Abstract
CD4+CD25+Foxp3+ regulatory T cells (Treg) play an important role in the induction and maintenance of immune tolerance. Although adoptive transfer of bulk populations of Treg can prevent or treat T cell–mediated inflammatory diseases and transplant allograft rejection in animal models, optimal Treg immunotherapy in humans would ideally use antigen-specific rather than polyclonal Treg for greater specificity of regulation and avoidance of general suppression. However, no robust approaches have been reported for the generation of human antigen-specific Treg at a practical scale for clinical use. Here, we report a simple and cost-effective novel method to rapidly induce and expand large numbers of functional human alloantigen-specific Treg from antigenically naive precursors in vitro using allogeneic nontransformed B cells as stimulators. By this approach naive CD4+CD25− T cells could be expanded 8-fold into alloantigen-specific Treg after 3 weeks of culture without any exogenous cytokines. The induced alloantigen-specific Treg were CD45RO+CCR7− memory cells, and had a CD4high, CD25+, Foxp3+, and CD62L (L-selectin)+ phenotype. Although these CD4highCD25+Foxp3+ alloantigen-specific Treg had no cytotoxic capacity, their suppressive function was cell-cell contact dependent and partially relied on cytotoxic T lymphocyte antigen-4 expression. This approach may accelerate the clinical application of Treg-based immunotherapy in transplantation and autoimmune diseases.
Introduction
CD4+CD25+Foxp3+ regulatory T cells (Treg) are negative regulators of immune responses to self- and foreign antigens and play a critical role in maintaining immune tolerance by suppressing pathologic immune responses in autoimmune diseases, transplant allograft rejection, and graft-versus-host disease (GVHD).1-3 On adoptive transfer in rodents, Treg were found to control experimental autoimmune diseases,4 inhibit GVHD,5,6 and prevent transplant allograft rejection,7,8 indicating that Treg-based therapy has a great therapeutic potential for these diseases in humans.
An important obstacle to Treg-based therapy has been the limited numbers of these cells that are available, as only approximately 1% to 2% of circulating human CD4+ T cells are Treg. Several groups have developed protocols to expand a large number of polyclonal CD4+CD25+ Treg in vitro with repeated stimulation by either CD3 and CD28 mAbs or artificial antigen-presenting cells (APCs) for activation through CD3 and CD28, together with exogenous high-dose interleukin-2 (IL-2).9-11 However, polyclonal Treg may cause global immune suppression.4,7 In addition, because there are only few antigen-specific Treg in the population of the polyclonal Treg, very large numbers of nonspecifically expanded Treg are required to inhibit bone-marrow allograft rejection in animal models.12 All of these characteristics of polyclonal Treg hamper their clinical applications.
In contrast, adoptive transfer of antigen-specific Treg has been shown to prevent and treat T cell–mediated inflammatory diseases with high efficiency. In animal models, small numbers of antigen-specific Treg can suppress experimental autoimmune diseases13 and prevent GVHD and allograft rejection in bone marrow and solid organ transplantation.14,15 Importantly, the transfer of antigen-specific Treg prevented target antigen-mediated T-cell responses, such as GVHD and allograft rejection, but did not compromise host general immunity, including the graft-versus-tumor activity and antiviral immunity.5,15-17 Based on these studies, antigen-specific Treg has substantial promise for human immunotherapy.
The reliable induction and expansion of rare antigen-specific Treg are technically challenging. Currently, several protocols for murine antigen-specific Treg induction and expansion have been reported in which either purified CD4+CD25− or CD4+CD25+ cells were cocultured with autologous dendritic cells (DCs) pulsed with alloantigen in the presence of high-dose IL-2 or directly cocultured with allogeneic DCs.14,18-20 Similar protocol has also been reported for generation of human antigen-specific Treg recently.21 In this protocol, antigen-specific CD4+CD25+ Treg can be generated using the coculture of CD4+CD25− T cells with allogeneic monocyte-derived DCs. However, the large-scale in vitro expansion of alloantigen-specific Treg is difficult because of certain features of DCs. For example, DCs are relatively rare in peripheral blood and are usually derived from apheresis or marrow sources, including monocytes.22,23 Further, DCs are not homogeneous and include multiple subsets with different functional capacities.24 Finally, there is no effective way to expand human DCs so far.25 In addition, the current approaches to generate human DCs in vitro are expensive and laborious.26
Schultze et al reported a simple and low-cost method to expand large number human CD40-activated B cells up to 105- to 106-fold from human peripheral blood mononuclear cells (PBMCs).27 These expanded B cells are effective as APCs and can efficiently induce antigen-specific T cells and cytotoxic T lymphocytes.26,27 In this study, we developed a novel protocol to induce and expand highly efficient human alloantigen-specific Treg in large-scale by coculture of naive CD4+CD25− T cells with human allogeneic CD40-activated B cells without any exogenous cytokines. The induced alloantigen-specific Treg were CD45RO+ and CCR7− memory cells, and expressed the common Treg markers (CD25 and Foxp3), as well as the lymph node homing receptor CD62L (L-selectin). Importantly, they were also identifiable by a CD4high surface phenotype. The suppressive function of these CD4highCD25+Foxp3+ alloantigen-specific Treg was cell-cell contact dependent but did not involve cell-mediated cytotoxicity. This novel approach for in vitro induction and expansion of alloantigen-specific Treg should facilitate the development of Treg-based clinical immunotherapy.
Methods
Generation of CD40-activated B cells
Human peripheral blood was obtained from healthy donors in accordance with the Declaration of Helsinki and with ethical committee approval from the Institutional Review Boards of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. PBMCs were isolated by density gradient centrifugation as previously reported.28,29 B cells from PBMCs were stimulated via CD40 using NIH3T3 cells transfected with the human CD40 ligand (t-CD40-L cells) as described previously.27 The transfected cells have been stable for human CD40L expression over a period of 5 years, and no other human molecules are expressed on t-CD40-L cells.27 The lethally irradiated (96 Gy) t-CD40-L cells were plated on 6-well plates (Corning Life Sciences, Acton, MA) at a concentration of 0.4 × 105 cells/well in medium containing 45% Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA), 45% F12 (Invitrogen) 10% fetal calf serum, 2 mM glutamine (Invitrogen), and 15 μg/mL gentamicin (Invitrogen). After an overnight culture at 37°C in 5% CO2, t-CD40-L cells were adherent and ready for B-cell culture. PBMCs at 2 × 106 cells/mL were cocultured at 37°C in 5% CO2 with t-CD40-L cells in the presence of IL-4 (2 ng/mL; R&D Systems, Minneapolis, MN) and cyclosporin A (5.5 × 10−7 M) in Iscove modified Dulbecco medium (Invitrogen) supplemented with 10% human AB serum, 50 μg/mL transferrin (Roche Diagnostics, Indianapolis, IN), 5 μg/mL insulin (Sigma-Aldrich, St Louis, MO), and 15 μg/mL gentamicin (Invitrogen). The concentration of cyclosporin A used here was found to only suppress T-cell proliferation without affecting B-cell growth. Cultured cells were transferred to the wells of new plates with fresh irradiated t-CD40-L cells every 3 to 5 days. Once the cultured PBMCs were 75% CD19, they were cultured at concentrations of 0.75 to 1.0 × 106 cells/mL. The number of viable cells and CD19+ B cells was analyzed by flow cytometry every 3 to 5 days. After 14 days of coculture, more than 95% of the viable suspended cells are CD19 positive. B cells were cryopreserved for future use. For coculture with CD4+ T cells, the cryopreserved CD40-activated B cells were always centrifuged on a Ficoll-Hypaque density gradients and washed twice in phosphate-buffered saline to remove nonviable cells, including remaining t-CD40-L cells. Alternatively, t-CD40-L cells were replaced by different concentrations of the soluble hexameric CD40-L (sCD40-L; Alexis Biochemicals, Lausen, Switzerland) to expand B cells.30
T-cell isolation
Human CD4+ or naive CD4+ T cells were isolated from healthy donor PBMCs by negative selection using a CD4+ T-cell isolation kit or a naive CD4+ T-cell isolation kit (Miltenyi Biotec, CA) for depletion of cells expressing CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ, and CD235a (glycophorin A) (for CD4+ T cells) or depletion of CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγ/δ, CD235a, and CD45RO (for naive CD4+ T cells). The CD25+ cells were further depleted by positive selection with directly conjugated anti-CD25 magnetic microbeads (Miltenyi Biotec, Auburn, CA) after the double-column depletion procedures. After the double-column depletion procedure, the CD4+CD25− or CD4+CD45RA+CD45RO−CD25− cells were routinely more than 99% pure by flow cytometric analysis. In some cases, the CD25− cells were sorted by FACSAria, and the purity of CD4+CD25− or CD4+CD45RA+CD45RO−CD25− cells was greater than 99.9%.
Allogeneic stimulation assay to induce and expand Treg
Freshly purified CD4+CD25− or CD4+CD45RA+CD45RO−CD25− T cells were cocultured with allogeneic CD40-activated B cells at a 10:1 T cell–to–B cell ratio in the RPMI 1640 medium with 10% heat-inactivated human AB serum. For some experiments, the T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) as previously reported before coculture with CD40-activated B cells.18 In the repeated stimulation experiments, the allogeneic CD40-activated B cells were added every 7 days of culture. In some experiments, human recombinant IL-2 (1000 IU/mL) was added in the culture medium. Functional and phenotypical hallmarks of the induced and expanded T cells were examined at the indicated time of culture. The expansion of the cells was determined by counting trypan blue–excluding cells.
Flow cytometric analysis
Cells were phenotypically analyzed using a FACSAria. The following fluorescence-conjugated monoclonal antibodies (mAbs) were used. Anti–CD4-PE-Cy5, anti–CD45RA-PE, and anti-CD45RO−APC were purchased from Invitrogen. Anti–CD25−APC, anti–CD62L-APC, anti–CD27-PE, anti–CD44-PE, anti–CCR7-PE, anti–cytotoxic T lymphocyte antigen-4 (CTLA-4)–PE, anti–GITR-PE, and their isotype-matched control Abs of irrelevant specificity were purchased from BD Biosciences (San Jose, CA). Intracellular staining was performed after cell fixation and permeabilization, using Fix and Perm reagents (BD Biosciences) as we reported before.28,29,31 The following mAbs were used: anti–CTLA-4-PE (BD Biosciences), anti–GITR-PE (BD Biosciences), anti–IL-10-PE (R&D Systems), anti–transforming growth factor-β (TGF-β-PE; IQ Products, Groningen, The Netherlands), and anti–IL-2 (BD Biosciences). For Foxp3 staining, the human Foxp3 staining kit (eBioscience, San Diego, CA) was used as we described before.32
Mixed lymphocyte reaction assays
The suppressor capacity of T cells induced and expanded in coculture with allogeneic CD40-activated B cells was studied in a mixed lymphocyte reaction (MLR) coculture suppression assay, as we described before with some modifications.23,32 CD4+CD25− or CD4+CD45RA+CD25− T cells were cocultured with allogeneic CD40-activated B cells (target) for 7 or 21 days, after which time CD4mediumCD25− and CD4highCD25+ T cells were sorted by FACSAria. The purity of sorted cells was routinely more than 99%. The sorted CD4highCD25+ and CD4mediumCD25− cells referred to as “suppressor” were titrated and added at the start of MLR assays, consisting of a total of 5 × 104 responder CD4+CD25− T cells from same donor of CD4medium/CD4high cells and 5 × 104 gamma-irradiated (30 Gy) target PBMCs from same donor of allogeneic B cells. Antigen specificity was examined in the cocultures that were performed with third-party stimulator PBMCs that were fully class I and II HLA-mismatched with the (target) allogeneic B cells. Proliferation was analyzed by [3H]-thymidine incorporation assay as described previously,33,34 with incorporation expressed as the mean plus or minus SEM cpm of 4 to 6 wells/condition.
Cytotoxic capacity of the induced and expanded cells was determined by the Live/Dead cell-mediated cytotoxicity kit (Invitrogen).35 Similar MLR coculture was set except that responder CD4+CD25− T cells were labeled with 3, 3′-dioctadecyloxacarbocyanine. After 2 and 3 days of MLR culture, cells were stained with propidium iodide at 37°C for 2 hours and then analyzed by flow cytometry. Back gating on the green fluorescent target cells, the propidium iodide-positive cells were evaluated for the percentage of lysed cells.
The contact dependency of CD4highCD25+ Treg was examined in Transwell experiments using 24-well plates. Briefly, 2 × 105 responder CD4+CD25− cells and 2 × 105 gamma-irradiated stimulator PBMCs (target) were cocultured in the lower compartment of the well. A total of 2 × 105 of CD4highCD25+ Treg were cultured in the Transwell insert (0.4 μm pore size; Millicell; Millipore, Billerica, MA). On day 3 of the cocultures, equivalent culture volumes were transferred from the lower compartment of the 24-well plate to a 96-well, round-bottom plate and analyzed for proliferation.
Blocking studies were performed in the presence of the neutralization mAbs directly against CTLA-4 (10 μg/mL; Ancell, Bayport, MN), IL-4 (10 μg/mL, R&D Systems), IL-10 (10 μg/mL; eBiosciences), glucocorticoid-induced TNF receptor (GITR; 2 μg/mL, R&D Systems), TGF-β (2 μg/mL, R&D Systems), and their relevant isotype controls.
Statistical analysis
Graphs and statistical analyses were performed with the use of Prism 4.00 for Windows software (GraphPad Software, San Diego, CA). P values of .05 or less were considered significant.
Results
CD40-activated B cells expanded by incubation with either CD40-ligand transfected cells or soluble hexameric CD40-ligand express high levels of MHC and costimulatory molecules
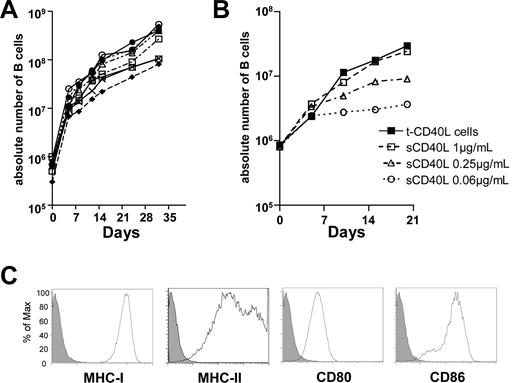
As in a previous report,27 nontransformed CD40-activated B cells could be expanded from circulating B cells contained in PBMCs by treatment with CD40-ligand (CD40-L) transfected NIH3T3 (t-CD40-L) cells, IL-4, and low concentrations of cyclosporin A. The purity of CD19+CD3− B cells was at least 83% by day 8 and more than 95% at day 12. By 28 to 32 days of culture, more than 99% of cells were the CD19+CD3− B cells (data not shown). To evaluate the expansion rate of B cells, we monitored the absolute number of CD19+CD3− cells generated from 5.0 mL of peripheral blood from 8 unselected healthy adult donors. We found that, after 32 days of culture, 8.1 to 54.3 × 107 CD40-activated B cells could be generated (Figure 1A). We next determined whether soluble hexameric CD40-ligand (sCD40-L) could replace t-CD40-L for B-cell activation and expansion, as t-CD40-L are xenogenic and would be potentially undesirable contaminants in adoptive immunity protocols in humans. We found that sCD40-L expanded B cells in a dose-dependent fashion (Figure 1B). At the concentration of 1.0 μg/mL, it was similarly effective as t-CD40-L in promoting B-cell expansion (Figure 1B). CD40-activated B cells generated using either sCD40-L or tCD40-L expressed high levels of major histocompatibility complex (MHC) class I and II molecules and costimulatory molecules CD80 and CD86 at 8 days (Figure 1C), and the expression of these molecules remained stable thereafter (data not shown).
CD40 activation is highly effective in generating large numbers of CD40-activated B cells that express high levels of MHC and costimulatory molecules. (A) Overall expansion of CD40-activated B cells from 8 different persons. CD40-activated B cells were generated by the coculture of PBMCs from 5 mL of peripheral blood with CD40L-transfected NIH3T3 (t-CD40-L) cells. (B) sCD40-L is as efficient as t-CD40-L cells at expanding human B cells in culture. CD40-activated B cells were generated by t-CD40-L cells or different concentrations of soluble hexameric CD40-L. The results shown are representative of 3 independent experiments. (C) Expression of CD80, CD86, and MHC class I and II on the CD40-activated B cells cultured for 8 days (solid histograms). The filled histograms were obtained with relevant isotype controls. Data shown here are representative of B-cell populations obtained from 8 different healthy adult donors.
CD40 activation is highly effective in generating large numbers of CD40-activated B cells that express high levels of MHC and costimulatory molecules. (A) Overall expansion of CD40-activated B cells from 8 different persons. CD40-activated B cells were generated by the coculture of PBMCs from 5 mL of peripheral blood with CD40L-transfected NIH3T3 (t-CD40-L) cells. (B) sCD40-L is as efficient as t-CD40-L cells at expanding human B cells in culture. CD40-activated B cells were generated by t-CD40-L cells or different concentrations of soluble hexameric CD40-L. The results shown are representative of 3 independent experiments. (C) Expression of CD80, CD86, and MHC class I and II on the CD40-activated B cells cultured for 8 days (solid histograms). The filled histograms were obtained with relevant isotype controls. Data shown here are representative of B-cell populations obtained from 8 different healthy adult donors.
Human alloreactive CD4high cells induced by CD40-activated B cells are Treg
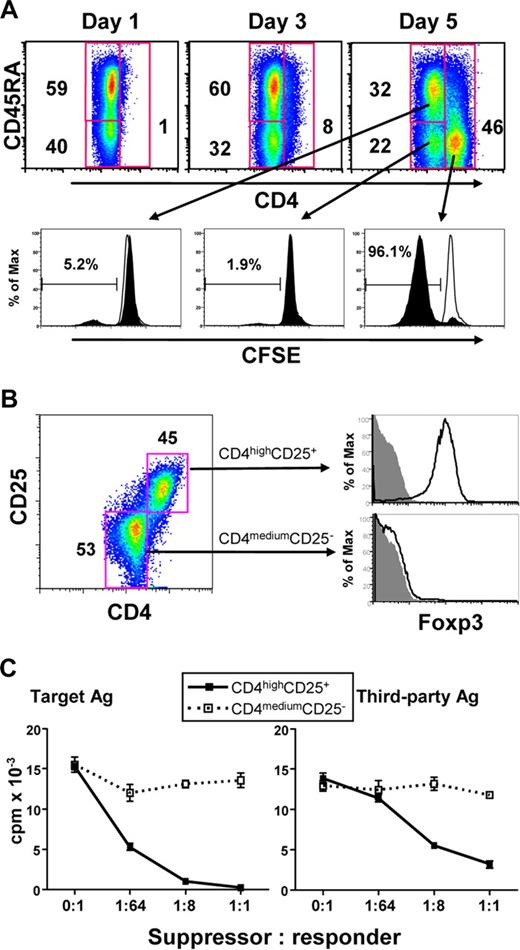
To determine whether allogeneic CD40-activated B cells can induce Treg from CD4+CD25− T cells, purified circulating CD4+CD25− T cells (purity > 99%) were stimulated with allogeneic CD40-activated B cells for 7 days. Surprisingly, a new cell subset with significantly up-regulated levels of CD4 surface expression was induced after 5 days of allostimulation, and most of these CD4high cells lost CD45RA expression (Figure 2A) and acquired CD45RO expression (data not shown). Furthermore, most of these CD4high cells also lost CFSE staining, whereas the CD4medium cells still maintained their CFSE content (Figure 2A), suggesting that the induced CD4high cells were proliferating alloreactive cells. These presumed alloreactive CD4high cells expressed CD25 and Foxp3, whereas CD4medium cells did not express these 2 Treg markers (Figure 2B). Together, these findings indicated that CD40-activated B cells preferentially expanded a CD4highCD25+Foxp3+ Treg cell population. Similar results were also found using highly purified CD4+CD25− T cells (purity > 99.9%) sorted by fluorescence-activated cell sorting (FACS) in this coculture system (data not shown). Thus, it is unlikely that the CD4highCD25+Foxp3+ Treg obtained were the result of an expansion of CD4+CD25+ T cells contaminating the initial culture.
Human alloreactive CD4high cells induced by CD40-activated B cells are Treg. (A) CD4 expression in CD4+CD25− T cells stimulated with allogeneic B cells for 5 days (top panels), and its relationship with cell proliferation based on the loss of CFSE label and CD45RA expression. Top panels represented the T cells gated on CD4. The percentage of CD4+ T cells in each gate is indicated. For the bottom panel, open histograms indicate the CFSE fluorescence intensities of the unstimulated control T cells, and the filled histograms represent the CFSE fluorescence intensities of the allostimulated T cells. The numbers in each histogram represent the percentage of cells that have undergone mitosis from each cell subset. (B) CD4high cells express both CD25 and Foxp3. The dot plot on the left shows CD25 expression after 5 days of allostimulation. Open histograms on the right show the Foxp3 expression, and filled histograms indicate the isotype controls. The results shown are representative of 4 different experiments. (C) CD4highCD25+ Treg generated from CD4+CD25− T cells potently suppressed MLR in an antigen-nonspecific manner. Freshly purified CD4+CD25− T cells were cocultured with CD40-activated allogeneic B cells for 7 days. The sorted CD4highCD25+ (■) and CD4mediumCD25− (□) cells were added into MLR culture system as described in “Mixed lymphocyte reaction assays.” Proliferation (y-axis) is shown for 3 days of MLR. The results shown are representative of 5 different experiments.
Human alloreactive CD4high cells induced by CD40-activated B cells are Treg. (A) CD4 expression in CD4+CD25− T cells stimulated with allogeneic B cells for 5 days (top panels), and its relationship with cell proliferation based on the loss of CFSE label and CD45RA expression. Top panels represented the T cells gated on CD4. The percentage of CD4+ T cells in each gate is indicated. For the bottom panel, open histograms indicate the CFSE fluorescence intensities of the unstimulated control T cells, and the filled histograms represent the CFSE fluorescence intensities of the allostimulated T cells. The numbers in each histogram represent the percentage of cells that have undergone mitosis from each cell subset. (B) CD4high cells express both CD25 and Foxp3. The dot plot on the left shows CD25 expression after 5 days of allostimulation. Open histograms on the right show the Foxp3 expression, and filled histograms indicate the isotype controls. The results shown are representative of 4 different experiments. (C) CD4highCD25+ Treg generated from CD4+CD25− T cells potently suppressed MLR in an antigen-nonspecific manner. Freshly purified CD4+CD25− T cells were cocultured with CD40-activated allogeneic B cells for 7 days. The sorted CD4highCD25+ (■) and CD4mediumCD25− (□) cells were added into MLR culture system as described in “Mixed lymphocyte reaction assays.” Proliferation (y-axis) is shown for 3 days of MLR. The results shown are representative of 5 different experiments.
To examine the function and alloantigen specificity of the induced CD4highCD25+Foxp3+ Treg from CD4+CD25− cells, the MLR assay was used. As shown in Figure 2C, after 7 days of allostimulation, CD4highCD25+ and CD4mediumCD25− cells were sorted by FACS and then added in the MLR assay. CD4medium cells did not suppress either the original target or third-party alloantigen-induced proliferation, whereas CD4highCD25+ cells suppressed both target- and third-party antigen–induced proliferations, although their suppressive effect on third-party alloantigen–induced proliferation was lower than that mediated by the target alloantigen (Figure 2C). Thus, CD4highCD25+ Treg generated from CD4+CD25− cells effectively suppressed in the MLR assay, but their suppression was not alloantigen-specific.
CD40-activated B cells can induce alloantigen-specific CD4highCD25+ Treg from naive CD4+CD25− cells
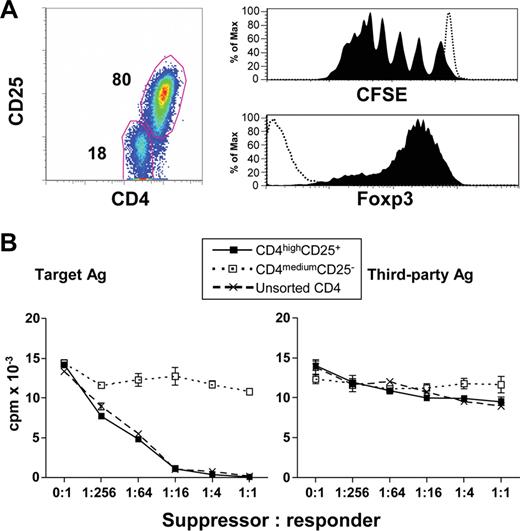
We next determined whether alloantigen-specific Treg could be generated from purified naive CD4+CD25− cells (CD4+CD45RA+CD45RO−CD25−) by coculture with allogeneic CD40-activated B cells. As in the case of unfractionated CD4+CD25− T cells, naive CD4+CD25− cells expanded by coculture with CD40-activated B cells also acquired a CD4high, CD25+, and Foxp3+ phenotype after 7 days of culture (Figure 3A). Furthermore, these CD4highCD25+Foxp3+ Treg underwent 7 or 8 cell divisions by 7 days of allostimulation (Figure 3A). In contrast, CD4medium cells neither divided nor expressed CD25 and Foxp3 (Figure 3A).
Human CD4high Treg induced from naive CD4+CD25− T cells by CD40-activated allogeneic B cells are alloantigen-specific Treg. (A) Characteristics of CD4high Treg induced from naive CD4+CD25− T cells. Freshly purified naive CD4+CD25− T cells were labeled with CFSE and cocultured with CD40-activated allogeneic B cells for 7 days. Representative data of CD4 and CD25 expression (left panel), CFSE dilution (top right panel), and Foxp3 expression (bottom right panel) from 6 independent experiments. Open histograms show the CFSE fluorescence intensity (top right panel) and Foxp3 expression (bottom right panel) of CD4mediumCD25− cells. Filled histograms represent the CFSE fluorescence intensity (top right panel) and Foxp3 expression (bottom right panel) of CD4highCD25+ cells. (B) CD4highCD25+ Treg generated from naive CD4+CD25− T cells potently suppressed MLR in an alloantigen-specific manner, and unsorted CD4+ T cells generated from naive CD4+CD25− T cells had similar suppressor capacities in MLR. Freshly purified CD4+CD45RA+CD25− T cells were cocultured with CD40-activated allogeneic B cells for 7 days. The sorted CD4highCD25+ (■) and CD4mediumCD25− (□), and unsorted CD4+ T cells (×) were added into MLR culture system as described in “Mixed lymphocyte reaction assays.” Proliferation (y-axis) was shown for day 3 of MLR. The results shown are representative of 8 independent experiments.
