB wild-type BM cells with 10 × 106 CIK cells generated from luc+ FVB mice (L2G85). To compare GVHD development, 10 × 106 L2G85 splenocytes were transplanted with BM cells (n = 5). (A) BLI of mice after transplantation. Donor-derived CIK cells in the allogeneic mice homed to and proliferated in secondary lymphoid organs, followed by the infiltration of gut and skin (top row), which was similar to GVHD progression (bottom row). However, in CIK cell–receiving mice, the speed of spread to peripheral tissue was slower and maximum peak signal from gut was lower than that in animals receiving fresh splenocytes. In syngeneic mice (middle row), there was no significant CIK cell proliferation and only a transient signal was observed on day 14 in cervical lymph nodes and abdomen. (B) Quantitation of photon emission of BLI. (C) Ex vivo imaging demonstrated compared with naive splenocytes, CIK cell homing to and proliferating in gastrointestinal tissues over time. (D) Histopathology of selected tissues on day 7 after transplantation. Magnification was ×200 or ×400 as indicated. Goblet cells (dashed arrow); crypt abscesses (solid arrow); lymphocytic infiltration surrounding the bile ducts (red arrow). (E) Mice receiving splenocytes (n = 3) or CIK cells (n = 3) were humanely killed on day 7 after BMT. GVHD histopathologic score was calculated on liver, small intestine, and large intestine damage on day 7 after BMT (CIK vs splenocytes, P = .002). Error bars represent SD.
Trafficking and survival of CIK cells upon adoptive transfer. Lethally irradiated host allogeneic (Balb/c, H-2d, n = 5) and syngeneic (FVB/N, H-2q, n = 5) recipients were injected with 5 × 106 FVB wild-type BM cells with 10 × 106 CIK cells generated from luc+ FVB mice (L2G85). To compare GVHD development, 10 × 106 L2G85 splenocytes were transplanted with BM cells (n = 5). (A) BLI of mice after transplantation. Donor-derived CIK cells in the allogeneic mice homed to and proliferated in secondary lymphoid organs, followed by the infiltration of gut and skin (top row), which was similar to GVHD progression (bottom row). However, in CIK cell–receiving mice, the speed of spread to peripheral tissue was slower and maximum peak signal from gut was lower than that in animals receiving fresh splenocytes. In syngeneic mice (middle row), there was no significant CIK cell proliferation and only a transient signal was observed on day 14 in cervical lymph nodes and abdomen. (B) Quantitation of photon emission of BLI. (C) Ex vivo imaging demonstrated compared with naive splenocytes, CIK cell homing to and proliferating in gastrointestinal tissues over time. (D) Histopathology of selected tissues on day 7 after transplantation. Magnification was ×200 or ×400 as indicated. Goblet cells (dashed arrow); crypt abscesses (solid arrow); lymphocytic infiltration surrounding the bile ducts (red arrow). (E) Mice receiving splenocytes (n = 3) or CIK cells (n = 3) were humanely killed on day 7 after BMT. GVHD histopathologic score was calculated on liver, small intestine, and large intestine damage on day 7 after BMT (CIK vs splenocytes, P = .002). Error bars represent SD.
Quantitative determination of photon emission by BLI demonstrated that the signal of allogeneic CIK cells increased rapidly up to day 10 and then declined thereafter. BLI signals over background persisted for approximately 90 to 120 days (Figure 2B).
To further evaluate the duration of CIK cell survival, we transplanted GFP+C57BL/6 CIK cells plus C57BL/6 BM cells into lethally irradiated allogeneic Balb/c mice and syngeneic C57BL/6 mice and sequentially analyzed the tissue distribution of CIK-derived cells (GFP+) by FACS, including peripheral blood, spleen, cervical and mesenteric lymph nodes, and thymus. CIK cells peaked by day 7 and persisted at least until day 90 in the peripheral blood in both allogeneic and syngeneic animals (Table 1). A high percentage of GFP+ cells ranging from 10% to 40% in the peripheral blood were detected at early time points after allogeneic BMT and then decreased over time, which was in concurrence with the quantification obtained by BLI (Figure 2B). Similarly, the percentage of syngeneic GFP+ CIK cells was relatively constant, ranging from 3% to 10% over time. Results from other sites were similar to those seen in peripheral blood except infiltration of the thymus. Allogeneic CIK cells infiltrated this organ ranging from 3% to 10% of total thymocytes; however, only a few syngeneic CIK cells were detected at this site (< 0.01%, data not shown).
