Abstract
Aberrant expression of Aurora kinases and inactivation of wild-type p53 by Mdm2 overexpression are frequent molecular events in acute myelogenous leukemia (AML), and preclinical data for inhibition of Aurora kinases or Mdm2 are promising. However, it remains largely unknown whether the viability of cells exposed to Aurora kinase inhibitors depends on the p53 status. We investigated the interaction of Aurora kinases and p53 pathways after their simultaneous blockades using a small-molecule pan-Aurora kinase inhibitor, MK-0457, and a selective small-molecule antagonist of Mdm2, Nutlin-3. We found that MK-0457, which itself activates p53 signaling, acts synergistically with Nutlin-3 to induce apoptosis in wild-type p53 AML cell lines OCI-AML-3 and MOLM-13 but not in p53-null HL-60 cells. MK-0457 and Nutlin-3 showed synergism in inducing p53, conformational change of Bax and Δψm loss, suggesting an involvement of p53-mediated mitochondrial apoptosis. Nutlin-3 constrained endoreduplication after Aurora inhibition via activation of a p53-dependent postmitotic checkpoint and p21 induction in pseudo-G1 cells. Our findings provide the molecular rationale for concomitant targeting of Aurora kinases and Mdm2 in AML where TP53 mutations are rare and downstream p53 signaling is mostly intact.
Introduction
The Aurora family of serine/threonine kinases is essential for mitotic progression.1 The mammalian kinases, Aurora A, B, and C, share similar catalytic domains with 67% to 76% amino acid sequence identity. Aurora A plays a crucial role in bipolar spindle formation and centrosome maturation, which secures segregation of chromosomes into daughter cells.2 Aurora B and C are chromosomal passenger proteins.1 Aurora B is required for chromosomal segregation and cytokinesis.1 Overexpression of kinase-inactive Aurora-B disrupts kinetochore-microtubule interactions, cleavage furrow formation, and cytokinesis, leading to polyploidy.3 The polyploid state can arrest cell-cycle progression through activation of a p53-dependent checkpoint.4 Aurora C has been described to complement Aurora B function in cytokinesis.5 Aurora kinases have been strongly associated with cancer. The Aurora kinases are overexpressed in a variety of solid tumors, including colon, breast, ovarian, gastric, and pancreatic tumors.6,7 It has also been shown that hematologic malignancies, including acute myelogenous leukemias (AML), acute lymphoblastic leukemias, as well as chronic myeloid leukemias, aberrantly express Aurora A and B kinases.8
MK-0457 (formerly VX-680) is a small-molecule pan-Aurora kinase inhibitor that blocks cell-cycle progression and induces apoptosis in a diverse range of human tumor types.9 Tumor cells treated with MK-0457 enter and exit mitosis with normal kinetics. However, after the completion of mitosis, the cells accumulate in a pseudo-G1 state with a 4N DNA content or proceed to S-phase in the absence of cell division. Continued proliferation in the presence of aberrant mitosis and failed cytokinesis presumably results in apoptosis.9 These cellular effects are closely associated with the disruption of Aurora B function.10 Whether cells arrest with a 4N DNA content in pseudo-G1 or endoreduplicate with the accumulation of more than 4N DNA content is thought to primarily depend on the status of the p53-dependent postmitotic checkpoint.10,11 p53 can respond to a failed cell division by inducing a G1-like arrest of tetraploid cells after an abnormal mitosis. Consistent with the role of p53 in constraining endoreduplication after Aurora inhibition, endoreduplication induced by Aurora kinase inhibition was enhanced when p53 was inactivated by genetic modification using either short interfering RNA, HPV-16-E6 oncoprotein, or dominant-negative p53.12,13 The mechanism for apoptotic effect of MK-0457 remains unclear. Although recent studies have suggested that the integrity of the postmitotic checkpoint may govern not only the degree of endoreduplication but also the viability of cells exposed to MK-0457,10 it is debatable whether the viability of cells exposed to Aurora kinase inhibitors depends on the p53 status.13,14 Furthermore, very little is known about the ultimate fate of the arrested cells. If cell death after Aurora inhibition depends on the absence or a compromised p53 signaling,13 it is possible that activation of p53 may inhibit MK-0457-induced apoptosis. This poses a major concern in AML, in which p53 mutation is rare and induction of apoptosis determines the response to conventional chemotherapy.15 To examine these issues further, we have explored the role of p53 in the response to MK-0457 using Nutlin-3,16 a potent and selective small-molecule antagonist of Mdm2. Nutlin-3 increases cellular p53 levels, a critical determinant of p53-dependent apoptosis, and efficiently induces p53-mediated apoptosis in AML cells harboring wild-type p53.17 The p53-mediated apoptosis pathway has been shown to be well preserved in model cell lines OCI-AML-3 and MOLM-13.17-19
We found that (1) concomitant inhibition of Mdm2-p53 interaction and Aurora kinases synergistically induces apoptosis in AML cells with wild-type p53; (2) Nutlin-3 enhances p53 signaling and mitochondrial apoptosis in concert with Aurora inhibition, involving activation of p53-dependent postmitotic checkpoints; and (3) Nutlin-3 aberrantly induces p21 in pseudo-G1 cells and blocks endoreduplication after Aurora inhibition. Our data suggest that combined targeting of Mdm2-p53 interaction and Aurora kinases would constitute a novel mechanism-based therapy with clinical potential in AML.
Methods
Reagents
The pan-Aurora inhibitor MK-0457 (formerly VX-680) and the selective small-molecule antagonist of Mdm2, Nutlin-3 (Axxora Life Sciences, San Diego, CA) were used.9,16 In some experiments, cells were cultured with 50 μM Z-VAD-FMK (Axxora Life Sciences). Z-VAD-FMK was added to the cells 1 hour before drug administration. The final dimethyl sulfoxide (DMSO) concentration in the medium did not exceed 0.1% (vol/vol). At this concentration, DMSO itself had no effect up to 72 hours on cell growth or viability of the AML cells used in this study.