Human CD4high Treg induced from naive CD4+CD25− T cells by CD40-activated allogeneic B cells are alloantigen-specific Treg. (A) Characteristics of CD4high Treg induced from naive CD4+CD25− T cells. Freshly purified naive CD4+CD25− T cells were labeled with CFSE and cocultured with CD40-activated allogeneic B cells for 7 days. Representative data of CD4 and CD25 expression (left panel), CFSE dilution (top right panel), and Foxp3 expression (bottom right panel) from 6 independent experiments. Open histograms show the CFSE fluorescence intensity (top right panel) and Foxp3 expression (bottom right panel) of CD4mediumCD25− cells. Filled histograms represent the CFSE fluorescence intensity (top right panel) and Foxp3 expression (bottom right panel) of CD4highCD25+ cells. (B) CD4highCD25+ Treg generated from naive CD4+CD25− T cells potently suppressed MLR in an alloantigen-specific manner, and unsorted CD4+ T cells generated from naive CD4+CD25− T cells had similar suppressor capacities in MLR. Freshly purified CD4+CD45RA+CD25− T cells were cocultured with CD40-activated allogeneic B cells for 7 days. The sorted CD4highCD25+ (■) and CD4mediumCD25− (□), and unsorted CD4+ T cells (×) were added into MLR culture system as described in “Mixed lymphocyte reaction assays.” Proliferation (y-axis) was shown for day 3 of MLR. The results shown are representative of 8 independent experiments.
We further examined the suppressive capacity and alloantigen specificity of the CD4highCD25+ Treg induced from naive precursors. These CD4highCD25+ Treg significantly suppressed the original target alloantigen-induced proliferation, whereas CD4mediumCD25− cells did not show substantial suppressive ability (Figure 3B). Importantly, the induced CD4highCD25+ Treg were unable to suppress a third-party alloantigen-induced proliferation (Figure 3B). These data demonstrate that CD4highCD25+ Treg induced from naive CD4+CD25− T cells by allogeneic CD40-activated B cells are alloantigen-specific.
The CD4highCD25+ Treg generated from naive precursors had very high suppressive potential: Even at a cell ratio of 1:256 for Treg/responder cells (CD4+CD25−), there was approximately 50% suppression of target alloantigen-stimulated proliferation. At a Treg/responder cell ratio of 1:16 or higher, the target alloantigen-stimulated proliferation was almost completely inhibited (Figure 3B). This highly suppressive potential was also evident with unsorted CD4+ T cells containing approximately 80% of CD4highCD25+ T cells and 20% of CD4mediumCD25− T cells, indicating that contaminating CD4mediumCD25− cells do not interfere with Treg activity and therefore do not need to be removed by FACS sorting (Figure 3B).
Characteristics of CD4highCD25+Foxp3+ alloantigen-specific Treg
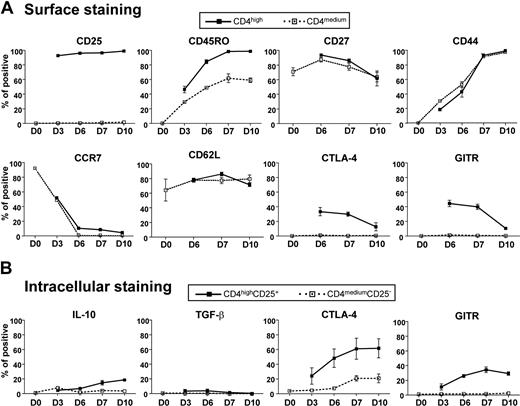
We further characterized the phenotype of the induced CD4highCD25+Foxp3+ alloantigen-specific Treg population. CD25 was significantly up-regulated from low basal levels by day 3 of culture, and more than 90% and 95% of CD4high cells expressed CD25 at day 3 and day 10, respectively, whereas there was no CD25 up-regulation on CD4medium cells for up to 10 days of culture (Figure 4A). The memory T-cell marker CD45RO was also up-regulated in both CD4high and CD4medium cells, but 95% of CD4high cells were CD45RO after 10 days of culture and only approximately 50% of CD4medium cells had this surface phenotype. Unlike previous reports indicating that the expression of CD27 and CD44 can discriminate functional CD4+CD25+ Treg in human and mice,36,37 we found no significant differences in CD27 and CD44 surface expression by CD4high Treg compared with CD4medium T cells or within the population of CD4highCD25+ Treg (Figure 4A). Most of the induced CD4high Treg lost their CCR7 expression after 6 days of culture, suggesting they had a memory/effector-like phenotype and tendency to migrate to inflamed tissues rather than undergo recirculation between the lymph nodes and blood.38 However, CD4high Treg still maintained high levels of CD62L expression, which probably would confer effective lymph node homing via high endothelial venules.
Characteristics of CD4highCD25+ alloantigen-specific Treg. Freshly purified naive CD4+CD25− T cells were cocultured with CD40-activated allogeneic B cells for the indicated time. The expression of cell surface markers (A) and intracellular cytokines (B) was determined and analyzed by FACS as described in “Flow cytometric analysis.” The percentage of positive cells for each cell surface marker or intracellular cytokine within the CD4highCD25+ and CD4mediumCD25− subsets are indicated. The results shown are representative of 4 independent experiments.
Characteristics of CD4highCD25+ alloantigen-specific Treg. Freshly purified naive CD4+CD25− T cells were cocultured with CD40-activated allogeneic B cells for the indicated time. The expression of cell surface markers (A) and intracellular cytokines (B) was determined and analyzed by FACS as described in “Flow cytometric analysis.” The percentage of positive cells for each cell surface marker or intracellular cytokine within the CD4highCD25+ and CD4mediumCD25− subsets are indicated. The results shown are representative of 4 independent experiments.
We next examined the expression of proteins previously implicated in the suppressive activity of Treg, including CTLA-4 (or CD152), GITR, IL-10, and TGF-β.39 Figure 4 shows that cell surface CTLA-4 and GITR were clearly detectable by day 3 and gradually increased such that approximately 30% and 45% of CD4high Treg expressed surface CTLA-4 and GITR, respectively, between day 6 and day 7. This was followed by a gradual decline in surface expression. Total CTLA-4 and GITR expression displayed different kinetics in that they gradually increased from day 3 so that approximately 60% and 30% of CD4highCD25+ Treg expressed CTLA-4 and GITR, respectively, after 10 days of culture based on intracellular staining (Figure 4B). In contrast, CD4mediumCD25− T cells expressed little or no CTLA-4 and GITR molecules on the surface or intracellularly (Figure 4). Both CD4mediumCD25− cells and CD4highCD25+ Treg expressed little or no detectable IL-10 and TGF-β during 10 days of culture (Figure 4B). Taken together, these data suggest that CTLA-4 or GITR, but not IL-10 and TGF-β, are potential mediators of CD4highCD25+ Treg suppressive activity.
CD4highCD25+ Treg lack cytotoxic capacity and suppress by a mechanism that requires cell-cell contact and involves, in part, CTLA-4 expression
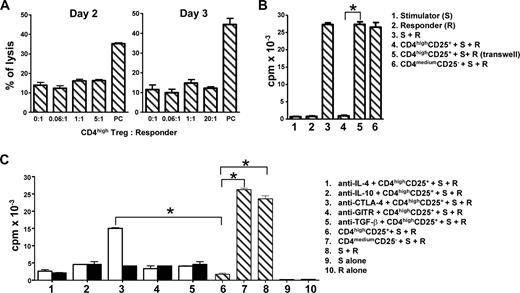
To determine the mechanism of CD4highCD25+ Treg suppression, we first determined whether CD4highCD25+ alloantigen-specific Treg had cytotoxic activity to responder cells (CD4+CD25−), as previous studies demonstrated that the suppression of Treg was dependent on their cytotoxicity.40,41 CD4highCD25+ Treg did not kill responder cells or induce their apoptosis during 2 to 3 days of MLR (Figure 5A), suggesting that the suppression of CD4highCD25+ alloantigen-specific Treg was not mediated by cell-mediated cytotoxicity.
CD4highCD25+ alloantigen-specific Treg have no cytotoxic capacity, and their suppressor function is dependent on cell-cell contact and partially relies on CTLA-4 expression. CD4highCD25+ Treg or CD4mediumCD25− T cells were sorted after 7 days of allostimulation as shown in Figure 3B. (A) Cytotoxic capacity of induced CD4highCD25+ Treg. (B) The alloantigen-specific suppressor function of CD4highCD25+ Treg is cell-cell contact dependent. (C) Neutralizing anti–CTLA-4 mAb partially reverses the alloantigen-specific suppression mediated by CD4highCD25+ Treg, but neutralizing mAbs to IL-4, IL-10, TGF-β, and GITR fails to reverse that suppression. Responder (R) CD4+CD25− and gamma-irradiated stimulator PBMC (S) were cocultured with or without sorted CD4highCD25+ Treg or CD4mediumCD25− T cells. The cytotoxic activities (A) of human IL-2–activated NK cells against K562 cells were set as positive controls (PC). Stimulator (S) or responder (R) cells alone were set as controls. For transwell experiments (B), the same amount of responder (R) and stimulator (S) cells were plated in the bottom wells of a transwell system. The top well insert was inoculated with same amount of sorted CD4highCD25+ Treg. For the blocking experiments (C), the neutralization mAbs (□) and their relevant isotype controls (■) were added in the coculture system. Proliferation (y-axis) is shown for day 3 of cultures. Data for 4 different experiments are shown (n = 4). The 2-tailed unpaired Student t tests were used for comparison. *P < .01.
CD4highCD25+ alloantigen-specific Treg have no cytotoxic capacity, and their suppressor function is dependent on cell-cell contact and partially relies on CTLA-4 expression. CD4highCD25+ Treg or CD4mediumCD25− T cells were sorted after 7 days of allostimulation as shown in Figure 3B. (A) Cytotoxic capacity of induced CD4highCD25+ Treg. (B) The alloantigen-specific suppressor function of CD4highCD25+ Treg is cell-cell contact dependent. (C) Neutralizing anti–CTLA-4 mAb partially reverses the alloantigen-specific suppression mediated by CD4highCD25+ Treg, but neutralizing mAbs to IL-4, IL-10, TGF-β, and GITR fails to reverse that suppression. Responder (R) CD4+CD25− and gamma-irradiated stimulator PBMC (S) were cocultured with or without sorted CD4highCD25+ Treg or CD4mediumCD25− T cells. The cytotoxic activities (A) of human IL-2–activated NK cells against K562 cells were set as positive controls (PC). Stimulator (S) or responder (R) cells alone were set as controls. For transwell experiments (B), the same amount of responder (R) and stimulator (S) cells were plated in the bottom wells of a transwell system. The top well insert was inoculated with same amount of sorted CD4highCD25+ Treg. For the blocking experiments (C), the neutralization mAbs (□) and their relevant isotype controls (■) were added in the coculture system. Proliferation (y-axis) is shown for day 3 of cultures. Data for 4 different experiments are shown (n = 4). The 2-tailed unpaired Student t tests were used for comparison. *P < .01.
We next determined whether CD4highCD25+ suppression could be mediated solely by soluble molecules released from Treg. As shown in Figure 5B, the suppression was lost when the responder cells were physically separated from the induced CD4highCD25+ Treg in a transwell culture system. The addition of neutralizing mAb for IL-10, TGF-β, IL-4, or GITR into MLR cultures had little or no effect on the ability of CD4highCD25+ Treg to suppress alloantigen-specific proliferation (Figure 5C). In contrast, antibody blockade of CTLA-4 partially reversed CD4highCD25+ Treg suppression (Figure 5C). Together, these data suggest that the CD4highCD25+ Treg-mediated suppression of alloantigen responses is cell-cell contact dependent and mediated, in part, by CTLA-4.
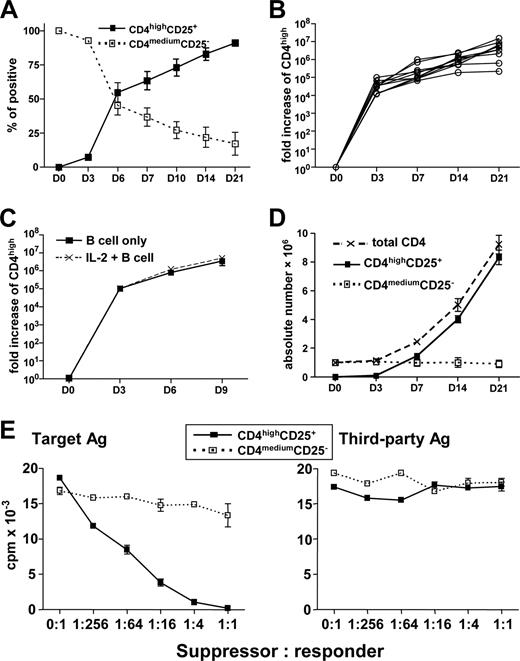
CD4highCD25+ Treg can be continuously expanded by CD40-activated B cells in large-scale without loss of function and exogenous IL-2 does not enhance cell expansion
We examined the ability of 3 weeks of coculture of naive CD4+CD25− T cells with allogeneic CD40-activated B cells to generate Treg, in which freshly generated CD40-activated B cells were added weekly. As shown in Figure 6A, CD4highCD25+ Treg gradually increased, and more than 92% of T cells in culture were the CD4highCD25+ Treg at day 21 (Figure 6A). Using 10 healthy randomly selected adult blood donors, we were able to expand CD4highCD25+ Treg 6.4 × 105- to 1.6 × 107-fold during 21 days of culture (Figure 6B). This expansion did not require exogenous IL-2, as its addition did not increase the generation of CD4highCD25+ Treg cells (Figure 6C). To more precisely determine the rate of expansion, we used a standard number of naive CD4+CD25− T cells (106) at the beginning of the culture and found that approximately 8.3 × 106 (range, 5.4-11.3 × 106) of CD4highCD25+ Treg could be generated from every 106 of naive CD4+CD25− T cells in 10 unselected donors (Figure 6D). Furthermore, expanded CD4highCD25+ Treg evaluated at 21 days of culture had similar suppressive ability and alloantigen specificity (Figure 6E) as Treg generated over a shorter period of in vitro culture. In addition, these Treg still maintained their high levels of Foxp3 expression (data not shown). Together, these results demonstrate that CD40-activated B cells can induce and expand CD4highCD25+Foxp3+ alloantigen-specific Treg at a scale that is probably to be relevant for clinical immunotherapy.
CD4highCD25+ alloantigen-specific Treg can be continuously expanded by CD40-activated B cells in large-scale without loss of function, and exogenous IL-2 does not enhance this cell expansion. Freshly purified naive CD4+CD25− T cells were cocultured with CD40-activated allogeneic B cells for the indicated time. (A) The percentages of CD4highCD25+ and CD4mediumCD25− cells in the cultures (n = 10). (B) Expansion of CD4highCD25+ alloantigen-specific Treg from 10 different persons. The expansion was normalized for the CD4highCD25+ cells, and the fold increase of the CD4highCD25+ was shown. (C) Naive CD4+CD25− were cocultured with CD40-activated allogeneic B cells with or without IL-2. The expansion was normalized for the CD4highCD25+ cells, and the fold increase of the CD4highCD25+ is shown (n = 4). (D) Absolute numbers of CD4highCD25+ alloantigen-specific Treg generated from 106 naive CD4+CD25− T cells (n = 10). (E) CD4highCD25+ alloantigen-specific Treg induced and expanded by CD40-activated B cells for 21 days remain functional. Freshly purified naive CD4+CD25− T cells (responder) were cocultured with CD40-activated allogeneic B cells (target antigen) to induce and expand CD4highCD25+ Treg for 21 days with replacement of B cells every 7 days. The sorted CD4highCD25+ and CD4mediumCD25− cells were added into the MLR culture system as described in “Mixed lymphocyte reaction assays.” Data shown here are representative of 3 independent experiments.
CD4highCD25+ alloantigen-specific Treg can be continuously expanded by CD40-activated B cells in large-scale without loss of function, and exogenous IL-2 does not enhance this cell expansion. Freshly purified naive CD4+CD25− T cells were cocultured with CD40-activated allogeneic B cells for the indicated time. (A) The percentages of CD4highCD25+ and CD4mediumCD25− cells in the cultures (n = 10). (B) Expansion of CD4highCD25+ alloantigen-specific Treg from 10 different persons. The expansion was normalized for the CD4highCD25+ cells, and the fold increase of the CD4highCD25+ was shown. (C) Naive CD4+CD25− were cocultured with CD40-activated allogeneic B cells with or without IL-2. The expansion was normalized for the CD4highCD25+ cells, and the fold increase of the CD4highCD25+ is shown (n = 4). (D) Absolute numbers of CD4highCD25+ alloantigen-specific Treg generated from 106 naive CD4+CD25− T cells (n = 10). (E) CD4highCD25+ alloantigen-specific Treg induced and expanded by CD40-activated B cells for 21 days remain functional. Freshly purified naive CD4+CD25− T cells (responder) were cocultured with CD40-activated allogeneic B cells (target antigen) to induce and expand CD4highCD25+ Treg for 21 days with replacement of B cells every 7 days. The sorted CD4highCD25+ and CD4mediumCD25− cells were added into the MLR culture system as described in “Mixed lymphocyte reaction assays.” Data shown here are representative of 3 independent experiments.
Discussion
In this study, we describe a reliable method to generate human alloantigen-specific Treg in vitro from naive CD4+ T-cell precursors. This, to best of our knowledge, is the first report about the large-scale generation of human antigen-specific Treg. Using allogeneic CD40-activated B cells, we induced naive CD4+ T-cell precursors to differentiate and expand into alloantigen-specific CD4highCD25+Foxp3+ Treg with robust regulatory activity that was alloantigen-specific. By repeatedly stimulating naive CD4+ T cells with allogeneic CD40-activated B cells for 21 days, we could routinely generate 6 to 11 × 106 alloantigen-specific Treg from every 106 naive CD4+CD25− T cells; these numbers of naive CD4+ T cells can typically be isolated from 5 to 8 mL of peripheral blood. Thus, this is a practical approach for the generation of relatively large numbers of human antigen-specific Treg, which should facilitate the development of clinical immunotherapy based on the adoptive transfer of Treg.
Our protocol is different from those previously reported for allogeneic Treg induction and expansion in that we used CD40-activated B cells as APCs rather than allogeneic monocyte-derived DCs or PBMCs.21,42 CD40-activated B cells have an important advantage for this purpose in that they can be readily expanded in vitro to a relatively large numbers (Figure 1), whereas, in contrast, monocytes differentiating in vitro into DCs do not undergo cell division.22 Cryopreserved CD40-activated B cells also retain their APC function on thawing and are relatively cost-effective to produce.26,27 In addition, because B cells stimulated with t-CD40-L cells or recombinant sCD40-L were equally effective at generating alloantigen-specific Treg, the use of sCD40-L may significantly improve the clinical applicability of the procedure.
We confirmed the work of Schultze et al26,27,43 : that activation of B cells by CD40 engagement using CD40-ligand transfected murine fibroblasts resulted in high levels of expression of MHC class I and class II and the CD80 and CD86 costimulatory molecules. Similar levels of expression of these molecules could be achieved using hexameric human CD40-ligand, indicating that engagement of CD40 was the essential signal and that other ligands potentially provided by murine fibroblasts were dispensable for B-cell activation. In contrast to prior work26,27,43 in which autologous CD40-activated B cells were used as APCs in conjunction with IL-2 and IL-7 to generate effector T-cell responses, we found that allogeneic Treg generation did not require the addition of exogenous cytokines. We speculate that the absence of exogenous cytokines, such as IL-7, and using allogeneic rather than autologous CD40-activated B cells in our system may account for why we observed the differentiation and marked expansion of allogeneic Treg rather than effector T cells in our CD40-activated B-cell/naive CD4+ T-cell coculture system. Our findings are also consistent with growing evidence that some subsets of B cells exert regulatory activity and that this may involve in certain contexts an enhancement of Treg activity.44
Using coculture of allogeneic CD40-activated B cells with total or naive CD4+CD25− T cells, we generated CD4highCD25+Foxp3+ Treg after 5 to 7 days of culture (Figures 2, 3). Importantly, we demonstrated that CD4highCD25+Foxp3+ Treg generated from naive CD4+CD25− T-cell precursors were alloantigen-specific (Figure 3), whereas those derived from total CD4+CD25− T cells, which included both naive and memory cells, had no antigen specificity (Figure 2). Treg generated from memory CD4+CD25− T cells were also found to have no antigen specificity and could suppress both target and third-party antigen stimulated MLR (data not shown). Consistent with our findings, other groups have also shown that Treg could be generated from both naive and memory CD4+ T cells after coculture with allogeneic DCs and that these 2 sources of Treg were similarly effective in suppression.21,45 However, differences in the antigen specificity of these Treg were not investigated in these studies.21,45 The reasons underlying the marked difference in antigen specificity between the Treg generated from total CD4+CD25− and naive CD4+CD25− T cells are still unclear, but it is plausible that Treg generated from antigen-experienced memory cells present in total CD4+CD25− T cells may account for this difference.
IL-2 is critical for maintaining Treg survival, although it is dispensable for the induction of CD4+CD25+Foxp3+ Treg.32,46 It was reported that the in vitro generation of polyclonal or alloantigen-specific human Treg required high-dose exogenous IL-2.11,36 However, in our culture system, it was unnecessary to add exogenous IL-2 for inducing and expanding alloantigen-specific CD4highCD25+Foxp3+ Treg (Figure 6D), most probably because the allogeneic CD40-activated B cells can secrete substantial amounts of IL-2 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). This lack of a requirement for exogenous cytokines could significantly reduce the cost for generation of alloantigen-specific Treg.
Importantly, we also observed a significant up-regulation of the CD4 molecule on T cells after the allostimulation of naive or total CD4+CD25− T cells with allogeneic CD40-activated B cells. Based on the expression of CD4 and CD25, the allostimulated human CD4+ T cells could be separated into 2 subsets: CD4highCD25+ and CD4mediumCD25− cells (Figures 2,3). We further demonstrated that the CD4highCD25+ but not CD4mediumCD25− cells were Treg that expressed Foxp3 and had highly suppressive capacities (Figures 2,3), raising the possibility that the CD4high might be a marker for human Treg in other contexts, a possibility that we are currently investigating.
The alloantigen-specific CD4highCD25+Foxp3+ Treg generated in our system were CD45RO+ and CCR7− memory cells and expressed a high level of lymph node homing receptor CD62L (Figure 3). This suggests that these cells might potentially be useful for migrating to peripheral lymphoid tissues draining graft sites to suppress T cell–mediated allograft rejection and GVHD. It has previously been demonstrated that ex vivo–expanded Treg can retain their regulatory activity and migrate appropriately into the peripheral lymphoid organs in the recipient if they express a high level of CD62L.12,47
We also demonstrated that induced alloantigen-specific CD4highCD25+Foxp3+ Treg expressed CTLA-4 and GITR but had minimal secretion of TGF-β or IL-10 (Figure 4). Other surface markers, such as CD27 and CD44, were previously reported by others to discriminate between functional Treg and non-Treg.36,37 However, we did not find any significant difference in the expression of CD27 and CD44 between the CD4highCD25+Foxp3+ Treg and CD4mediumCD25−Foxp3− non-Treg produced with the B-cell coculture system.
In functional analysis, we demonstrated that the alloantigen-specific CD4highCD25+Foxp3+ Treg generated in our system could completely block the alloantigen-stimulated MLR (Figure 3). With these Treg, the marked suppressive effects could occur with as little as about 718 suppressors (1:64 ratio) in a culture of 50 000 responding CD4+CD25− T cells and 50 000 allogeneic PBMC stimulators. These effects are more potent than those previously reported for freshly isolated or expanded human polyclonal and alloantigen-specific Treg9-11,21 and again suggest the potential clinical utility of the Treg generated by CD40-activated B cells in adoptive immunotherapy.
The mechanisms of Treg suppressive function still remain largely unknown. Evidence from in vitro and in vivo studies in both human and rodents indicate that direct cell-cell contact is required and that some immunoregulatory cytokines may also be involved in the suppression of effector T-cell activity.39 We also demonstrated that the suppressive functions of CD4highCD25+Foxp3+ Treg were cell-cell contact dependent and partially relied on CTLA-4 expression, consistent with previous observations in natural and in vitro expanded human Treg.36,48,49 Consistent with the previous finding that IL-10, TGF-β, and GITR are dispensable for the suppressive function of CD4+CD25+ Treg,9,11,21 we also demonstrated that these 3 molecules were not required for suppression by CD4highCD25+Foxp3+ Treg (Figure 5). Some previous studies have also suggested that the suppression of Treg may be mediated by their cytotoxicity,40,41 but we did not find that our induced CD4highCD25+Foxp3+ Treg had cytotoxic activities (Figure 5). In addition, the possibility of involvement of Th2 response in MLR was also excluded because blockade of IL-4 failed to inhibit the suppression of CD4highCD25+Foxp3+ Treg (Figure 5).
In conclusion, we have developed a relatively simple and low-cost protocol using allogeneic CD40-activated B cells to induce and expand highly efficient human alloantigen-specific CD4highCD25+Foxp3+ Treg from naive CD4+CD25− T cells in large scale. This may facilitate the clinical applications of Treg-based immunotherapy using in vitro induced and expanded alloantigen-specific Treg to induce donor-specific transplantation tolerance, although it remains to be shown whether this applies to the in vivo situation.50 Similar strategies, that is, induction and expansion of autoantigen-specific Treg using antigen-pulsed autologous CD40-activated B cells, could be exploited in the treatment of autoimmune diseases in which the target self-antigens are known.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Yan Chen for technical help.
This work was supported in part by Seed Funding for Basic Research, University Research Committee, the University of Hong Kong (HKU), Hong Kong SAR, PR China (W.T.); Competitive Earmarked Research Grant, Research Grants Council of Hong Kong, Hong Kong SAR, PR China, HKU 777407M (W.T.); Research Fund for the Control of Infectious Diseases, Hong Kong SAR, PR China (07060482, W.T.); HKU postgraduate studentships (J.Z., H.M.); and National Institutes of Health grant P01 AI-050153 (D.B.L.).
National Institutes of Health
Authorship
Contribution: W.T. conceived and designed the study, designed and performed the experiments, analyzed data, performed statistical analysis, and wrote the paper; Y.-L.L. supervised the study and wrote the paper; J.Z. performed experiments; Y.L., P.-L.C., and K.D. were involved in the early initiation of this study; H.M. performed the cytotoxicity experiments; P.S. provided the sCD40-L and commented on the paper; and D.B.L. supervised the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wenwei Tu, Department of Paediatrics and Adolescent Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Room L7-58, 7/F Laboratory Block, Faculty of Medicine Building, 21 Sassoon Road, Hong Kong; e-mail: wwtu@hkucc.hku.hk; or Yu-Lung Lau, Department of Paediatrics and Adolescent Medicine, Li Ka Shing Faculty of Medicine, University of Hong Kong, Room L7-58, 7/F Laboratory Block, Faculty of Medicine Building, 21 Sassoon Road, Hong Kong; e-mail: lauylung@hkucc.hku.hk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal