Polyphosphate, a linear polymer of inorganic phosphate found in bacteria, fungi, plants, and recently, human platelets, enhances fibrin clot structure and stability and may play a central role in both normal and pathologic coagulation.
A significant number of studies implicate fibrin structure as a major determinant of a clot's mechanical and fibrinolytic stability. In vitro studies demonstrate that clots composed of a dense fibrin network are more resistant to fibrinolysis and deformation than clots composed of a porous fibrin network. These observations are now translating to the clinical arena as studies with plasma show that hemostasis relies, at least in part, on fibrin structure and stability; patients whose plasma produces clots with abnormal structure and/or stability have an increased risk of thrombosis or bleeding. The list of molecules, ions, and conditions known to affect fibrin structure is lengthy, and includes local pH and concentrations of thrombin and calcium (reviewed in Wolberg1 ). More recent studies have focused on how cells influence fibrin structure via their ability to support thrombin generation and express fibrin-binding integrins.2-5 Platelets, in particular, regulate fibrin structure and stability via interactions between αIIbβ3 and fibrin(ogen),2-4 and may also regulate structure indirectly via their ability to support thrombin generation.
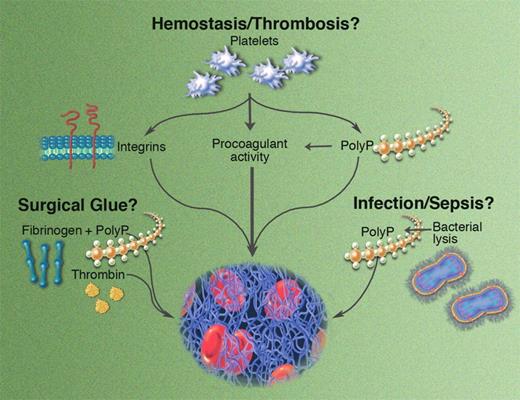
Potential roles for polyP in hemostasis and thrombosis. Illustration by Marie Dauenheimer.
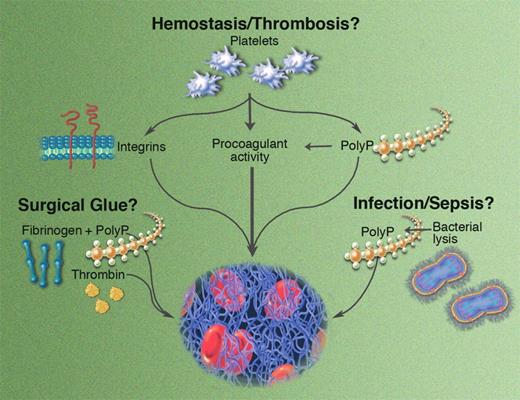
Potential roles for polyP in hemostasis and thrombosis. Illustration by Marie Dauenheimer.
In this issue of Blood, Smith and Morrissey report a novel mechanism by which platelets modulate fibrin structure and lysis. Their data show that polyphosphate (polyP) found in the dense granules of human platelets influences fibrin formation in a calcium-dependent manner. Clots formed in the presence of polyP are more turbid, have increased mass/length ratios, and are composed of abnormally thick fibrin fibers. Interestingly, while clots with these optical and structural properties are typically associated with an increased susceptibility to lysis, clots formed in the presence of polyP exhibit prolonged lysis times. Because polyP is secreted from dense granules immediately following platelet activation, its release coincides with release of factor V, TFPI, TAFI, and other proteins at the onset of coagulation, making it available at a critical juncture of the clotting reaction. Indeed, this publication by Smith and Morrissey follows an earlier one by the same group showing that polyP also activates the contact pathway, accelerates factor V conversion to factor Va, abrogates the anticoagulant effects of TFPI, and accelerates TAFI production via increased thrombin generation.6 Thus, the breadth of polyP's effects is wide, influencing multiple facets of coagulation.
As if the potential for these physiologic effects wasn't interesting enough, the authors propose 2 additional roles for polyP. First, the ability of polyP to enhance fibrin clot stability independent of its effects on thrombin generation suggests that polyP could be exploited as a prohemostatic adjuvant in surgical fibrin glue. Presumably, its physiologic roots would thwart an innate immunologic response, yielding a potent drug with low toxicity. Of course, polyP may have additional, as-yet-undescribed functions during clotting and/or wound healing that would need to be explored prior to its use as a therapeutic agent. Second, the authors note that in addition to platelets, polyP is widely distributed across other taxonomic kingdoms, including plants, fungi, and bacteria. Release of polyP from certain microorganisms may contribute to the organism's pathogenesis or modulate host defense during infection.
This original work sparks several important questions about the mechanism of action and potential function of polyP. The observation that polyP produces clots that contain thicker fibers and yet are resistant to fibrinolysis is paradoxical and should be further investigated using microscopy techniques that enable the direct visualization of clot formation and lysis in real time. Importantly, neither of these publications on polyP release from platelets examined its effects on hemostasis in an animal model. Does polyP regulate clotting during tissue-factor–initiated thrombin generation and/or clot formation in vivo? How does its relative influence differ between platelet-rich arterial clots and fibrin-rich venous clots? Does the polyP content in dense granules differ between individuals? For example, the bleeding defects seen in patients with dense granule defects may stem, in part, from a failure of polyP release to stabilize the fibrin network, whereas the release of abnormally high polyP levels from dense granules may increase platelet procoagulant activity—and therefore thrombotic risk. Could the clearance rate of polyP from plasma be a regulatory mechanism influencing clot structure and/or stability in different individuals? Undoubtedly, the answers to these and other questions will enrich our understanding of not only how fibrin structure and stability are determined, but also under what circumstances these fibrin properties influence hemostasis and thrombosis.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health