Cell lines, primary samples, and cultures
Three human AML cell lines were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS). OCI-AML-3 and MOLM-13 cells have wild-type p53, whereas p53 is disabled in HL-60 by large deletion of the TP53.17 Cell lines were harvested in log-phase growth, seeded at a density of 2 × 105 cells/mL, and exposed to MK-0457 and/or Nutlin-3 simultaneously. Heparinized peripheral blood samples were obtained from previously untreated AML patients with more than 70% leukemia cells after informed consent approved by the University of Texas M.D. Anderson Cancer Center Institutional Review Board was obtained in accordance with the Declaration of Helsinki. Mononuclear cells were purified by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) density-gradient centrifugation, and nonadherent cells were resuspended in RPMI 1640 medium supplemented with 10% FCS at a density of 5 × 105 cell/mL. HCT116 p21+/+ and p21−/− cells were kindly provided by B. Vogelstein (Johns Hopkins University School of Medicine, Baltimore, MD).20 The p21-deleted cells, created by homologous recombination techniques, were cultured in Dulbecco modified Eagle medium containing 10% FCS. Cell viability was evaluated by triplicate counts of trypan blue dye–excluding cells.
Transfection of Aurora B small interfering RNA
OCI-AML-3 cells were transfected by the Amaxa electroporator Nucleofector I, using the Nucleofector Kit V (program T-003) according to the manufacturer's procedure (Amaxa Biosystems, Gaithersburg, MD). To evaluate the transfection efficiency, cells were transfected with the BLOCK-iT Fluorescent Oligo (Invitrogen, Carlsbad, CA); efficiency of transfection was estimated to be approximately 60%, with approximately 90% cell viability. Small interfering RNA (siRNA) Aurora B was performed with validated Stealth RNAi DuoPak (12 938-004, Invitrogen). OCI-AML-3 cells (106 cells resuspended in 1 mL) were transfected by electroporation with scramble Med GC Duplex Stealth RNAi Negative Control (12 935-300; Invitrogen) or with Aurora B RNAi. Seventy-two hours after transfection, p53 expression was analyzed by Western blot analysis.
Apoptosis analysis
Evaluation of apoptosis by the annexin V–propidium iodide (PI) binding assay was performed as described.17 The extent of apoptosis was quantified as percentage of annexin V–positive cells, and the extent of drug-specific apoptosis was assessed by the formula: % specific apoptosis = (test − control) × 100/(100 − control). In the formula, the numerator is the actual amount of killing that occurred and the denominator is the potential amount of killing that could occur. To measure mitochondrial membrane potential (Δψm), cells were loaded with MitoTracker Red CMXRos (300 nM) and MitoTracker Green (100 μM, both from Invitrogen) for 1 hour at 37°C. The Δψm was then assessed by measuring CMXRos retention (red fluorescence) while simultaneously adjusting for mitochondrial mass (green fluorescence).17 All experiments were conducted in triplicate.
Western blot analysis and immunoprecipitation
Western blot analysis was performed as previously described.17 The following antibodies were used: rabbit polyclonal antiphospho-histone H3 (Cell Signaling Technology, Danvers, MA); rabbit polyclonal anti-p53 (FL-393; Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-Mdm2 (D-12; Santa Cruz Biotechnology); mouse monoclonal anti-p21 (Ab-1; EMD Biosciences, San Diego, CA); rabbit polyclonal anti-Puma (Ab-1; EMD Biosciences); mouse monoclonal anti-Bax (YTH-6A7; Trevigen, Gaithersburg, MD); rabbit polyclonal anti-Bax (Cell Signaling Technology); and mouse monoclonal anti–β-actin (AC-74; Sigma-Aldrich). Immunoprecipitation with the conformation-specific antibody YTH-6A7 was performed in targeting buffer (10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4, 20 mM KCl, 5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic acid) at 4°C. Immunoprecipitates were collected by incubating with protein A/G-agarose (Santa Cruz Biotechnology) for 3 hours, followed by centrifugation for 1 minute. The pellets were washed twice with wash buffer A (10 mM HEPES, pH 7.4, 150 mM NaCl, 2% 3-cholamidopropyl)dimethylammonio-1-propanesulfonate), 3 times with wash buffer B (10 mM HEPES, pH 7.4, 150 mM NaCl, 0.2% 3-cholamidopropyl)dimethylammonio-1-propanesulfonate), then an additional 3 times with wash buffer C (100 mM Tris-HCl, pH 8.0, 100 mM NaCl). Immunoprecipitates were released from the beads in sodium dodecyl sulfate loading buffer and analyzed by Western blotting with the rabbit polyclonal Bax antibodies.
Cell-cycle analysis
Cells were permeabilized in 70% ice-cold ethanol, incubated overnight with PI solution (25 μg/mL PI), and analyzed as described previously.17 Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets and aggregates, and a minimum of 3 × 104 gated cells was collected per sample.
Quantitation of intracellular proteins by flow cytometry
For intracellular p53 detection, cells were fixed with 2% paraformaldehyde and permeabilized with 100% ice-cold methanol and incubated for 1 hour at 4°C with antibody against p53 or its isotypic control (BD Biosciences, San Jose, CA).21 Involvement of Bax conformational change was analyzed by an antibody directed against the NH2-terminal region of Bax (YTH-6A7; Trevigen).21 Cellular fixation, permeabilization, and staining with primary antibody or an isotypic control were performed using the Dako IntraStain kit (Dako North America, Carpinteria, CA), according to the manufacturer's instructions. After washing, cells were incubated with Alexa Fluor 488 chicken anti–mouse secondary antibodies (Invitrogen) for 30 minutes at 4°C. Total Bax levels were determined by using polyclonal anti-Bax antibodies (Cell Signaling Technology) and Alexa Fluor 488 chicken anti–rabbit secondary antibodies (Invitrogen). When necessary, cells were further stained with PI for simultaneous DNA content analysis. All flow cytometric data were analyzed with FlowJo Software (TreeStar, Ashland, OR).
Statistical analysis
The statistical analysis was performed using the 2-tailed Student t test. Statistical significance was considered when P was less than .01. Unless otherwise indicated, average values were expressed as mean plus or minus standard deviation (SD). Synergism, additive effects, and antagonism were assessed as previously described.21 The combination index (CI), a numerical description of combination effects, was calculated using the more stringent statistical assumption of mutually nonexclusive modes of action. When CI = 1, this equation represents the conservation isobologram and indicates additive effects. CI values less than 1.0 indicate a more than expected additive effect (synergism), whereas CI values more than 1.0 indicate antagonism between the 2 drugs.
Results
Aurora kinase inhibition by MK-0457 enhances p53-mediated apoptosis in AML cell lines
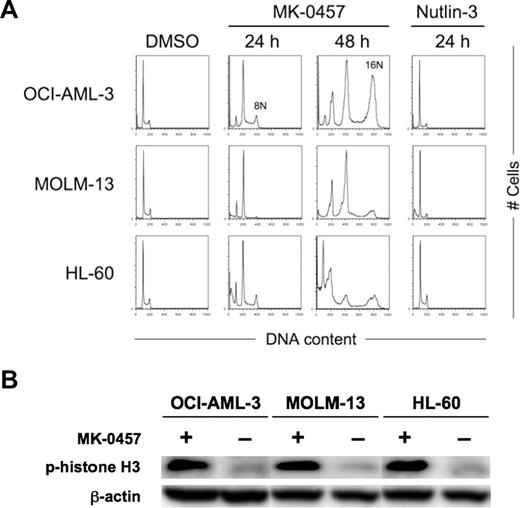
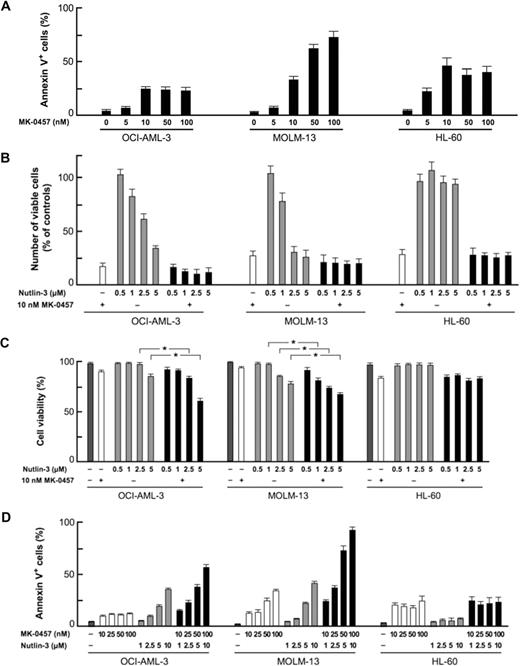
We first examined the effect of the Aurora kinase inhibitor on the growth and viability of cultured AML cell lines. OCI-AML-3 and MOLM-13 cells have wild-type p53 and HL-60 cells lack p53. MK-0457 exhibited antiproliferative activity at as low as 10 nM, where it induces endoreduplication of the cells (Figure 1A). MK-0457 at 10 nM efficiently ablated the expression of the phosphorylated forms of histone H3 on Ser10 in these cell lines (Figure 1B), suggesting effective inhibition of Aurora B kinase. MK-0457 also showed cytotoxic activity associated with induction of apoptosis (Figure 2A). These results are consistent with previously published data.9 A dose-response relationship between MK-0457 concentration and apoptosis induction was seen in MOLM-13 cells, whereas the apoptotic effect reached to the maximum at 10 nM in OCI-AML-3 and HL-60 cells (Figure 2A). Increased concentrations (∼ 1 μM) of MK-0457 did not augment apoptosis induction in OCI-AML-3 and HL-60 cells. To clarify whether MK-0457 enhances p53-dependent apoptosis, we next combined 10 nM of MK-0457 and a range of concentrations of Nutlin-3. The combination added minimal antiproliferative effect compared with treatment with MK-0457 alone (Figure 2B), probably because 10 nM of MK-0457 strongly inhibited the proliferation of AML cells. MK-0457 enhanced Nutlin-induced cytotoxicity in wild-type p53 cells (Figure 2C), implying that MK-0457 could augment p53-mediated induction of apoptosis. To statistically determine the combined effects of MK-0457 and Nutlin-3 on apoptosis induction, we exposed cells to MK-0457 (0, 10, 25, 50, or 100 nM) and Nutlin-3, using a fixed-ratio experimental design (1:100 in OCI-AML-3 and 1:50 in MOLM-13 cells). The interaction study between MK-0457 and Nutlin-3 showed synergistic effects on induction of apoptosis in OCI-AML-3 and MOLM-13 cells (Figure 2D). The CI values were 0.49 for ED50, 0.60 for ED75, and 0.74 for ED90 in OCI-AML-3 cells and were 0.26, 0.17, and 0.12 in MOLM-13 cells. The averaged CI values calculated from the values for ED50, ED75, and ED90 were 0.62 in OCI-AML-3 cells and 0.18 in MOLM-13 cells. These findings suggest that MK-0457 synergizes with Nutlin-3 to induce apoptosis in AML cells with wild-type p53. Such a synergism was not seen in p53-null HL-60 cells (Figure 2D).
Inhibition of Aurora kinase activity induces endoreduplication in AML cells. (A) AML cells were treated for the indicated time with 10 nM MK-0457 or 2.5 μM Nutlin-3. MK-0457 induced time-dependent endoreduplication of the cells irrespective of the p53 status. Nutlin-3 caused G1-phase cell-cycle arrest only in wild-type p53 OCI-AML-3 and MOLM-13 cells. Results are representative of 3 independent experiments. (B) Expression of phosphorylated histone H3 in AML cells after 24 hours of treatment with 10 nM MK-0457. MK-0457 abrogated histone H3 phosphorylation on Ser10 . Results are representative of 3 independent experiments.
Inhibition of Aurora kinase activity induces endoreduplication in AML cells. (A) AML cells were treated for the indicated time with 10 nM MK-0457 or 2.5 μM Nutlin-3. MK-0457 induced time-dependent endoreduplication of the cells irrespective of the p53 status. Nutlin-3 caused G1-phase cell-cycle arrest only in wild-type p53 OCI-AML-3 and MOLM-13 cells. Results are representative of 3 independent experiments. (B) Expression of phosphorylated histone H3 in AML cells after 24 hours of treatment with 10 nM MK-0457. MK-0457 abrogated histone H3 phosphorylation on Ser10 . Results are representative of 3 independent experiments.
Inhibition of Aurora kinase activity enhances p53-dependent apoptosis in AML cells. (A) AML cells were incubated with the indicated concentrations of MK-0457 for 48 hours, and the annexin V–positive fractions were measured by flow cytometry. Data are mean plus or minus SD. A dose-response relationship ranging from 5 to 100 nM was seen in MOLM-13 cells, whereas the effect reached the maximum at 10 nM in OCI-AML-3 and HL-60 cells. (B) Effects of MK-0457 and Nutlin-3 on viable cell number. Cells were incubated with a range of concentrations of Nutlin-3 in the absence or presence of 10 nM MK-0457 for 48 hours, and the cell viability was determined by the trypan blue exclusion method. Results are expressed as the percentage of the viable cell number in an untreated group and represent the average of triplicate cultures. (C) MK-0457 enhanced Nutlin-induced cytotoxicity in wild-type p53 OCI-AML-3 and MOLM-13 cells. Cells were incubated with a range of concentrations of Nutlin-3 in the absence or presence of 10 nM MK-0457 for 48 hours, and the cell viability was determined by the trypan blue exclusion method. Data are mean plus or minus SD. (D) MK-0457 enhanced Nutlin-induced apoptosis in wild-type p53 OCI-AML-3 and MOLM-13 cells but not in mutant p53 HL-60 cells. Cells were incubated with the indicated concentrations of MK-0457 or Nutlin-3, and the annexin V–positive fractions were measured by flow cytometry at 24 hours. Data are mean plus or minus SD.
Inhibition of Aurora kinase activity enhances p53-dependent apoptosis in AML cells. (A) AML cells were incubated with the indicated concentrations of MK-0457 for 48 hours, and the annexin V–positive fractions were measured by flow cytometry. Data are mean plus or minus SD. A dose-response relationship ranging from 5 to 100 nM was seen in MOLM-13 cells, whereas the effect reached the maximum at 10 nM in OCI-AML-3 and HL-60 cells. (B) Effects of MK-0457 and Nutlin-3 on viable cell number. Cells were incubated with a range of concentrations of Nutlin-3 in the absence or presence of 10 nM MK-0457 for 48 hours, and the cell viability was determined by the trypan blue exclusion method. Results are expressed as the percentage of the viable cell number in an untreated group and represent the average of triplicate cultures. (C) MK-0457 enhanced Nutlin-induced cytotoxicity in wild-type p53 OCI-AML-3 and MOLM-13 cells. Cells were incubated with a range of concentrations of Nutlin-3 in the absence or presence of 10 nM MK-0457 for 48 hours, and the cell viability was determined by the trypan blue exclusion method. Data are mean plus or minus SD. (D) MK-0457 enhanced Nutlin-induced apoptosis in wild-type p53 OCI-AML-3 and MOLM-13 cells but not in mutant p53 HL-60 cells. Cells were incubated with the indicated concentrations of MK-0457 or Nutlin-3, and the annexin V–positive fractions were measured by flow cytometry at 24 hours. Data are mean plus or minus SD.
Inhibition of Aurora kinases induces p53 and augments Nutlin-induced, p53-mediated apoptosis in AML cells
Inhibition of Aurora kinases has been suggested to activate the p53-dependent postmitotic checkpoint,10,11 and recent papers have demonstrated that inhibition of Aurora kinase can induce p53 in AML cells.14 Treatment of wild-type p53 AML cells with 10 nM of MK-0457 induced p53 (Figure 3A). Induced p53 levels were highest in cells treated with MK-0457 and Nutlin-3, suggesting their cooperation to activate p53 signaling (Figure 3A). p53 induction by MK-0457 and its augmentation by combination treatment with Nutlin-3 were detectable as early as 6 hours of exposure. In accordance with p53 accumulation, MK-0457 induced increased expression of p53-related proteins Mdm2, p21, and Puma (Figure 3B). To test the hypothesis that Aurora B expression modulates p53, Aurora B levels were reduced in OCI-AML-3 cells using siRNA. Aurora B siRNA led to a significant inhibition of Aurora B expression relative to cells transfected with a control siRNA 72 hours after transfection (Figure 3C). In contrast, we did not detect interference with the synthesis of the housekeeping cellular protein β-actin. Aurora B siRNA led to enhanced p53 expression (Figure 3C). p53 activates the proapoptotic protein Bax, resulting in Bax conformational change, mitochondrial membrane permeabilization, and apoptosis.22 Involvement of Bax conformational change and mitochondrial membrane permeabilization were determined in AML cells treated with 10 nM of MK-0457. A small fraction of cells (5%-10%) showed activation of Bax along with Δψm loss after 12 hours of treatment (Figure 3D). Activation of Bax and Δψm loss were also seen in p53-null HL-60 cells, suggesting that Aurora kinase inhibition induces mitochondrial apoptosis via both p53-dependent and -independent pathways. The low percentages of cells with active Bax or Δψm loss at 12 hours were consistent with small extent of apoptosis after the longer exposure to MK-0457 (Figure 2A,D). To elucidate the early stage of apoptosis and the combined effects of MK-0457 and Nutlin-3 on Bax activation and mitochondrial membrane permeabilization, we exposed MOLM-13 cells to 10 nM of MK-0457, 2.5 μM of Nutlin-3, or both for 6 hours. Few control cells were stained with this antibody (0.9% ± 0.2%), and MK-0457 induced a small degree of conformational change of Bax (3.2% ± 0.2%; Figure 3E). In MOLM-13 cells, Nutlin-3 immediately (∼ 1 hour) accumulates p53, which can directly activate Bax.17,22 We have also shown that total Bax levels remained unchanged in the first 24 hours after exposure.17 As predicted, an increase in the percentage of active Bax-positive cells was seen after incubation with Nutlin-3 (9.2% ± 0.6%). Interestingly, MK-0457 considerably enhanced Bax conformational change by Nutlin-3 (26.6% ± 2.1%; Figure 3E). When Bax antibodies that detect total Bax were used, no differences in the fluorescence pattern between control and drug-treated cells were observed (Figure 3E). Immunoprecipitation/immunoblotting results confirmed the cooperative nature of MK-0457/Nutlin-3 interaction on Bax activation (Figure 3F). MK-0457 and Nutlin-3 also showed strong synergism in inducing Δψm loss (Figure 3G). These results suggest that Aurora inhibition actively enhancesp53-mediated mitochondrial apoptosis. The enhancement of Nutlin-induced Bax conformational change and Δψm loss by MK-0457 was also seen in OCI-AML-3 cells but not in p53-null HL-60 cells.
Aurora kinase inhibition induces p53 and enhances p53-mediated mitochondrial apoptosis. (A) Cells were treated for 12 hours with 10 nM MK-0457 and 2.5 μM Nutlin-3 (1 μM in MOLM-13 cells), either as individual agents or in combination. DMSO-treated cells served as control. (B) Expression of p53-related proteins in OCI-AML-3 cells, which were treated for 24 hours with 2.5 μM Nutlin-3 and 10 nM MK-0457, either as individual agents or in combination. MK-0457 as well as Nutlin-3 induced increased protein expression of Mdm2, p21, and a BH3-only member of the Bcl-2 family protein Puma in OCI-AML-3 cells. The intensity of the p53 signals was quantified by densitometry using ImageJ 1.38x software, and the relative intensity compared with β-actin was calculated. (C) Western blot analysis of OCI-AML-3 cells electroporated with either control or Aurora B siRNA. Twenty-four hours after siRNA electroporation, Aurora B, p53, and β-actin levels were determined. (D) AML cells were cultured for 12 hours in the absence (□) or presence (■) of 10 nM MK-0457. Δψm was assessed by flow cytometry. Data are mean plus or minus SD of triplicate measurements. Comparable results were obtained in 2 other independent experiments. *P ≤ .01. (E) MK-0457 and Nutlin-3 synergistically induce Bax conformational change. MOLM-13 cells were treated with 10 nM MK-0457 and 2.5 μM Nutlin-3 for 6 hours, either as individual agents or in combination, and Bax conformational change was determined by staining with the active conformation-specific anti-Bax antibody YTH-6A7 (shaded histogram) or a corresponding isotype control (open histogram). To block caspase activation-mediated conformational change of Bax, cells were preincubated for 1 hour with 50 μM Z-VAD-FMK. Results are representative of 3 independent experiments. (F) After 1 hour of preincubation with 50 μM Z-VAD-FMK, MOLM-13 cells were treated with 10 nM MK-0457 and 2.5 μM Nutlin-3 for 6 hours, either as individual agents or in combination. Active Bax was immunoprecipitated (IP) from total cell lysates with the conformation-specific antibody YTH-6A7. The amount of immunoprecipitated Bax and the levels of Bax and β-actin in whole-cell lysates (WCL) were determined by Western blot analysis. (G) MK-0457 and Nutlin-3 synergistically induce Δψm loss. MOLM-13 cells were cultured for 6 hours in the presence of DMSO, 10 nM MK-0457, 2.5 μM Nutlin-3, or a combination of MK-0457 and Nutlin-3. Δψm was assessed by flow cytometry. Data are mean plus or minus SD of triplicate measurements. Comparable results were obtained in 2 other independent experiments. *P ≤ .01.
Aurora kinase inhibition induces p53 and enhances p53-mediated mitochondrial apoptosis. (A) Cells were treated for 12 hours with 10 nM MK-0457 and 2.5 μM Nutlin-3 (1 μM in MOLM-13 cells), either as individual agents or in combination. DMSO-treated cells served as control. (B) Expression of p53-related proteins in OCI-AML-3 cells, which were treated for 24 hours with 2.5 μM Nutlin-3 and 10 nM MK-0457, either as individual agents or in combination. MK-0457 as well as Nutlin-3 induced increased protein expression of Mdm2, p21, and a BH3-only member of the Bcl-2 family protein Puma in OCI-AML-3 cells. The intensity of the p53 signals was quantified by densitometry using ImageJ 1.38x software, and the relative intensity compared with β-actin was calculated. (C) Western blot analysis of OCI-AML-3 cells electroporated with either control or Aurora B siRNA. Twenty-four hours after siRNA electroporation, Aurora B, p53, and β-actin levels were determined. (D) AML cells were cultured for 12 hours in the absence (□) or presence (■) of 10 nM MK-0457. Δψm was assessed by flow cytometry. Data are mean plus or minus SD of triplicate measurements. Comparable results were obtained in 2 other independent experiments. *P ≤ .01. (E) MK-0457 and Nutlin-3 synergistically induce Bax conformational change. MOLM-13 cells were treated with 10 nM MK-0457 and 2.5 μM Nutlin-3 for 6 hours, either as individual agents or in combination, and Bax conformational change was determined by staining with the active conformation-specific anti-Bax antibody YTH-6A7 (shaded histogram) or a corresponding isotype control (open histogram). To block caspase activation-mediated conformational change of Bax, cells were preincubated for 1 hour with 50 μM Z-VAD-FMK. Results are representative of 3 independent experiments. (F) After 1 hour of preincubation with 50 μM Z-VAD-FMK, MOLM-13 cells were treated with 10 nM MK-0457 and 2.5 μM Nutlin-3 for 6 hours, either as individual agents or in combination. Active Bax was immunoprecipitated (IP) from total cell lysates with the conformation-specific antibody YTH-6A7. The amount of immunoprecipitated Bax and the levels of Bax and β-actin in whole-cell lysates (WCL) were determined by Western blot analysis. (G) MK-0457 and Nutlin-3 synergistically induce Δψm loss. MOLM-13 cells were cultured for 6 hours in the presence of DMSO, 10 nM MK-0457, 2.5 μM Nutlin-3, or a combination of MK-0457 and Nutlin-3. Δψm was assessed by flow cytometry. Data are mean plus or minus SD of triplicate measurements. Comparable results were obtained in 2 other independent experiments. *P ≤ .01.
Induction of p53 blocks endoreduplication of AML cells by MK-0457 and enhances apoptosis
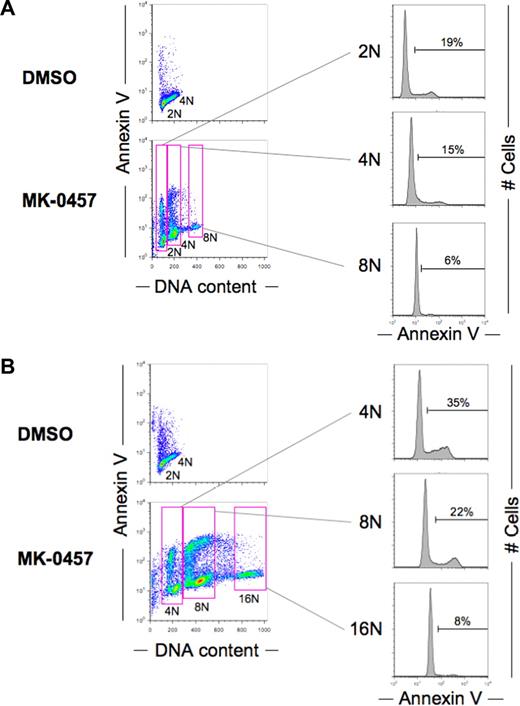
Inhibition of Aurora kinases has been described to induce endoreduplication.9,13 Treatment of AML cell lines with 10 nM of MK-0457 resulted in increased percentages of cells with high DNA content up to 16N at 48 hours (Figure 1A). To clarify an association of endoreduplication with apoptosis, annexin V–positive fractions were correlated with DNA content at 2 time points (36 and 48 hours) after exposure to MK-0457. At the earlier time point (36 hours), cells endoreduplicated with the accumulation of 8N DNA content, but cells with 16N DNA content were not seen. At 48 hours after exposure, the endoreduplication further progressed with the accumulation of 8N and 16N DNA content. As shown in Figure 4, higher DNA content was associated with lower percentages of annexin V–positive cells. The longer exposure increased the percentages of annexin V–positive cells in cells with 4N or 8N DNA content. These findings suggest that apoptosis occurs among the arrested cells. The finding was consistently found in all 3 cell lines. It has been described that postmitotic checkpoint depends on p53.11-13 To determine whether induction of p53 prevents endoreduplication, cells were concomitantly treated with MK-0457 and Nutlin-3. Nutlin-3 blocked endoreduplication associated with Aurora inhibition; and more interestingly, it enhanced MK-0457–induced apoptosis (Figure 5A,B). The blockade of endoreduplication and enhancement of apoptosis were not seen in p53-null HL-60 cells (Figures 2D, 5C).
Cells with higher DNA content are resistant to MK-0457–induced apoptosis. MOLM-13 cells were treated with 10 nM MK-0457 for 36 hours (A) or 48 hours (B). The annexin V–positive fractions in correlation with DNA content were measured by flow cytometry. Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets. DMSO-treated cells served as control. Results are representative of 3 independent experiments.
Cells with higher DNA content are resistant to MK-0457–induced apoptosis. MOLM-13 cells were treated with 10 nM MK-0457 for 36 hours (A) or 48 hours (B). The annexin V–positive fractions in correlation with DNA content were measured by flow cytometry. Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets. DMSO-treated cells served as control. Results are representative of 3 independent experiments.
Nutlin-induced p53 blocks endoreduplication and enhances apoptosis in wild-type p53 AML cells treated with MK-0457. (A,B) OCI-AML-3 cells (A) or MOLM-13 cells (B) were treated for 48 hours with 2.5 μM Nutlin-3 and 10 nM of MK-0457, either as individual agents or in combination. Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets. DMSO-treated cells served as control. Combination treatment results in reduced endoreduplication with increased percentage of annexin V–positive, apoptotic cell fraction. (C) HL-60 cells were treated for 48 hours with 2.5 μM Nutlin-3 and 10 nM MK-0457, either as individual agents or in combination. Nutlin-3 did not influence the degree of endoreduplication after MK-0457 treatment. Results are representative of 3 independent experiments.
Nutlin-induced p53 blocks endoreduplication and enhances apoptosis in wild-type p53 AML cells treated with MK-0457. (A,B) OCI-AML-3 cells (A) or MOLM-13 cells (B) were treated for 48 hours with 2.5 μM Nutlin-3 and 10 nM of MK-0457, either as individual agents or in combination. Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets. DMSO-treated cells served as control. Combination treatment results in reduced endoreduplication with increased percentage of annexin V–positive, apoptotic cell fraction. (C) HL-60 cells were treated for 48 hours with 2.5 μM Nutlin-3 and 10 nM MK-0457, either as individual agents or in combination. Nutlin-3 did not influence the degree of endoreduplication after MK-0457 treatment. Results are representative of 3 independent experiments.
Combination treatment with MK-0457 and Nutlin-3 induces aberrant expression of p21 in cells with more than or equal to 4N DNA content
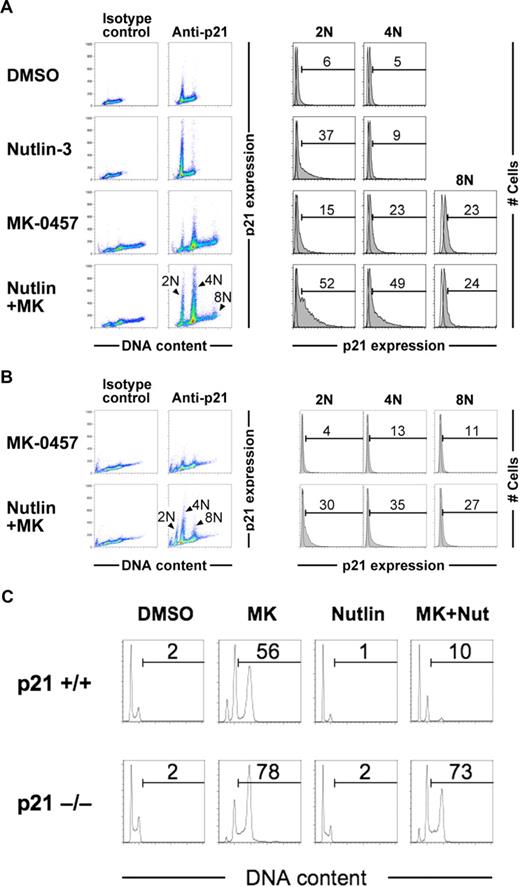
p53-dependent induction of p21 generally occurs in a cell cycle–dependent manner, with high expression levels in G1 phase.18,19 On the other hand, the p53-dependent postmitotic checkpoint has been reported to induce the G1 arrest of tetraploid cells after cytokinetic failure, involving p53-dependent induction of p21.11,13,23 To elucidate whether Nutlin-3 activates the postmitotic checkpoint in MK-0457–treated cells, we investigated p21 levels in association with DNA content after 24 hours of exposure in OCI-AML-3 cells. Nutlin-3 induced p21 preferentially in G1-phase cells, as reported previously (Figure 6A).18,19 Interestingly, Nutlin-3 induced p21 in cells with 4N DNA content as well as G1-phase cells when combined with MK-0457 (Figure 6A), suggesting an activation of the tetraploid checkpoint. Such an aberrant p21 expression was observed in cells with 4N and 8N DNA content after 48-hour concomitant exposure to Nutlin-3 and MK-0457 (Figure 6B). To test the hypothesis that p21 expression constrains MK-0457–induced endoreduplication, a well-characterized p21 knockout cell line (HCT116) was used. The p21-deleted cells (p21−/−) underwent endoreduplication more readily than their wild-type counterparts in response to MK-0457 (Figure 6C). Importantly, Nutlin-3 cotreatment efficiently inhibited MK-0457–induced endoreduplication in p21+/+ but not in p21−/− cells, suggesting a critical role of p21 in endoreduplication blockade. Taken together, Nutlin-3 would activate the p53-dependent postmitotic checkpoints, resulting in the blockade of endoreduplication after Aurora inhibition.
Nutlin-3 induces p21 in pseudo-G1 cells when combined with MK-0457. (A) OCI-AML-3 cells were treated for 24 hours with 2.5 μM Nutlin-3 and 10 nM MK-0457, either as individual agents or in combination. p21 expression levels and DNA content were measured by flow cytometry. Open histograms show staining with isotype controls. Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets. Results are representative of 3 independent experiments. (B) After 48 hours of treatment, aberrant p21 expression was observed in cells with 8N as well as 4N DNA content. (C) Parental HCT116 cells (p21+/+) or p21-deficient HCT116 cells (p21−/−) were treated for 48 hours with 10 nM MK-0457 and 10 μM Nutlin-3, either as individual agents or in combination. Nutlin-3 caused G1-phase cell- cycle arrest and inhibited MK-0457-induced endoreduplication more prominently in p21+/+ cells than p21−/− cells. Results are representative of 3 independent experiments.
Nutlin-3 induces p21 in pseudo-G1 cells when combined with MK-0457. (A) OCI-AML-3 cells were treated for 24 hours with 2.5 μM Nutlin-3 and 10 nM MK-0457, either as individual agents or in combination. p21 expression levels and DNA content were measured by flow cytometry. Open histograms show staining with isotype controls. Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets. Results are representative of 3 independent experiments. (B) After 48 hours of treatment, aberrant p21 expression was observed in cells with 8N as well as 4N DNA content. (C) Parental HCT116 cells (p21+/+) or p21-deficient HCT116 cells (p21−/−) were treated for 48 hours with 10 nM MK-0457 and 10 μM Nutlin-3, either as individual agents or in combination. Nutlin-3 caused G1-phase cell- cycle arrest and inhibited MK-0457-induced endoreduplication more prominently in p21+/+ cells than p21−/− cells. Results are representative of 3 independent experiments.
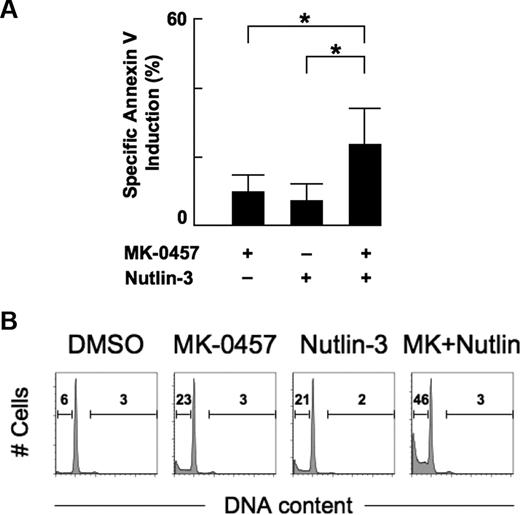
Aurora kinase inhibition by MK-0457 enhances p53-mediated apoptosis in primary AML cells
We examined the combined apoptotic effect of MK-0457 and Nutlin-3 on primary cells from 8 patients with AML. Cells were treated for 48 hours with 10 nM MK-0457 and 1 μM Nutlin-3 either as individual agents or in combination. The percentages of spontaneous apoptosis as determined by annexin V positivity in the series was 16.1% plus or minus 10.3% (range, 6.7%-32.2%). Treatment of cells with MK-0457 alone showed modest apoptotic effect (10.1% ± 4.8% specific apoptosis). The percentages of specific apoptosis at 48 hours after exposure to Nutlin-3 in the absence or presence of MK-0457 were 7.6% plus or minus 4.9% and 23.8% plus or minus 10.3%, respectively, showing a significant difference (P < .01, Figure 7A). Cell cycle was also analyzed in 3 samples. The cell-cycle distribution profiles of cells treated with MK-0457 were similar to those of DMSO-treated cells (Figure 7B). MK-0457 did not induce endoreduplication in these samples. Our findings suggest that MK-0457 may enhance Nutlin-induced apoptosis in primary AML cells.
Nutlin-induced apoptosis is enhanced by combination with MK-0457 in primary AML cells. (A) Primary AML cells from 8 patients were incubated for 48 hours with 10 nM MK-0457 and 1 μM Nutlin-3, either as individual agents or in combination, and the annexin V–positive fractions were measured by flow cytometry. Data are mean plus or minus SD. *P ≤ .01. (B) Representative cell-cycle distribution patterns in primary AML cells after treatment. Cells were treated for 48 hours with 10 nM MK-0457 and 1 μM Nutlin-3, either as individual agents or in combination. Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets. DMSO-treated cells served as control. MK-0457 did not induce significant increase in percentages of cells with more than or equal to 4N DNA content, whereas the MK-0457/Nutlin-3 combination treatment results in increased percentage of sub-G1 cells.
Nutlin-induced apoptosis is enhanced by combination with MK-0457 in primary AML cells. (A) Primary AML cells from 8 patients were incubated for 48 hours with 10 nM MK-0457 and 1 μM Nutlin-3, either as individual agents or in combination, and the annexin V–positive fractions were measured by flow cytometry. Data are mean plus or minus SD. *P ≤ .01. (B) Representative cell-cycle distribution patterns in primary AML cells after treatment. Cells were treated for 48 hours with 10 nM MK-0457 and 1 μM Nutlin-3, either as individual agents or in combination. Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets. DMSO-treated cells served as control. MK-0457 did not induce significant increase in percentages of cells with more than or equal to 4N DNA content, whereas the MK-0457/Nutlin-3 combination treatment results in increased percentage of sub-G1 cells.
Discussion
It has been reported that treatment with MK-0457 results in polyploidy and inhibits the growth of several tumor types in cell culture, with the induction of apoptosis most prominent in leukemia, lymphoma, and colorectal cell lines.9 Treated cells either undergo eventual apoptosis after additional cell cycles, where the inherited polyploid genome and multiple centrosomes result in mitotic catastrophe, or a pseudo-G1 arrest, primarily induced by a p53-dependent postmitotic checkpoint.11 Although little is known about the ultimate fate of the arrested cells, our data suggest that p53 activation by Mdm2 inhibition efficiently induces apoptosis in cells with 4N and 8N DNA content after MK-0457 treatment. Because the enhancement of apoptosis was associated with aberrant p21 induction and limited endoreduplication, Nutlin-3 would activate the p53-dependent postmitotic checkpoints and cause apoptosis via potent induction of p53. We think that our findings have important clinical implications because most chemotherapeutic agents mediate cell death through DNA damage and p53 activation. Because p53 mutations are rare in AML, combined targeting of Mdm2-p53 interaction and Aurora kinases could offer considerable therapeutic promise in AML. In support of this idea, it has been described that wild-type p53 AML cells are prone to apoptosis after Aurora inhibition and that combinations of Aurora inhibitors with DNA damaging agents show enhanced antiproliferative effects.8,14,24 On the contrary, in some conditions, a compromised p53 signaling has been associated with increased sensitivity to Aurora inhibition.13,24 Perhaps a threshold exists for p53 above which the p53-dependent postmitotic checkpoint actively mediates apoptosis.
MK-0457 additionally inhibits the activity of Fms-related tyrosine kinase-3 (FLT3), which is frequently increased in patients with AML, as well as Bcr/AblT351I kinase.9,25 The FLT3-activating mutations are associated with poor prognosis in AML,26 and the T315I BCR-ABL mutation mediates clinical resistance to imatinib, nilotinib, and dasatinib.27 The inhibition of FLT3 might contribute to the dose-dependent apoptotic effect exclusively seen in MOLM-13, which carries internal tandem duplication of FLT3.28 It is also possible that the off-target inhibition might partially constrain endoreduplication in MOLM-13 cells treated with MK-0457. It has been shown that the pharmacologic inhibition of FLT3 can induce G1-phase cell-cycle arrest and apoptosis in AML cell lines expressing a constitutively activated FLT3.29,30 MK-0457 has been shown to strongly inhibit Flt3 with a inhibition constant (Ki) of 30 nM and to efficiently ablate colony formation of primary AML cells with FLT3/internal tandem duplication mutation.9 Encouragingly, we found a highly synergistic apoptotic effect between MK-0457 and Nutlin-3 in MOLM-13 cells (CI values < 0.3). Our findings support the rationale of targeting the Mdm2-p53 interaction and Aurora kinases as a therapeutic strategy for AML.
In cells treated for 24 hours with MK-0457 alone, p53 accumulated enough, but induction of p53-related proteins was impaired compared with Nutlin-treated cells. It is unlikely that the impaired induction of p53-related proteins merely reflects slow accumulation of p53 in MK-0457–treated cells because increased p21 and Mdm2 levels occur immediately (∼ 2 hours) after p53 accumulation.17 p53 induction by MK-0457 was detectable at 6 hours of exposure. Intriguingly, the transcriptionally compromised p53 was reactivated by Nutlin-3. Nutlins are nongenotoxic and activate p53 by preventing it from binding to Mdm2.16 They do not bind to p53 or interfere with its phosphorylation status.16,31 Although the mechanisms remain to be elucidated, our data suggest that the molecular mechanisms underlying p53 accumulation after MK-0457 treatment overlap only partially with those controlling its activation as a transcriptional activator. Several conditions where accumulation of p53 protein occurs without induction of its transcriptional activity have been reported, such as hypoxia and inhibition of DNA synthesis.32-34 Because the transcriptionally compromised p53 in hypoxic condition was capable of being partly reactivated by Nutlin-3 treatment (K.K., unpublished data, 2007), p53 activation by inhibition of Mdm2-p53 interaction may restore a dissociation between p53 accumulation and effector in some circumstances.
In conclusion, concomitant inhibition of Mdm2-p53 interaction and Aurora kinases activates p53-dependent postmitotic checkpoints at pseudo-G1 and induces proapoptotic p53 signaling and mitochondrial apoptosis. Our data suggest that combined targeting of Mdm2-p53 interaction and Aurora kinases could offer therapeutic promise in AML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Donald Bergstrom of Merck & Co (Blue Bell, PA) for kindly providing MK-0457, and Ms Teresa McQueen (Section of Molecular Hematology and Therapy, Department of Stem Cell Transplantation and Cellular Therapy, University of Texas M.D. Anderson Cancer Center, Houston, TX) for her technical assistance.
This work was supported in part by grants from the National Institutes of Health (AML-PO1; CA55164, CA49639, CA89346, and CA16672), the Paul and Mary Haas Chair in Genetics at M.D. Anderson Cancer Center (M.A.), and the Kanae Foundation for Life & Socio-Medical Science (Tokyo, Japan) and Novartis Foundation (Tokyo, Japan) for the Promotion of Science (K.K.).
National Institutes of Health
Authorship
Contribution: K.K. designed and performed research, analyzed data, and wrote the paper; M.K. designed the research and analyzed data; T.T. performed research; H.N. contributed to discussion; and M.A. designed research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael Andreeff, Section of Molecular Hematology and Therapy, M D Anderson Cancer Center, University of Texas, 1515 Holcombe Boulevard, Unit 448, Houston, TX 77030; e-mail: mandreef@mdanderson.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal