Abstract
IL17-producing (Th17) cells are a distinct lineage of T helper cells that regulate immunity and inflammation. The role of antigen-presenting cells in the induction of Th17 cells in humans remains to be fully defined. Here, we show that human dendritic cells (DCs) are efficient inducers of Th17 cells in culture, including antigen-specific Th17 cells. Although most freshly isolated circulating human Th17 cells secrete IL17 alone or with IL2, those induced by DCs are polyfunctional and coexpress IL17 and IFNγ (Th17-1 cells). The capacity of DCs to expand Th17-1 cells is enhanced upon DC maturation, and mature DCs are superior to monocytes for the expansion of autologous Th17 cells. In myeloma, where tumors are infiltrated by DCs, Th17 cells are enriched in the bone marrow relative to circulation. Bone marrow from patients with myeloma contains a higher proportion of Th17-1 cells compared with the marrow in preneoplastic gammopathy (monoclonal gammopathy of undetermined significance [MGUS]). Uptake of apoptotic but not necrotic myeloma tumor cells by DCs leads to enhanced induction of Th17-1 cells. These data demonstrate the capacity of DCs to induce expansion of polyfunctional IL17-producing T cells in humans, and suggest a role for DCs in the enrichment of Th17-1 cells in the tumor bed.
Introduction
Dendritic cells (DCs) are highly differentiated antigen-presenting cells (APCs) that play a key role in the initiation and regulation of T-cell immunity to pathogens and tumors while at the same time preventing immune responses against self-tissues or environmental antigens.1 The repertoire of T cells induced upon activation includes several types, such as T helper 1 (Th1), Th2, and Th17 as well as regulatory T (Treg) cells, and is likely to further increase in complexity. The balance of induction of different T-cell types is thought to depend on cytokines and other signals derived from APCs, which then activate specific transcription factors that mediate the differentiation of naive T cells.2
Th17 cells are recognized as a distinct lineage of T helper cells producing IL17, IL17F, and IL22, which play an important role in immunity to certain pathogens and autoimmune inflammation.3-10 In view of their importance to immunopathology, several studies have examined the factors regulating the differentiation of murine Th17 cells.11-17 In contrast, the data about IL17 producers in humans is somewhat limited, and some aspects of their biology may differ from studies in mice.3,18-23 Th1 and Th17 cells were initially viewed as distinct and possibly antagonistic differentiation pathways.24 However, particularly in humans, a substantial proportion of IL17 producers in the tissue of patients with autoimmune disease (eg, Crohn disease, uveitis) have been found to coexpress IFNγ and IL17 (known as Th17-1 cells).23,25,26 Th17 cells coexpressing IFNγ have also been described in murine models of graft-versus-host disease.27 The data about the role of Th17 cells in tumor immunity is limited,28,29 and in this context, the heterogeneity of IL17 producers in human tumor tissues has not been described in detail. Recent studies have documented the capacity of murine DCs to activate Th17 cells.30,31 However, the role of APCs in the activation of IL17-producing cells in humans is less studied.3 In prior studies, we have described an important role for DCs in the activation of effector and Treg cells in the context of human myeloma.32-34 Here, we have examined the role of DCs in the induction of human IL17-producing cells, and the properties of IL17 producers in patients with multiple myeloma.
Methods
Healthy donors and patients with myeloma
Peripheral blood was collected from healthy donors after informed consent was obtained in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) at the Rockefeller University. Paired samples of blood and bone marrow aspirates were obtained from patients with myeloma after informed consent approved by IRB at St Vincent's Cancer Center. Healthy donor buffy coats purchased from the New York Blood Center (New York, NY) were also used as a source of mononuclear cells.
Generation of monocyte-derived DCs
DCs were generated from blood monocytes as described.35 Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donor blood by density gradient centrifugation using ficoll hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden). CD14+ cells were separated from the PBMCs using CD14 microbeads and columns (Miltenyi Biotec, Auburn, CA). The CD14− cells obtained from the PBMCs were cultured in RPMI with L-glutamine (Mediatech, Herndon, VA) supplemented with 5% pooled human serum (Labquip, Niagara Falls, NY) and used as the source of T cells. Some of the CD14+ cells were used as the source of monocytes for T-cell stimulation. In order to generate DCs, CD14+ cells were cultured in the presence of IL4 (20 ng/mL; R&D Systems, Minneapolis, MN) and granulocyte-macrophage colony-stimulating factor (GM-CSF; 20 ng/mL; Immunex, Seattle, WA) in 1% plasma as described.35 On day 5 of culture, the DCs were either left untreated (immature) or matured using the inflammatory cytokine cocktail consisting of prostaglandin E2 (1 μg/mL; Sigma-Aldrich, St Louis, MO), IL1β (10 ng/mL), IL-6 (1000 U/mL), and tumor necrosis factor-α (10 ng/mL; all from R&D Systems). For some experiments, DCs were matured using LPS (20 ng/mL; Sigma-Aldrich) or poly I:C (25 μg/mL; InvivoGen, San Diego, CA).
DC–T-cell coculture
T cells were purified from the CD14− fraction using the pan–T-cell isolation kit (Miltenyi Biotec, Auburn, CA). CD14+ monocytes or monocyte-derived immature DCs or DCs matured using inflammatory cytokines, LPS, or poly I:C were used to stimulate T cells. DCs were added to the T cells at a ratio of 1:30. For some experiments, T cells were depleted of the CD45RO fraction using magnetic beads prior to coculture. The depletion of the CD45RO+ population was monitored by flow cytometry. For some experiments, monocyte-derived dendritic cells (Mo-DCs) were cultured with T cells in the presence of the following neutralizing antibodies—anti-IL1b (10 μg/mL), anti-IL6 (10 μg/mL), anti-IL23p19 (10 μg/mL; all from R&D Systems)—or the respective isotype control antibodies. For some experiments, DCs and T cells were separated by a transwell insert (0.2 mm inserts; Nunc, Roskilde, Denmark). In some conditions, T cells were labeled with CFSE (carboxyfluorescein diacetate succinimidyl ester; 0.5 μM; Molecular Probes, Eugene, OR) to monitor proliferation, prior to DC–T-cell coculture.
Intracellular flow cytometry to detect cytokine production by T cells
Fresh PBMCs from healthy donors were stimulated with PMA (0.5 μg/mL) and ionomycin (0.5 μg/mL) in the presence of monensin (GolgiStop; BD Biosciences, San Jose, CA) for 5 hours. The cells were stained with the aqua LIVE/DEAD fixable dead cell dye (Molecular Probes) to distinguish the living cells from the dead cells. The cells were fixed and permeabillized using the BD Cytofix/Cytoperm solution and stained with the following antibodies: IL17-Alexa488, IL17-Alexa647 (eBioscience, San Diego, CA), IL2-PE, IFNγ-APC, IFNγ-PE-Cy7, CD3-Alexa700, CD4-APC-Cy7 (all from BD Biosciences), and CD8-PE-Texas Red (Caltag Laboratories, Burlingame, CA). The cells were acquired using BD LSRII instrument and FACSDIVA software (BD Biosciences). The data were analyzed using FlowJo software (TreeStar, Ashland, OR). For some experiments, the presence of IL17 was also analyzed in the supernatants of T-cell cultures after stimulation with PMA ionomycin by enzyme-linked immunosorbent assay (ELISA; eBiosciences, San Diego, CA) using the manufacturer's recommendations.
Real-time quantitative RT-PCR
RNA was extracted from cells by using the RNeasy Mini Kit (Qiagen, Valencia, CA). Retinoid-related orphan receptor gamma (RORC) expression was quantified by using Assays-on-Demand primer-probes from Applied Biosystems (identification no. Hs01076112; Foster City, CA). The reverse transcriptase–polymerase chain reaction (RT-PCR) was performed by using EZ PCR Core Reagents (Applied Biosystems) according to the manufacturer's directions. The samples were amplified and quantified on an Applied Biosystems PRISM 7700 by using the following thermal cycler conditions: 2 minutes at 50°C; 30 minutes at 60°C; 5 minutes at 95°C; and 40 cycles of 15 seconds at 95°C followed by 60 seconds at 60°C. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene, was used to normalize each sample. The data were analyzed, and samples were quantitated by the software provided with the Applied Biosystems PRISM 7700.
Detection and expansion of Candida-specific Th17 cells
For expansion of Candida-specific Th17 cells, monocytes, or monocyte-derived immature DCs were pulsed with Candida antigen mix (20 ng/mL; Greer Labs, Lenoir, NC). After 4 hours, the immature DCs were matured using inflammatory cytokine cocktail as described above. The monocytes and the DCs were cultured with the Candida mix for 24 hours prior to being used to stimulate T cells. Pan–T-selected T cells were labeled with CFSE (0.5 μM CFSE; Molecular Probes) and cultured alone, in the presence of 20 μg/mL of Candida mix, with monocytes and cytokine-matured DCs alone as well as monocytes and cytokine-matured DCs loaded with Candida antigen mix. After 5 days of coculture, some of the T cells were stimulated with PMA and ionomycin for 5 hours with GolgiStop and cytokine production was measured by flow cytometry as described previously. IL-2 (50 U/mL) was added to the rest of the cultures. After 7 more days in culture, intracellular cytokine staining was performed after stimulation with PMA and ionomycin to analyze the expansion of antigen specific T cells by flow cytometry. To examine the antigen specificity of the proliferating T cells, in some experiments, the cocultured T cells were stimulated overnight with either DCs alone or DCs loaded with candida antigen mix in the presence of GolgiStop, and intracellular cytokine staining was performed as described previously.
Stimulation with tumor-loaded DCs
For some experiments, DCs were loaded with dying myeloma tumor cells prior to coculture. Multiple myeloma (MM) cell lines (U266 cells from ATCC [Manassas, VA] and OPM2 cells kindly provided by J. Shaughnessy [Little Rock, AR] were killed with γ-irradiation (apoptotic cells) or freeze-thaw (necrotic cells) as described,32 and fed to immature DCs at a DC-tumor ratio of 1:1. Tumor-loaded DCs were then used to stimulate autologous T cells, as described earlier.32 After 5 days of culture, the presence of IL17-producing T cells was monitored by flow cytometry after stimulation with PMA/ionomycin, as described earlier.
Results
Phenotype of freshly isolated circulating human IL17-producing T cells
Current data about the polyfunctionality of IL17 producers in humans is limited.23,25 Therefore, we first analyzed the frequency of T cells in healthy donor PBMCs (n = 20) producing IL17, IFNγ, and IL2 in response to stimulation with PMA and ionomycin. The proportion of IL17-producing T cells was variable (mean ± SD 0.3% ± 0.3% of PBMCs; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). The majority of IL17-producing T cells were CD4+ T cells, consistent with prior studies (Figure S1B). Most of the circulating Th17 cells secreted either IL17 alone, or with IL2, with only 8% (± 3.5%) cells coexpressing both IL17 and IFNγ, known as Th17-1 cells (Figure S1C). Therefore, although the frequency of circulating human Th17 cells is variable between individuals, in most healthy individuals only a small proportion of circulating Th17 cells are Th17-1 cells.
DC-mediated expansion of Th17 cells
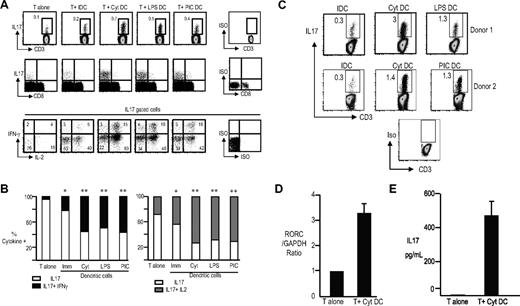
Next, we analyzed the capacity of monocyte-derived DCs to expand human Th17 cells in culture. T cells were cultured with either autologous immature DCs, DCs matured using inflammatory cytokines, or Toll receptor ligands (LPS or poly-IC). After 5 days, some of the cultured cells were analyzed for the presence of Th17 cells. Exogenous IL2 was added to the remainder of the cultures, which were reassayed 7 days later. The capacity of DCs to activate IL17-producing T cells was linked to their maturation status and was evident within 5 days of culture (Figure 1A,B). The capacity of mature DCs to mediate greater expansion of Th17 cells than immature DCs was also evident after another week of culture in the presence of IL2 (Figure 1C). Importantly, a substantial proportion of Th17 cells expanded with DCs were polyfunctional and secreted IFNγ in addition to IL17 and IL2 (Figure 1A,B). The degree of Th17-1 induction with different maturation stimuli tested was comparable, but always superior to immature DCs (Figure 1A,B). DC-mediated expansion of IL17-producing T cells was also verified by measuring the expression of Th17-polarizing transcription factor RORC by TaqMan (Figure 1D) and the presence of IL17 by ELISA (Figure 1E). In a prior study, it was suggested that human DCs could expand Th17 cells only from CD45RO+ T cells.26 However, we could also observe expansion of Th17 cells using CD45RO-depleted cells as a starting population (Figure S2). Therefore, mature DCs are efficient APCs for the induction of human Th17-1 cells.
DC-mediated activation of human IL17-producing T cells. T cells were culture alone (T alone) or with immature DCs (T + IDC), cytokine-matured DCs (T + Cyt DC), lypopolyscharide-matured DCs (T + LPS DC) or poly I:C–matured DCs (T + PIC DC). At 5 days later, the T cells were examined for the production of IL17 using PMA/ionomycin in the presence of GolgiStop for 5 hours. The cells were labeled with aqua LIVE/DEAD dye to distinguish dead cells, fixed, permeabilized, and simultaneously stained for the presence of IL17, IL2, and IFNγ. (A) Representative plot from one of the experiments showing the frequency of the IL17 + T cells in the cultures (top panel). Middle panel shows the phenotype of IL17-producing cells (data gated on CD3+ T cells). Bottom panel shows the cytokine profile of the IL17-producing cells in the various conditions. Data gated on CD3+IL17+ cells and analyzed for the expression of IL2 and IFNγ. The numbers represent the percentage of Th17 cells expressing IL17 alone or with IL2 or IFNγ. (B) Panel on the left is a bar graph showing the percentage of IL17 cells that make IL17 with or without IFNγ. Panel on the right shows the percentage of IL17 cells that make IL17 with or without IL2. The graphs represent data from 7 different healthy donors. *P < .05 when compared with T cells alone. **P < .05 when compared with T cells alone as well as T cells cultured with immature DCs. (C) Expansion of IL17-producing T cells by DCs. T cells were cultured with DCs as above for 5 days. After 5 days, IL2 was added to the DC–T-cell cocultures, and the T cells were cultured for an additional 7 days. On day 12 of culture, the T cells were stimulated with PMA and ionomycin to assess the frequency of IL17-producing T cells. Figure shows frequency of IL17-producing cells after coculture with cytokine-matured as well as LPS- and poly I:C–matured DCs. (D) T cells cultured alone (T alone) and those from cocultures with Cyt-DCs as in panel C were stimulated with PMA/ionomycin for 5 hours, and the expression of RORC was analyzed by TaqMan. (E) T cells cultured alone (T alone) and those from cocultures with Cyt-DCs as in panel C were stimulated with PMA/ionomycin for 5 hours, and supernatants were assayed for the presence of IL17 by ELISA. Error bars in panels D and E indicate SD.
DC-mediated activation of human IL17-producing T cells. T cells were culture alone (T alone) or with immature DCs (T + IDC), cytokine-matured DCs (T + Cyt DC), lypopolyscharide-matured DCs (T + LPS DC) or poly I:C–matured DCs (T + PIC DC). At 5 days later, the T cells were examined for the production of IL17 using PMA/ionomycin in the presence of GolgiStop for 5 hours. The cells were labeled with aqua LIVE/DEAD dye to distinguish dead cells, fixed, permeabilized, and simultaneously stained for the presence of IL17, IL2, and IFNγ. (A) Representative plot from one of the experiments showing the frequency of the IL17 + T cells in the cultures (top panel). Middle panel shows the phenotype of IL17-producing cells (data gated on CD3+ T cells). Bottom panel shows the cytokine profile of the IL17-producing cells in the various conditions. Data gated on CD3+IL17+ cells and analyzed for the expression of IL2 and IFNγ. The numbers represent the percentage of Th17 cells expressing IL17 alone or with IL2 or IFNγ. (B) Panel on the left is a bar graph showing the percentage of IL17 cells that make IL17 with or without IFNγ. Panel on the right shows the percentage of IL17 cells that make IL17 with or without IL2. The graphs represent data from 7 different healthy donors. *P < .05 when compared with T cells alone. **P < .05 when compared with T cells alone as well as T cells cultured with immature DCs. (C) Expansion of IL17-producing T cells by DCs. T cells were cultured with DCs as above for 5 days. After 5 days, IL2 was added to the DC–T-cell cocultures, and the T cells were cultured for an additional 7 days. On day 12 of culture, the T cells were stimulated with PMA and ionomycin to assess the frequency of IL17-producing T cells. Figure shows frequency of IL17-producing cells after coculture with cytokine-matured as well as LPS- and poly I:C–matured DCs. (D) T cells cultured alone (T alone) and those from cocultures with Cyt-DCs as in panel C were stimulated with PMA/ionomycin for 5 hours, and the expression of RORC was analyzed by TaqMan. (E) T cells cultured alone (T alone) and those from cocultures with Cyt-DCs as in panel C were stimulated with PMA/ionomycin for 5 hours, and supernatants were assayed for the presence of IL17 by ELISA. Error bars in panels D and E indicate SD.
DCs are superior to monocytes for the expansion of Th17 cells
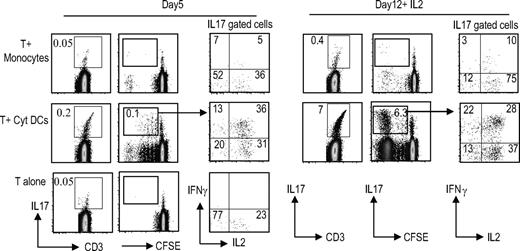
Prior studies have suggested that monocytes are effective APCs for expansion of human Th17 cells in culture.20,22 However, these studies tested expansion of allogeneic lymphocytes in the presence of anti-CD3 beads. In order to compare the capacity of different APCs to stimulate autologous IL17 producers, we monitored the expansion of Th17 cells in culture after stimulation with monocytes versus cytokine-matured DCs. Cultures were examined for the presence of Th17 cells after 5 days of coculture as well as at day 12 in the presence of IL2. Under these conditions, DCs were superior to monocytes both for the expansion of IL17-producing cells in culture, and the induction of polyfunctional Th17 cells (Figure 2).
DCs are superior to monocytes for the expansion of TH17 cells. T cells were labeled with the CFSE dye to monitor proliferation and cultured either alone (T alone) or with cytokine-matured DCs (T + Cyt DC) or monocytes (T + monocyte). On day 5, some of the cells were examined for proliferation as well as IL17 production using PMA/ionomycin stimulation as before. IL2 was added to the rest of the cultures. On day 12, the DC–T-cell cocultures were again examined for proliferation of the T cells as measured by the CFSE dilution and the presence of IL17 cells as well as for the cytokine profile of the IL17-producing cells with PMA/ionomycin stimulation. Figure represents one of 5 similar experiments.
DCs are superior to monocytes for the expansion of TH17 cells. T cells were labeled with the CFSE dye to monitor proliferation and cultured either alone (T alone) or with cytokine-matured DCs (T + Cyt DC) or monocytes (T + monocyte). On day 5, some of the cells were examined for proliferation as well as IL17 production using PMA/ionomycin stimulation as before. IL2 was added to the rest of the cultures. On day 12, the DC–T-cell cocultures were again examined for proliferation of the T cells as measured by the CFSE dilution and the presence of IL17 cells as well as for the cytokine profile of the IL17-producing cells with PMA/ionomycin stimulation. Figure represents one of 5 similar experiments.
Mechanism of DC-mediated expansion of Th17 cells
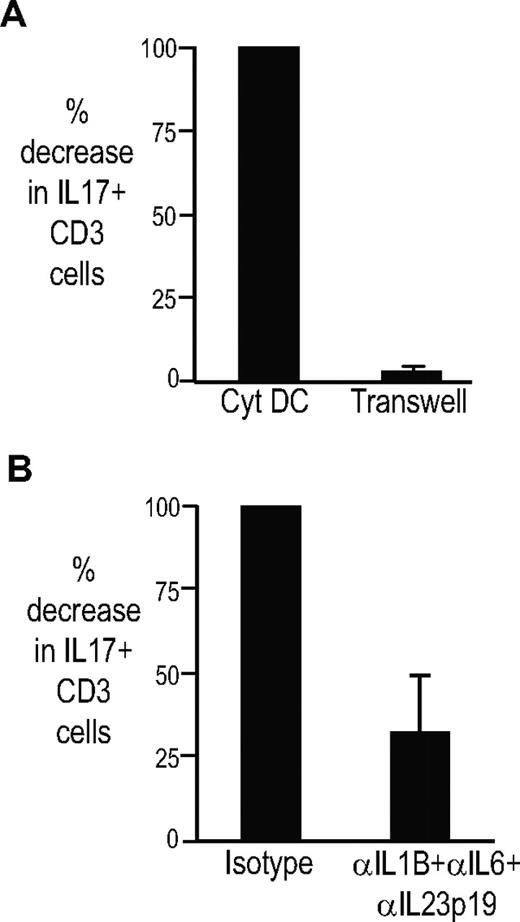
The capacity of DCs to expand Th17 cells depends on cell-cell contact (or short-range interactions), as cocultures in transwells abrogated both the expansion of Th17 cells as well as the induction of polyfunctional Th17-1 cells (Figure 3A; data not shown). The addition of blocking antibodies against IL1β, IL6, and IL23 also led to partial inhibition of the induction of Th17 cells (Figure 3B). Cytokine neutralization inhibited the induction of both single-positive and polyfunctional Th17 cells (data not shown). Therefore, DC-mediated expansion of Th17 cells under these conditions was cell-contact dependent and partly dependent on polarizing cytokines.
Mechanism of activation of Th17 cells by DCs. T cells were stimulated with cytokine-matured DCs alone or in the presence of blocking antibodies against IL1b, IL6, and IL23p19 or isotype antibodies. In some conditions, the T cells were separated from DCs using transwells. At 5 days after coculture, the T cells were stimulated with PMA/ionomycin to assess the presence of IL17-producing cells. The figure shows the percentage of decrease in IL17 cells when the DCs are separated from the T cells by transwell as well as in the presence of the blocking antibodies (n = 2). Figure shows mean plus standard deviation.
Mechanism of activation of Th17 cells by DCs. T cells were stimulated with cytokine-matured DCs alone or in the presence of blocking antibodies against IL1b, IL6, and IL23p19 or isotype antibodies. In some conditions, the T cells were separated from DCs using transwells. At 5 days after coculture, the T cells were stimulated with PMA/ionomycin to assess the presence of IL17-producing cells. The figure shows the percentage of decrease in IL17 cells when the DCs are separated from the T cells by transwell as well as in the presence of the blocking antibodies (n = 2). Figure shows mean plus standard deviation.
Expansion of antigen-specific Th17 cells
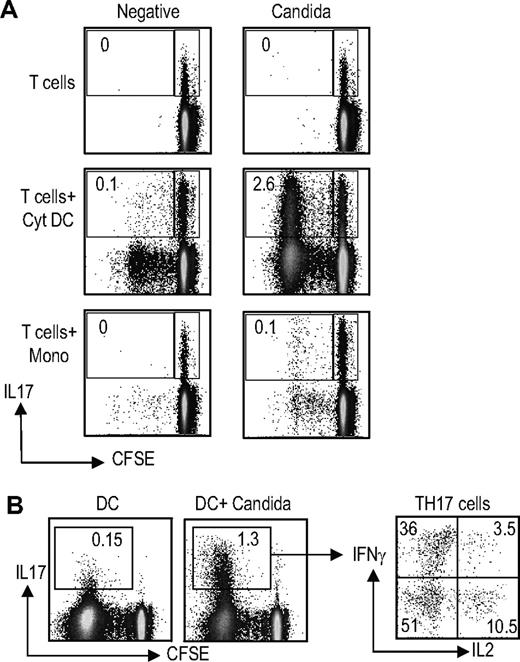
Recent studies have shown that a significant proportion of human Candida-specific T cells consists of Th17 cells.21 Therefore, we examined the capacity of inflammatory cytokine matured DCs (Cyt-DCs) loaded with Candida antigen to expand Th17 cells in culture (Figure 4). Antigen-specific proliferation was monitored with dilution of CFSE. After 5 days of culture, proliferation of Candida-specific IL17 producers could be detected in response to Candida-pulsed Cyt-DCs. The expansion of Candida-specific Th17 cells on day 5 of culture in response to Cyt-DCs was superior to that with monocytes (Figure 4A). Here again, the proliferating IL17-producing T cells were polyfunctional, and secreted IFNγ or IL2 in addition to IL17 (data not shown). Upon further culture for another week in the presence of IL2, the capacity of Candida-pulsed monocytes to expand Th17 cells could also be demonstrated, consistent with other studies (data not shown). In order to further analyze the antigen specificity of Th17 cells expanded using DCs, the T-cell cultures were restimulated with unpulsed or Candida-pulsed DCs (Figure 4B). Expansion of Candida-specific Th17-1 cells could be clearly demonstrated in these cultures.
Expansion of antigen-specific Th17 cells by DCs. (A) Comparison of monocytes versus Cyt-DCs for the expansion of antigen-specific Th17 cells. CFSE-labeled T cells were cultured alone (T cells), or cocultured with cytokine-matured DCs (T cells + Cyt DC) or monocytes (T cells + Mono) with or without loading with Candida antigen (20 μg/mL overnight). At 5 days later, T cells were stimulated with PMA/ionomycin in the presence of monensin. Candida antigen–specific T cells (those that proliferated and diluted their CFSE in response to stimulation with DCs loaded with Candida antigen) were examined for the production of IL17 and IFNγ as well as IL2 using flow cytometry. Figure represents one of 5 similar experiments. (B) T cells from cultures expanded using Candida-loaded Cyt-DCs as in panel A were restimulated with unpulsed DCs or Candida-loaded DCs. The presence of IL17-, IFNγ-, and IL2-producing cells was analyzed using flow cytometry.
Expansion of antigen-specific Th17 cells by DCs. (A) Comparison of monocytes versus Cyt-DCs for the expansion of antigen-specific Th17 cells. CFSE-labeled T cells were cultured alone (T cells), or cocultured with cytokine-matured DCs (T cells + Cyt DC) or monocytes (T cells + Mono) with or without loading with Candida antigen (20 μg/mL overnight). At 5 days later, T cells were stimulated with PMA/ionomycin in the presence of monensin. Candida antigen–specific T cells (those that proliferated and diluted their CFSE in response to stimulation with DCs loaded with Candida antigen) were examined for the production of IL17 and IFNγ as well as IL2 using flow cytometry. Figure represents one of 5 similar experiments. (B) T cells from cultures expanded using Candida-loaded Cyt-DCs as in panel A were restimulated with unpulsed DCs or Candida-loaded DCs. The presence of IL17-, IFNγ-, and IL2-producing cells was analyzed using flow cytometry.
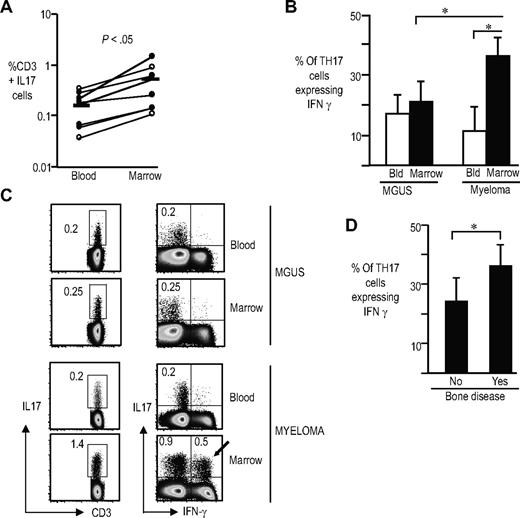
Frequency and phenotype of Th17 cells in patients with myeloma
Most studies of human Th17 cells to date have focused on patients with autoimmunity. In order to analyze the proportion of Th17 cells in the setting of human cancer, we analyzed these cells in paired samples from the blood and marrow of patients with plasma cell dyscrasias. Clinical features of patients studied are shown in Table 1. Overall, the proportion of Th17 cells in the bone marrow of patients was higher than the corresponding frequencies of these cells in the blood (Figure 5A). Functional properties of Th17 cells in the marrow were different from those in the blood. Bone marrow IL17 producers in the myeloma marrow contained a significantly higher proportion of polyfunctional Th17-1 cells than those in circulation (Figure 5B). This enrichment of Th17-1 cells was specific for MM, and not observed in the bone marrow from monoclonal gammopathy of undetermined significance (MGUS; Figure 5B,C). Consistent with this, the proportion of Th17-1 cells was higher in patients with lytic bone disease (Figure 5D). Therefore IL17 producers in the myeloma marrow are enriched for polyfunctional Th17-1 cells, similar to the phenotype of cells induced by DCs.
Enrichment of Th17-1 cells in the bone marrow of patients with myeloma. Paired samples from blood and bone marrow of patients with plasma cell dyscrasias were stimulated with PMA/ionomycin for 5 hours in the presence of GolgiStop. The presence of IL17-producing cells were examined by intracellular flow cytometry. The cells were labeled with aqua LIVE/DEAD dye to gate out the dead cells, fixed and permeabilized, and stained with antibodies against CD3, CD4, CD8, IL17, IL2, and IFNγ. (A) Frequency of IL17-producing cells in the blood compared with the bone marrow. Open symbols indicate MGUS; closed symbols, myeloma. P < .05 Horizontal bars represent the mean for IL17 cells in blood and marrow. (B) Proportion of the IL17-producing cells producing IFNγ (Th17-1 cells) in the blood or marrow of patients with myeloma (n = 4) and MGUS (n = 4). *P < .05 for marrow versus blood in myeloma, and for MGUS marrow versus myeloma marrow. (C) Representative fluorescence-activated cell sorter (FACS) plot showing frequency of Th17 as well as Th17-1 cells in paired blood and marrow samples from patients with myeloma or MGUS. The numbers represent the percentage of IL17-producing CD3+ T cells. (D) Comparison of the proportion of Th17-1 cells in patients with or without lytic bone disease. Error bars represent the mean and standard deviation.
Enrichment of Th17-1 cells in the bone marrow of patients with myeloma. Paired samples from blood and bone marrow of patients with plasma cell dyscrasias were stimulated with PMA/ionomycin for 5 hours in the presence of GolgiStop. The presence of IL17-producing cells were examined by intracellular flow cytometry. The cells were labeled with aqua LIVE/DEAD dye to gate out the dead cells, fixed and permeabilized, and stained with antibodies against CD3, CD4, CD8, IL17, IL2, and IFNγ. (A) Frequency of IL17-producing cells in the blood compared with the bone marrow. Open symbols indicate MGUS; closed symbols, myeloma. P < .05 Horizontal bars represent the mean for IL17 cells in blood and marrow. (B) Proportion of the IL17-producing cells producing IFNγ (Th17-1 cells) in the blood or marrow of patients with myeloma (n = 4) and MGUS (n = 4). *P < .05 for marrow versus blood in myeloma, and for MGUS marrow versus myeloma marrow. (C) Representative fluorescence-activated cell sorter (FACS) plot showing frequency of Th17 as well as Th17-1 cells in paired blood and marrow samples from patients with myeloma or MGUS. The numbers represent the percentage of IL17-producing CD3+ T cells. (D) Comparison of the proportion of Th17-1 cells in patients with or without lytic bone disease. Error bars represent the mean and standard deviation.
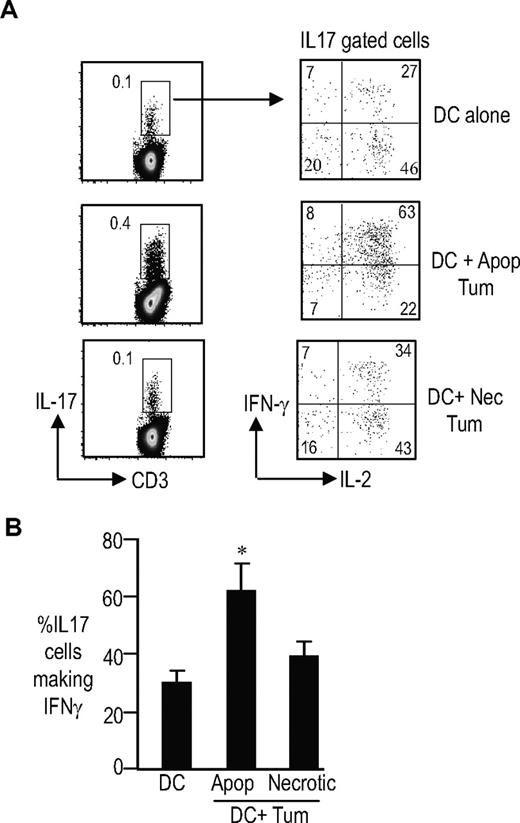
Expansion of Th17 cells by DCs loaded with dying tumor cells
Prior studies have shown that the tumor bed in myeloma is highly enriched in DCs. Relative enrichment of Th17-1 cells in the tumor bed suggested that interactions between tumor cells and DCs may contribute to the observed phenotype of IL17 producers. Several studies have documented an increase in several inflammatory cytokines, including IL6, IL1, and TNF in MM.36 As noted earlier, we have shown that culture of DCs with these inflammatory cytokines indeed enhances their capacity to activate Th17-1 cells. Effect of dying cells on DC-mediated antigen presentation is an area of investigation in several labs, but has focused largely on the induction of effector and regulatory T cells.33,34,37 We hypothesized that the uptake of dying tumor cells by tumor-infiltrating DCs might also impact DC-mediated induction of Th17-1 cells, in addition to the effect of inflammatory cytokines. To test this further, we loaded DCs with apoptotic or necrotic tumor cells and analyzed the induction of IL17 producers. DCs loaded with apoptotic tumor cells led to greater expansion of Th17-1 cells than unloaded DCs (Figure 6A,B). Interestingly, this was not observed with DCs loaded with necrotic cells. Thus, uptake of dying tumor cells by DCs in the tumor bed may also contribute to the observed enrichment of Th17-1 cells in the tumor bed. Together, these studies suggest that the mode of tumor cell death (apoptosis versus necrosis) has an impact on the ability of tumor-loaded DCs to induce Th17 cells.
Induction of Th17/1 cells by DCs loaded with apoptotic but not necrotic tumor cells. T cells were cultured with immature DCs (DC) or immature DCs fed apoptotic (DC + Apop tum) or necrotic myeloma tumor cells (DC + necrotic Tum) for 5 to 7 days. The frequency of IL17-producing cells as well as the profile of the Th17 cells was analyzed by intracellular cytokine flow cytometry after stimulation of the cells with PMA and ionomycin in the presence of GolgiStop. Data shown are for DCs fed with U266 myeloma cells. Similar findings were seen after uptake of OPM2 cells (data not shown). (A) The top panel shows the frequency of Th17 cells; the bottom panel shows the expression of IFNγ or IL2 in IL17-expressing cells. (B) Summary of experiments on 4 separate donors showing the percentage of TH17–1 T cells when T cells are cultured with DCs or DCs loaded with apoptotic or necrotic myeloma tumor cells. *P < .001 for DC + apoptotic cells compared with DC alone and DC + necrotic cell conditions. The data represent means plus SD.
Induction of Th17/1 cells by DCs loaded with apoptotic but not necrotic tumor cells. T cells were cultured with immature DCs (DC) or immature DCs fed apoptotic (DC + Apop tum) or necrotic myeloma tumor cells (DC + necrotic Tum) for 5 to 7 days. The frequency of IL17-producing cells as well as the profile of the Th17 cells was analyzed by intracellular cytokine flow cytometry after stimulation of the cells with PMA and ionomycin in the presence of GolgiStop. Data shown are for DCs fed with U266 myeloma cells. Similar findings were seen after uptake of OPM2 cells (data not shown). (A) The top panel shows the frequency of Th17 cells; the bottom panel shows the expression of IFNγ or IL2 in IL17-expressing cells. (B) Summary of experiments on 4 separate donors showing the percentage of TH17–1 T cells when T cells are cultured with DCs or DCs loaded with apoptotic or necrotic myeloma tumor cells. *P < .001 for DC + apoptotic cells compared with DC alone and DC + necrotic cell conditions. The data represent means plus SD.
Discussion
These data demonstrate that human monocyte-derived mature DCs are effective APCs for the induction of IL17-producing T cells, including antigen-specific Th17 cells in culture. Th17 cells have been implicated in the regulation of autoimmune reactions as well as host defense against pathogens such as Candida, and against tumors.3,4,38 DCs are currently under active clinical evaluation as adjuvants for immune therapy, as well as tools for ex vivo expansion of T cells for adoptive therapy.39 Therefore, the capacity of DCs to induce antigen-specific Th17 cells has obvious implications for optimally harnessing their properties against pathogens and tumors in the clinic.
An important aspect of DC-mediated expansion of IL17-producing T cells is the expansion of polyfunctional Th17 cells that coexpress IFNγ and/or IL2 and IL17. Th1 and Th17 cells have been typically viewed as antagonistic lineages, particularly in the mouse.24 This may be in part because transcription factors that drive the differentiation of murine Th1 or Th17 lineages (Tbet or RORγt, respectively) also inhibit the differentiation along the other pathway.40,41 However, T cells that coexpress both IFNγ and IL17 (Th17-1 cells) are quite prominent in the involved tissue in autoimmunity such as Crohn disease,23 and as shown here, in the tumor bed in human myeloma. Although such Th17-1 cells may also be observed in the mouse, the proportion of these cells may be higher in humans. Further studies are needed to better understand the biology of human Th17-1 cells, particularly in the tumor microenvironment.
The capacity of DCs to expand Th17 cells was linked to their activation status, and DCs matured with inflammatory cytokine cocktail led to greater expansion than immature DCs. Typically, the role of Th17-polarizing cytokines (eg, IL6) has been studied in terms of activation of critical transcription factors (eg, STAT3) in T cells.12,13,42 However, our data suggest that these cytokines may also help Th17 induction via activation of DCs. Inflammatory cytokines (eg, TNF) have also been shown to enhance the Th17-inducing capacity of monocytes.43 Recent studies have shown that bacterial peptidoglycan can prime human monocyte-derived DCs (in the presence of SEB) to mediate expansion of Th17 cells.26 It has been suggested that LPS-activated allogeneic monocytes, in the presence of anti-CD3, were efficient APCs for expansion of Th17 cells and superior to Mo-DCs.20,22 In contrast, we tested stimulation of autologous T cells without anti-CD3 beads or SEB. Under these conditions, mature DCs were more efficient for the induction of IL17 producers, particularly the Th17-1 cells.
In view of the link between chronic inflammation and cancer, Th17 cells may also play a role in regulating host defense against cancer. We find that in contrast to circulating Th17 cells, the IL17 producers in the marrow bed of patients with MM are predominantly polyfunctional Th17-1 cells. This was not seen in the marrow of patients with MGUS. Recently, serum levels of IL17 were shown to be elevated in patients with myeloma, which is consistent with our studies.44 IL17-producing cells can in principle contribute to myeloma pathology in several ways. For example, IL17 has been shown to have strong proangiogenic effects, and the elevated levels of IL17 in myeloma were correlated with elevated levels of angiogenic cytokines.44,45 IL17 has also been shown to be a potent osteoclastogenic factor,46,47 and therefore polyfunctional IL17 producers may also play a role in myeloma bone disease. Interestingly, both IL17 and IFNγ (as secreted by these polyfunctional cells) were recently shown to synergize in the induction of giant cell formation and inducing fusion of DCs.48,49 DC fusion has been implicated in the formation of osteoclasts in an inflammatory setting,50 and may well contribute to osteoclastogenesis in MM. IL17 can also induce the secretion of cytokines such as IL6 from stromal cells,45 which may promote myeloma growth. Therefore, our finding that Th17-1 cells are increased in the bone marrow in myeloma compared with MGUS is consistent with both the angiogenic and osteoclastogenic switch observed at this transition. Importantly, these data emphasize the need to directly study the T cells from the tumor bed in patients as opposed to those in circulation.33,51,52
Prior studies have shown that myeloma tumor bed is heavily infiltrated by DCs,53-56 which, as shown here, are the most efficient inducers of human Th17-1 cells. These data support a model in which Th17 cells are recruited to the tumor bed by Th17-attracting chemokines (eg, CCL20, recently shown to be enriched in the myeloma tumor bed)57 and activated to a Th17-1 phenotype locally by tumor-infiltrating DCs. The capacity of DCs to induce Th17-1 cells may be further enhanced by the uptake of apoptotic tumor cells, as well as inflammatory cytokines (eg, IL1, IL6, TNF)58 in the tumor bed. It is also possible that additional stimuli implicated in the induction of Th17 cells, such as pathogens,45 are present in the myeloma marrow, but these remain to be identified.
The finding that uptake of apoptotic but not necrotic tumor cells leads to enhanced induction of Th17-1 cells is of particular interest because altered clearance of apoptotic cells has already been linked to autoimmunity.59 These studies therefore provide a link between the clearance of dying cells and induction of Th17 cells in autoimmunity. The mechanistic basis of enhanced Th17 induction by apoptotic but not necrotic cells need further study, but may relate to altered signaling of IL12-related cytokines in response to uptake of apoptotic cells.60,61
In summary, we have shown that human DCs are efficient APCs for the induction of polyfunctional Th17-1 cells, and that such cells are the dominant population of IL17 producers in the tumor bed in human myeloma. DCs may therefore be efficient tools for the activation of antigen-specific Th17-1 cells in the clinic. It is notable, however, that at present, data from preclinical models are somewhat conflicting regarding whether IL17-producing T cells (or the IL23-IL17 axis) is beneficial or harmful in the context of immunity to pathogens (eg, Candida)30,62,63 or tumors.64-67 One possibility is that the biologic effects of Th17 cells may depend on the distinct subset of Th17 cells, which in turn may depend on the nature of APCs eliciting the response. Targeting the induction of Th17-1 cells may also be valuable in the context of autoimmunity.68 The capacity of human DCs to induce these cells as shown here makes them attractive targets for the manipulation of this pathway in the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ralph M. Steinman for his mentorship and critical review of this manuscript. This manuscript is dedicated to all of our patients and volunteers who have participated in our clinical studies.
M.V.D. is supported in part by funds from the National Institutes of Health (NIH; CA106802, CA109465), Damon Runyon Cancer Research Foundation (New York, NY), and Dana Foundation (New York, NY). K.M.D. is supported in part by funds from the NIH (AI054375), Dana Foundation, and the Alexandrine and Alexander Sinsheimer Award (New York, NY).
National Institutes of Health
Authorship
Contribution: K.M.D. designed the study, performed experiments, analyzed data, and wrote the paper; S.B., A.K., and P.M. performed some experiments; A.M., D.V., and S.J. performed clinical research; and M.V.D. codesigned the study and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kavita M. Dhodapkar, The Rockefeller University, 1230 York Avenue, Box 176, New York, NY 10065; e-mail: dhodapk@rockefeller.edu.