Sequential analysis of the percentage of CIK-derived cells found in peripheral blood and their subpopulations after allogeneic and syngeneic bone marrow transplantations
| Days after BMT . | Allogeneic CIK . | Syngeneic CIK . | ||||||
|---|---|---|---|---|---|---|---|---|
| CIK-derived cells, % . | CD8+NKG2D+, % . | CD8+NKG2D−, % . | CD4+, % . | CIK-derived cells, % . | CD8+NKG2D+, % . | CD8+NKG2D−, % . | CD4+, % . | |
| 0* | 51.5 ± 6.2 | 39.5 ± 5.4 | 5.0 ± 2.0 | 51.5 ± 6.2 | 39.5 ± 5.4 | 5.0 ± 2.0 | ||
| 7 | 26.3 ± 0.8 | 54.0 ± 3.2 | 21.8 ± 0.2 | 22.1 ± 1.1 | 1.9 ± 1.1 | 75.5 ± 2.5 | 15.4 ± 2.9 | 4.0 ± 1.4 |
| 14 | 12.7 ± 3.0 | 57.5 ± 0.5 | 14.0 ± 2.4 | 18.6 ± 4.0 | 8.1 ± 2.1 | 80.6 ± 4.9 | 7.0 ± 0.1 | 6.4 ± 2.4 |
| 30 | 1.2 ± 0.7 | 60.5 ± 3.7 | 21.1 ± 6.1 | 17.2 ± 2.0 | 3.2 ± 1.3 | 82.6 ± 3.9 | 4.8 ± 1.1 | 6.3 ± 1.0 |
| 60 | 2.1 ± 0.7 | 51.5 ± 5.5 | 5.8 ± 1.3 | 41.9 ± 7.6 | 1.2 ± 0.2 | 76.1 ± 4.8 | 2.3 ± 0.5 | 9.5 ± 1.0 |
| 90 | 1.3 ± 0.1 | 50.1 ± 3.1 | 4.3 ± 1.4 | 46.7 ± 2.8 | 2.3 ± 1.0 | 77.9 ± 12.9 | 3.0 ± 1.1 | 7.2 ± 2.9 |
| Days after BMT . | Allogeneic CIK . | Syngeneic CIK . | ||||||
|---|---|---|---|---|---|---|---|---|
| CIK-derived cells, % . | CD8+NKG2D+, % . | CD8+NKG2D−, % . | CD4+, % . | CIK-derived cells, % . | CD8+NKG2D+, % . | CD8+NKG2D−, % . | CD4+, % . | |
| 0* | 51.5 ± 6.2 | 39.5 ± 5.4 | 5.0 ± 2.0 | 51.5 ± 6.2 | 39.5 ± 5.4 | 5.0 ± 2.0 | ||
| 7 | 26.3 ± 0.8 | 54.0 ± 3.2 | 21.8 ± 0.2 | 22.1 ± 1.1 | 1.9 ± 1.1 | 75.5 ± 2.5 | 15.4 ± 2.9 | 4.0 ± 1.4 |
| 14 | 12.7 ± 3.0 | 57.5 ± 0.5 | 14.0 ± 2.4 | 18.6 ± 4.0 | 8.1 ± 2.1 | 80.6 ± 4.9 | 7.0 ± 0.1 | 6.4 ± 2.4 |
| 30 | 1.2 ± 0.7 | 60.5 ± 3.7 | 21.1 ± 6.1 | 17.2 ± 2.0 | 3.2 ± 1.3 | 82.6 ± 3.9 | 4.8 ± 1.1 | 6.3 ± 1.0 |
| 60 | 2.1 ± 0.7 | 51.5 ± 5.5 | 5.8 ± 1.3 | 41.9 ± 7.6 | 1.2 ± 0.2 | 76.1 ± 4.8 | 2.3 ± 0.5 | 9.5 ± 1.0 |
| 90 | 1.3 ± 0.1 | 50.1 ± 3.1 | 4.3 ± 1.4 | 46.7 ± 2.8 | 2.3 ± 1.0 | 77.9 ± 12.9 | 3.0 ± 1.1 | 7.2 ± 2.9 |
Lethally irradiated host mice (allogeneic Balb/c (H-2d) recipients and syngeneic C57BL/6 (H-2b) recipients) were given 5 × 106 C57BL/6 wild-type BM cells with 10 × 106 CIK cells generated from GFP+ C57BL/6 mice. The percentage of GFP+ cells in peripheral blood and their subpopulations was analyzed by FACS at indicated time points.
The CIK subpopulations at day 0 were day 14 of CIK-cultured cells in vitro.
Allogeneic CIK cells traffic to GVHD target organs
To analyze the tissue distribution of CIK cells in the abdomen in greater detail, we performed ex vivo BLI on freshly prepared intraperitoneal organs such as the liver, spleen, and gastrointestinal tract (GIT). Ex vivo images of GVHD controls revealed that infused donor splenocytes homed to and proliferated in secondary lymphoid tissues by day 3, such as the spleen, mesenteric lymph nodes, and Peyer patches, followed by infiltration of the GIT by day 5 with strong signals that increased over time until the control animals expired (Figure 2C). The pattern of allogeneic CIK progression was similar to that of fresh splenocytes; however, the GIT infiltration of allogeneic CIK cells was slower and waxed and waned compared with splenocytes. Strikingly, the signal from the small and large intestines in the mice receiving allogeneic CIK cells was much weaker (Figure 2C, day 7 in CIK) and transient (Figure 2C, day 9 in CIK), leading to allogeneic CIK cells causing much less GVHD. After the signal from the gut disappeared, the signals from the mesenteric lymph nodes, Peyer patches, and spleen remained high and then allogeneic CIK cells transiently infiltrated the GIT by day 21.
Ex vivo imaging of mice receiving syngeneic CIK cells was only performed on day 14, because this was the only day that we observed a signal over the abdomen. The signal proved to be from the mesenteric lymph nodes, not from the GIT.
Many crypt abscesses indicative of GVHD were observed in the large intestine from mice that received allogeneic splenocytes. In contrast, there were only a few crypt abscesses in the mice receiving CIK cells with minimal tissue damage. Likewise, liver histopathology displayed strong lymphocytic infiltration surrounding the bile ducts in mice receiving splenocytes, whereas mice treated with CIK cells showed no such cellular infiltration (Figure 2D). GVHD pathologic scores based on liver and small and large intestine damage clearly demonstrated that allogeneic CIK cells did not induce significant GVHD (CIK vs GVHD, P = .002), with almost the same tissue damage as BM alone (CIK vs BM alone, P = .158; Figure 2E).
Allogeneic CIK proliferation in vivo is driven by both major and minor histocompatibility antigens
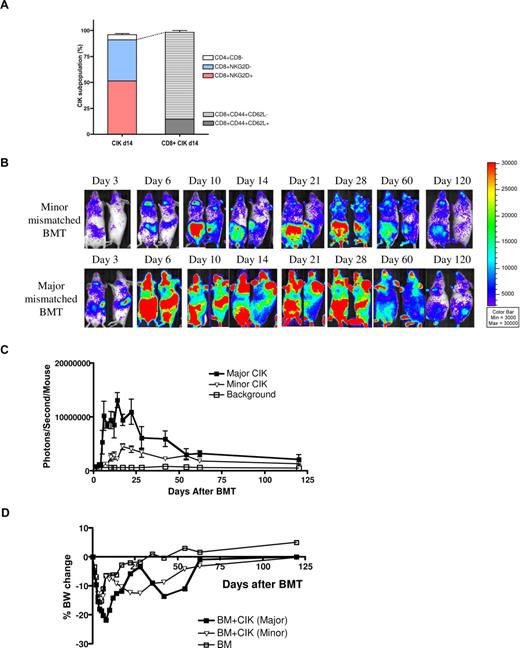
CIK cells are a heterogeneous population including major subsets such as CD8+NKG2D+, CD8+NKG2D−, and CD4+CD8− T cells. In 15% to 20% of CD8+ CIK cells, both CD44 and CD62L were expressed (Table 1, Figure 3A). After allogeneic BMT the CD8+NKG2D+ subset persisted and the percentage of CD4+ T cells gradually increased, whereas CD8+NKG2D− cells declined over time.
Comparison of CIK cells trafficking between major- and minor-mismatched recipients. Balb/c (H-2d, major mismatch, n = 5) and Balb/b (H-2b, minor mismatch, n = 5) recipients were lethally irradiated, and then injected with 5 × 106 C57BL/6 (H-2b) wild-type BM with 10 × 106 CIK cells generated from B6-L2G85 (H-2b) luc+ mice. (A) CIK subpopulations. (B) BLI images and (C) quantitative analysis of BLI over time. (D) Percent body weight change over time. Error bars represent SD.
Comparison of CIK cells trafficking between major- and minor-mismatched recipients. Balb/c (H-2d, major mismatch, n = 5) and Balb/b (H-2b, minor mismatch, n = 5) recipients were lethally irradiated, and then injected with 5 × 106 C57BL/6 (H-2b) wild-type BM with 10 × 106 CIK cells generated from B6-L2G85 (H-2b) luc+ mice. (A) CIK subpopulations. (B) BLI images and (C) quantitative analysis of BLI over time. (D) Percent body weight change over time. Error bars represent SD.
To more similarly mimic the human BMT setting, we studied the kinetics of CIK cell trafficking and survival in a minor mismatch model. CIK cells from B6-L2G85 luc+ animals were transplanted into Balb/c (major mismatch) and Balb/b (minor mismatch) recipients. The pattern of homing and proliferation of the minor-mismatched CIK cells was similar to the major-mismatched model; however, the speed of spread to peripheral tissues was slower and maximum peak of photon emission was lower than that of major MHC-mismatched CIK cells (Figure 3B,C). These observations indicated that the proliferation of allogeneic CIK cells was driven not only by MHC disparity, but also by mismatch of minor antigens.
Body weight recovery in the minor-mismatched model was more complete than in the major-mismatched model, especially at early time points after BMT; however, the body weight loss was observed again after day 12 (Figure 3D). These findings were in accordance with the delayed BLI signal increase over the abdomen, suggesting that CIK cells induced only very mild GVHD.
Early proliferation of CIK cells compared with splenocytes in vivo
The above observations of BLI studies suggested that the proliferation of CIK cells after allogeneic BMT might be slower than that of splenocytes, which could be one of the reasons why allogeneic CIK cells cause less GVHD. To further evaluate cellular proliferation of these different cell populations, we transplanted CFSE-labeled CIK cells generated from congenic C57BL/6 (H-2b, Thy1.1) or splenocytes plus wild-type C57BL/6 (H-2b, Thy1.2) BM into lethally irradiated Balb/c mice. The congenic markers Thy1.1 and Thy1.2 were used to distinguish CIK cells or fresh splenocytes from BM-derived T cells. On day 3 after transplantation, we analyzed the rate of cellular division of CIK cells and splenocyte-derived cells in the spleen using a CFSE-based cell proliferation assay. In animals that received allogeneic splenocytes, the CFSE level was very low in both CD4+ and CD8+ T cells, suggesting that these cells had already divided at least 10 times after only 3 days in vivo. In contrast, CD8+ T cells derived from CIK cells, which were the main subpopulation in CIK cell cultures, divided with much slower kinetics (Figure 4A). The proliferation index of CIK cells was statistically significantly lower than that of splenocytes in both CD8 and CD4 (Figure 4B). These results further demonstrate that the division rate of CIK cells in vivo was much less than that of naive splenocytes and led to much less GVHD.
Cell division rate of CIK cells in vivo compared with naive splenocytes. Lethally irradiated Balb/c recipient mice were injected with 5 × 106 C57BL/6 (H-2b, Thy1.2) wild-type BM cells with either CFSE-labeled 10 × 106 CIK cells or splenocytes generated from congenic C57BL/6 (H-2b, Thy1.1). (A) On day 3, the cell division rate from splenocytes (top row) and CIK cells (bottom row) in the spleen was analyzed by a CFSE-based cell proliferation assay. Similar results were obtained in 3 allogeneic and 3 syngeneic receipient mice. (B) Proliferation index of CD4 and CD8 T cells of splenocytes and CIK cells. Error bars represent SD. *P < .05; **P < .01.
Cell division rate of CIK cells in vivo compared with naive splenocytes. Lethally irradiated Balb/c recipient mice were injected with 5 × 106 C57BL/6 (H-2b, Thy1.2) wild-type BM cells with either CFSE-labeled 10 × 106 CIK cells or splenocytes generated from congenic C57BL/6 (H-2b, Thy1.1). (A) On day 3, the cell division rate from splenocytes (top row) and CIK cells (bottom row) in the spleen was analyzed by a CFSE-based cell proliferation assay. Similar results were obtained in 3 allogeneic and 3 syngeneic receipient mice. (B) Proliferation index of CD4 and CD8 T cells of splenocytes and CIK cells. Error bars represent SD. *P < .05; **P < .01.
Rate of early apoptotic cells in CIK cells compared with splenocytes
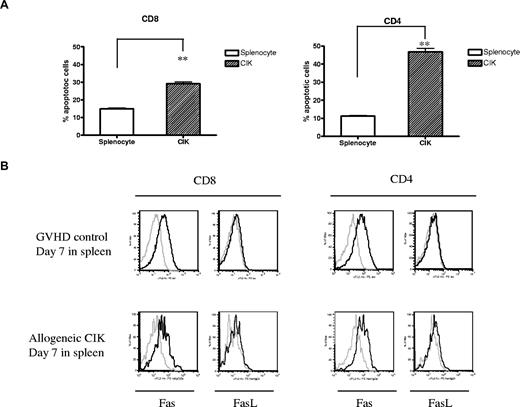
Another possible explanation as to why CIK cells cause less GVHD is that CIK cells may undergo more rapid apoptosis than splenocytes in vivo. On day 7 after BMT, using the congeneic markers, we measured the early apoptotic cells of donor-derived CIK cells using annexin V and PI staining. Percentages of early apoptotic cells in the mice receiving CIK cells, which were defined as the fraction of annexin V–positive and PI-negative cells, were significantly higher than those of mice receiving fresh splenocytes in both CD8 (P = .006) and CD4 (P = .003) subpopulations (Figure 5A).
Rate of apoptosis of transferred T cells. Lethally irradiated host mice (Balb/c, H-2d) were injected with 5 × 106 C57BL/6 (H-2b, Thy1.2) wild-type BM cells with either 10 × 106 CIK cells generated from congeneic C57BL/6 (H-2b, Thy1.1) or 10 × 106 splenocytes. (A) Early apoptotic cells from CIK cells (n = 3) and GVHD effector cells (n = 3) from the spleen were analyzed on day 7 after BMT separately in CD8+ and CD4+ lymphocytes by FACS using annexin V and PI staining. Error bars represent SD. **P < .01. (B) Comparison of Fas and FasL expression between GVHD controls and CIK cell–receiving mice on day 7 after BMT. One representative result of 3 similar experiments is shown.
Rate of apoptosis of transferred T cells. Lethally irradiated host mice (Balb/c, H-2d) were injected with 5 × 106 C57BL/6 (H-2b, Thy1.2) wild-type BM cells with either 10 × 106 CIK cells generated from congeneic C57BL/6 (H-2b, Thy1.1) or 10 × 106 splenocytes. (A) Early apoptotic cells from CIK cells (n = 3) and GVHD effector cells (n = 3) from the spleen were analyzed on day 7 after BMT separately in CD8+ and CD4+ lymphocytes by FACS using annexin V and PI staining. Error bars represent SD. **P < .01. (B) Comparison of Fas and FasL expression between GVHD controls and CIK cell–receiving mice on day 7 after BMT. One representative result of 3 similar experiments is shown.
Fas- and Fas ligand–mediated apoptosis play an important role in GVHD development.14 Therefore, we compared the level of expression of Fas and Fas ligand between GVHD control mice and CIK-receiving mice. Expression of Fas was up-regulated almost equivalently and the expression level of Fas ligand remained unchanged in both groups on splenocytes on day 7 after BMT (Figure 5B).
CIK cells express lower levels of homing molecules toward inflamed GVHD target organs
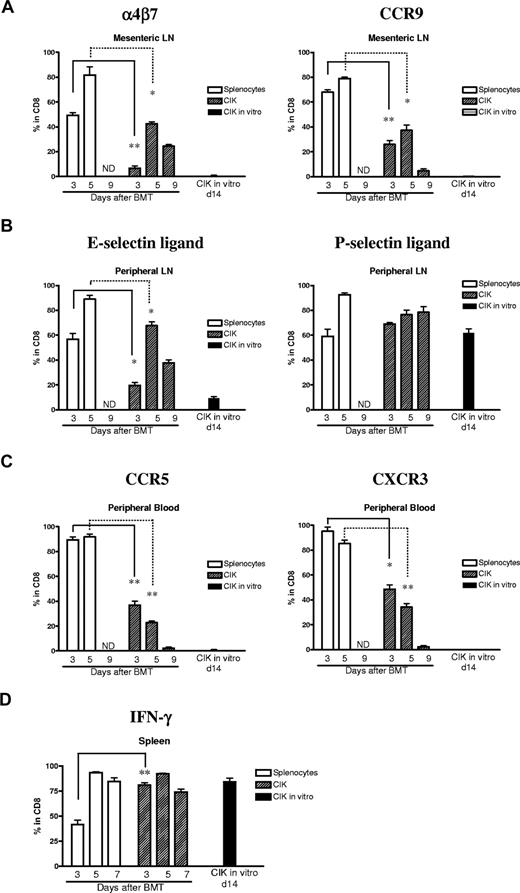
Several studies have demonstrated that after allogeneic stimulation there is up-regulation of homing molecules and chemokine receptors which allow lymphocytes to enter GVHD target organs.15,16 To test the possibility that expression patterns of such molecules in CIK cells differ from those of GVHD effector cells, we compared the expression of α4β7, CCR9, E-selectin ligand, P-selectin ligand, CCR5, and CXCR3 on donor CIK cells or splenocytes over time. The gut homing molecule, α4β7, was expressed on less than 5% of naive T cells and CIK cells in vitro. Cells expressing this molecule were evaluated in the regional lymph nodes after BMT. The up-regulation of α4β7 on splenocytes in GVHD control animals was much faster and its peak level higher (> 80%) than those of CIK cells in the mesenteric lymph nodes, and a similar tendency was also observed in another gut homing molecule, CCR9 (Figure 6A). Further, we assessed 2 skin homing molecules, E-selectin ligand and P-selectin ligand, respectively. E-selectin ligand was also up-regulated more on splenocytes than CIK cells; however, P-selectin ligand was expressed at almost the same level between the groups receiving naive splenocytes and CIK cells over time (Figure 6B). All homing molecules were expressed at very low levels (0%-5%) on CIK cells in vitro except P-selectin ligand.
Expression of chemokine receptors and homing molecules between CIK cells and splenocytes. Lethally irradiated host mice (Balb/c, H-2d) were injected with C57BL/6 (H-2b, Thy1.2) wild-type 5 × 106 BM cells with either 10 × 106 CIK cells or splenocytes generated from congenic C57BL/6 (H-2b, Thy1.1). Expression of chemokine receptors, homing molecules, and IFN-γ expressed on donor-derived cells were analyzed at indicated days and tissues after BMT by FACS. (A) α4β7 and CCR9. (B) E-selectin ligand and P-selectin ligand. (C) CCR5 and CXCR3. (D) IFN-γ. ND indicates not done. Samples were not obtained because all GVHD control mice died by day 9. LN indicates lymph node. Error bars represent SD. *P < .05; **P < .01.
Expression of chemokine receptors and homing molecules between CIK cells and splenocytes. Lethally irradiated host mice (Balb/c, H-2d) were injected with C57BL/6 (H-2b, Thy1.2) wild-type 5 × 106 BM cells with either 10 × 106 CIK cells or splenocytes generated from congenic C57BL/6 (H-2b, Thy1.1). Expression of chemokine receptors, homing molecules, and IFN-γ expressed on donor-derived cells were analyzed at indicated days and tissues after BMT by FACS. (A) α4β7 and CCR9. (B) E-selectin ligand and P-selectin ligand. (C) CCR5 and CXCR3. (D) IFN-γ. ND indicates not done. Samples were not obtained because all GVHD control mice died by day 9. LN indicates lymph node. Error bars represent SD. *P < .05; **P < .01.
We also evaluated CXCR3 and CCR5, homing molecules that direct cells to inflamed tissues, and observed that these molecules were highly expressed (> 80%) even by day 3 in GVHD control group CD8+ T cells in peripheral blood. In marked contrast, the expression of CXCR3 and CCR5 on CIK cells was at most 30% to 50% by day 3 and then declined to near baseline levels by day 9 (Figure 6C).
CIK cells retain high levels of IFN-γ in vivo
We have previously shown that CIK cells in vitro produced high levels of IFN-γ, which has a protective effect against GVHD at early time points after BMT.17 CIK cells in vivo retained high amounts of IFN-γ secretion throughout BMT, whereas the expression of IFN-γ was significantly lower by day 3 in GVHD control T cells in spleen than CIK cells at the same time point (Figure 6D).
Exogenous IL-2 does not worsen GVHD in mice receiving allogeneic CIK cells
As IL-2 is the essential cytokine for CIK cell growth in vitro, we further evaluated whether exogenous administration of IL-2 (50 000 U/mouse per day)18 affected CIK kinetics in vivo. No significant differences were observed in BLI, survival, body weight loss, proliferation index, and the rate of apoptosis between the mice receiving allogeneic CIK cells with or without IL-2 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Allogeneic CIK cells in vivo do not exacerbate GVHD even if high doses of exogenous IL-2 are administered.
CIK cells retain antitumor function in vivo
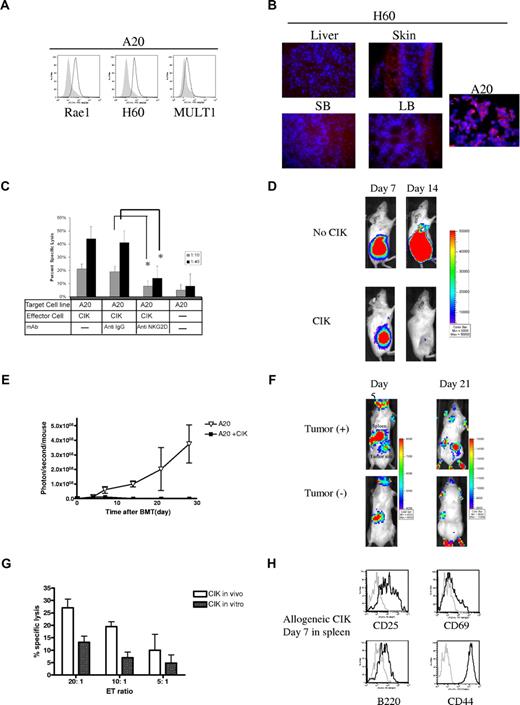
To determine the GVL function of CIK cells, we used BLI to assess tumor responses and CIK cell trafficking to tumor sites. Animals were injected with A20 luc+ leukemia/lymphoma B cells subcutaneously followed by wild-type allogeneic CIK cells in some animals with TCD-BM. The A20 cells expressed the NKG2D ligands Rae1, H60, and MULT1 (Figure 7A). To investigate whether allogeneic CIK cells kill A20 tumor cells by a NKG2D-mediated mechanism we evaluated in vitro cytotoxicity in the presence and absence of a blocking antibody against NKG2D. The addition of blocking mAbs directed against NKG2D significantly attenuated cytotoxicity, whereas an isotype control antibody had little effect (Figure 7C). In addition, immunohistochemistry staining of GVHD target tissues at days 3 and 5 after irradiation and BMT demonstrated that large and small bowel epithelium, skin, and liver did not up-regulate expression of NKG2D ligands MULT1 nor Rae1 or H60 (Figure 7B, Figure S2). This may further explain why allogeneic CIK cells caused less GVHD while retaining GVT effects.
CIK cells retain tumor killing activity and home to tumor site in vivo. (A) NKG2D ligands were expressed on A20 tumor cells, (B) but not on GVHD target tissues such as small (SB) and large bowel (LB), skin, and liver on day 3 after irradiation. (C) Cytolysis against A20 cells. Effectors (allogeneic CIK cells) were combined with targets at indicated ratio either alone or in the presence of isotype control antibodies or NKG2D antibodies. *P < .05. (D) A20 luc+ leukemia/lymphoma cells (106; H-2d) were injected into Balb/c (H-2d) hosts in the right flank subcutaneously after lethal irradiation, followed by injection of 5 × 106 FVB wild-type (H-2q) TCD-BM with or without 10 × 106 CIK cells generated from FVB wild-type animals. Tumor regression was observed in CIK cell–receiving mice. (E) Emitted photons from luc+ tumor cells over time. (F) A20 cells (106) were injected into the right flank of Balb/c hosts subcutaneously after lethal irradiation, followed by injection of 5 × 106 FVB wild-type TCD-BM with 10 × 106 CIK cells generated from luc+ L2G85 animals. Accumulation of luc+ CIK cells was observed in the tumor site. (G) CIK cells were collected on day 7 after BMT and compared with expanded CIK cells prior to injection. Lethally irradiated Balb/c (H-2d) mice were given 5 × 106 C57BL/6 BM plus 10 × 106 CIK cells generated from GFP+C57BL/6 splenocytes. On day 7, GFP+ cells were recovered from the host spleen by FACS sorting and then used in a 51Cr release cytotoxicity assay against A20 cells. Error bars represent SD. (H) Activation status of allogeneic CIK cells from the host spleen in vivo was analyzed on day 7 after BMT.
CIK cells retain tumor killing activity and home to tumor site in vivo. (A) NKG2D ligands were expressed on A20 tumor cells, (B) but not on GVHD target tissues such as small (SB) and large bowel (LB), skin, and liver on day 3 after irradiation. (C) Cytolysis against A20 cells. Effectors (allogeneic CIK cells) were combined with targets at indicated ratio either alone or in the presence of isotype control antibodies or NKG2D antibodies. *P < .05. (D) A20 luc+ leukemia/lymphoma cells (106; H-2d) were injected into Balb/c (H-2d) hosts in the right flank subcutaneously after lethal irradiation, followed by injection of 5 × 106 FVB wild-type (H-2q) TCD-BM with or without 10 × 106 CIK cells generated from FVB wild-type animals. Tumor regression was observed in CIK cell–receiving mice. (E) Emitted photons from luc+ tumor cells over time. (F) A20 cells (106) were injected into the right flank of Balb/c hosts subcutaneously after lethal irradiation, followed by injection of 5 × 106 FVB wild-type TCD-BM with 10 × 106 CIK cells generated from luc+ L2G85 animals. Accumulation of luc+ CIK cells was observed in the tumor site. (G) CIK cells were collected on day 7 after BMT and compared with expanded CIK cells prior to injection. Lethally irradiated Balb/c (H-2d) mice were given 5 × 106 C57BL/6 BM plus 10 × 106 CIK cells generated from GFP+C57BL/6 splenocytes. On day 7, GFP+ cells were recovered from the host spleen by FACS sorting and then used in a 51Cr release cytotoxicity assay against A20 cells. Error bars represent SD. (H) Activation status of allogeneic CIK cells from the host spleen in vivo was analyzed on day 7 after BMT.
To explore the in vivo effect of allogeneic CIK cells we used A20 expressing yfp/luc. Animals received luc-expressing A20 cells followed by TCD-BM and in some animals wild-type CIK cells. Tumor signal on day 7 was similar in both groups. By day 14, tumor growth was eradicated in the mice receiving CIK cells in a dose-dependent manner (Figure 7D, Figure S3) and the effect persisted (Figure 7E).
To investigate whether luc+ CIK cells trafficked to specific tumor sites, we visualized CIK cell trafficking by BLI in mice bearing wild-type A20 cells. By day 5, a strong signal was observed from the lower right dorsal area, where tumors were injected, with a bright signal from the spleen in both tumor-bearing and non–tumor-bearing animals. A sustained strong BLI signal from the tumor site was evident on day 21 (Figure 7F), leading to prolonged suppression of tumor growth.
To demonstrate that CIK cells recovered from animals retained tumor cytotoxicity, we transplanted 5 × 106 C57BL/6 TCD-BM with 10 × 106 GFP+ C57BL/6 CIK cells into lethally irradiated Balb/c recipients. GFP+ CIK cells were extracted on day 7 from the spleen by FACS sorting and evaluated in cytotoxicity experiments against A20 tumor cells. Both cells cultured in vitro and cells recovered in vivo demonstrated cytotoxicity against A20 cells (Figure 7G). The GFP+ CIK cells recovered from the animals maintained the activation markers seen in vitro: CD25, CD44, CD69, and B220 (Figure 7H).
Discussion
Little is known about the fate of activated tumoricidal T cells, such as CIK cells after adoptive transfer in vivo. Previous work has demonstrated that CIK cells have much less capacity for induction of GVHD compared with splenocytes, yet have retained antitumor activity.2,9 We have previously characterized the temporal events involved in GVHD induction by splenocytes using BLI12 and hypothesized that differences in the in vivo trafficking and survival of adoptively transferred CIK cells may provide insights into their biologic characteristics. In this study we used both FVB (H-2q) and C57BL/6 (H-2b) animals to generate CIK cells with similar results. Up to 20 × 106 CIK cells could be injected into irradiated Balb/c animals with minimal GVHD, whereas only 2.5 × 106 splenocytes induced acute lethal GVHD. Using BLI, we evaluated the trafficking and survival of CIK cells compared with splenocytes in both allogeneic and syngeneic recipients. CIK cells had dramatically increased proliferation in allogeneic (both major- and minor-mismatched) recipients compared with syngeneic recipients and could be detected for at least 90 days. Although IL-2 is an essential cytokine for CIK growth in vitro, exogenous administration of IL-2 had no impact on CIK kinetics or capacity for GVHD induction in vivo.
Both allogeneic CIK cells and naive splenocytes homed to and proliferated in GVHD target organs, although both the proliferation rate and signal intensity in GVHD target tissues were significantly less in animals that received luc+ CIK cells compared with splenocytes. By evaluating BLI images and isolating donor-derived cells, we first demonstrated that the division rate of CIK cells in vivo was much slower than that of naive T cells, especially in the CD8+ subpopulation. Second, in vivo CIK cells were more susceptible to apoptosis compared with naive splenocytes. When GVHD developed, activated naive T cells underwent apoptosis by Fas/Fas ligand–mediated apoptosis14,19 and IFN-γ enhanced Fas-mediated apoptosis of activated donor T cells.20 Interestingly, a much higher percentage of early apoptotic cells was detected in the mice receiving CIK cells; however, this difference was not due to increased levels of Fas and Fas ligand expression. Third, significant differences of IFN-γ secretion were observed between the mice receiving CIK cells and those receiving splenocytes by day 3 after BMT. Although the role of IFN-γ on GVHD progression is controversial, IFN-γ has protective effects against GVHD, especially at early time points after BMT.17 CIK cells consistently produced IFN-γ even at very early time points after BMT. Our previous work has demonstrated the importance of IFN-γ production because CIK cells from IFN-γ knock-out animals were capable of inducing lethal GVHD.9 Finally, we demonstrated that CIK cells expressed lower levels of homing markers and chemokine receptors, which direct cells toward inflamed GVHD organs, compared with splenocytes in vivo. Waldman et al recently demonstrated that α4β7 was a crucial homing molecule toward gut and liver for GVHD effector cells by using β7 subunit knockout mice.21 In the same way using knockout mice, CCR5 and CXCR3, which are induced by inflammation, were shown to be key molecules in GVHD induction.22,23 Partial expression of CCR5 and CXCR3 on day 3 in CIK cells might be due to the irradiation-induced tissue damage used as BMT conditioning. The skin homing molecule, E-selectin ligand, was up-regulated more on GVHD effector cells than CIK cells over time. Another skin homing molecule, P-selectin ligand, was maintained at high levels after BMT in mice receiving CIK cells. This might explain why the signal from the skin even in mice receiving CIK cells was relatively high; however, clinical symptoms of skin GVHD were not observed. We did not observe significant up-regulaton of NKG2D ligands at sites of potential GVHD induction, suggesting that even cells that are capable of infiltrating these target tissues were not activated through NKG2D-mediated mechanisms which are critically important for CIK cell–mediated cytoxicity. These collective findings help explain the lesser tissue damage observed in CIK-treated animals.
CD4+CD25+ regulatory T cells (Tregs) have been shown to reduce GVHD.11,24 Some groups have reported that Tregs can be expanded from CD4 cells in the presence of anti–CD3 antibody and IL-2 with or without rapamycin.25,26 We found between 5% and 15% CD4+CD25+ cells in CIK cultures in vitro. To distinguish between activated CD4 cells and Tregs, we evaluated Foxp3 expression, which has been identified as a more specific marker for Tregs.27 No Foxp3-positive cells were observed in CD4+CD25+ cells in CIK cell cultures, nor in CD8+CD25+ cells, which also have immunoregulatory function (data not shown).28 Another possible mechanism to explain the reduced GVHD in CIK cell–treated animals was potential changes in the surface molecules that induced T-cell tolerance such as PD-1 and CTLA-4, both of which are involved in attenuation of GVHD.29,30 Therefore, we compared the expression levels of these molecules in mice receiving CIK cells compared with splenocytes on day 7 after BMT. However, no significant differences were noted between the CIK cells and splenocytes.
In addition to GVHD induction we evaluated the impact of allogeneic CIK cells on tumor growth. CIK cells significantly reduced the growth of A20 B-cell leukemia/lymphoma cells in a dose-dependent manner. We observed the direct trafficking of CIK cells to tumor sites where A20 leukemia/lymphoma cells were injected subcutaneously, and they persisted there for several weeks. The BLI signal from the tumor site was much higher than that of peritumoral normal skin, suggesting that CIK cells are capable of specifically migrating to extravascular tumor sites. As CIK cells kill tumors mainly through NKG2D-mediated cytotoxicity, it is interesting to note that NKG2D ligands are highly expressed in a variety of both solid and liquid tumors31 but not on many normal tissues, including GVHD target tissues even after irradiation. Recently, several strategies using CIK cells have been developed to enhance cytotoxic activity against tumors. First, Zhang et al demonstrated that CIK cells cocultured with dendritic cells (DCs) pulsed with tumor lysates had enhanced tumor killing activity,32 suggesting that CIK cells eliminate tumors not only by innate immunity mediated by NKG2D but also by acquired immune systems. Second, our group showed that bispecific antibody coadministered with CIK cells, which redirect CIK cells to tumor targets with affinity to both CD3 and cancer antigens such as Her2neu, resulted in significantly higher cytotoxicity in vitro and in vivo in a rodent model.33,34 Furthermore, Thorne et al demonstrated that CIK cells infected with oncolytic vaccinia virus had synergistic antitumor effects in vitro and in vivo.35
CIK cells have been used in patients with hematologic malignancies after both autologous36 and allogeneic BMT37 to treat or possibly reduce the risk of disease recurrence instead of conventional donor lymphocyte infusions (DLIs) to treat relapsed patients after BMT. Based on our animal studies, infusions with a higher number of allogeneic CIK cells might be tolerated to seek better GVT effects without causing GVHD.
In conclusion, CIK cells traffic and survive in allogeneic recipients for prolonged periods with much less GVHD due to the reduced proliferation capacity of this cell population. CIK cells retain strong tumor killing activity and homing capacity to the tumor site for a prolonged period after BMT. These studies further demonstrate that CIK cells may be an effective alternative to treat or prevent relapse after allogeneic transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tsuyoshi Takahashi, Vu H. Nguyen, and Dennis Leveson-Gower for helpful discussion and excellent technical assistance. We are also grateful to Jacob Y. Shin and Enosh M. Baker for providing suggestions and helpful instruction.
This work was supported by the Dana Foundation (New York, NY) and by National Institutes of Health (Bethesda, MD) grants PO1-CA49605 and RO1-CA80006.
National Institutes of Health
Authorship
Contribution: R.N. designed and performed research, analyzed and wrote the manuscript; J.B., A.B., R.Z., J.O., E.S., and K.M. helped design experiments, performed research, and analyzed data; and R.S.N. provided overall research advice and helped write the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert S. Negrin, Center for Clinical Science Research Building, Room 2205, 269 W Campus Drive, Stanford University, Stanford, CA 94305; e-mail: negrs@stanford.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal