Abstract
Hydroxamic acid analog pan-histone deacetylase (HDAC) inhibitors (HA-HDIs) have shown preclinical and clinical activity against human acute leukemia. Here we describe HA-HDI–resistant human acute myeloid leukemia (AML) HL-60 (HL-60/LR) cells that are resistant to LAQ824, vorinostat, LBH589, and sodium butyrate. HL-60/LR cells show increased expression of HDACs 1, 2, and 4 but lack HDAC6 expression, with concomitant hyperacetylation of heat shock protein 90 (hsp90). Treatment with HA-HDI failed to further augment hsp90 acetylation, or increase the levels of p21 or reactive oxygen species (ROSs), in HL-60/LR versus HL-60 cells. Although cross-resistant to antileukemia agents (eg, cytarabine, etoposide, and TRAIL), HL-60/LR cells are collaterally sensitive to the hsp90 inhibitor 17-AAG. Treatment with 17-AAG did not induce hsp70 or deplete the hsp90 client proteins AKT and c-Raf. HL-60/LR versus HL-60 cells display a higher growth fraction and shorter doubling time, along with a shorter interval to generation of leukemia and survival in nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice. Thus, resistance of AML cells to HA-HDIs is associated with loss of HDAC6, hyperacetylation of hsp90, aggressive leukemia phenotype, and collateral sensitivity to 17-AAG. These findings suggest that an hsp90 inhibitor-based antileukemia therapy may override de novo or acquired resistance of AML cells to HA-HDIs.
Introduction
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) are enzymes that are recruited by multiprotein transcriptional complexes to the promoters of genes, where they regulate transcription by mediating the acetylation and deacetylation, respectively, of specific lysine residues in the N-termini of histone tails and/or in transcription factors (eg, p53, E2F1, GATA1, RelA, YY1, and Mad/Max) without directly binding to the DNA.1,2 Three classes of HDACs have been identified. They include classes I, II (A and B), and III (SIR2, sirtuins).1-3 Several chromosomal translocations in acute myeloid leukemia (AML) that produce chimeric fusion oncoproteins have been shown to recruit HDACs to the promoters to repress genes involved in cell-cycle growth inhibition, differentiation, and apoptosis.4,5 Consistent with this, inhibition of HDAC activity has been demonstrated to modify deregulated gene transcription, and thereby induce growth arrest, differentiation, and apoptosis in a relatively selective manner in cancer versus normal host cells.1,2,4 Several structurally diverse classes of naturally occurring and synthetic compounds have been identified as HDAC inhibitors (HDIs).1,2 Among these, the hydroxamic acid analog pan-histone deacetylase inhibitors (HA-HDIs; eg, vorinostat [SAHA]) and the cinnamic acid hydroxamates LAQ824 and LBH589 have been shown to inhibit both classes I and II HDACs, including HDAC6—a predominantly cytosolic HDAC that acts as a deacetylase for hsp90, cortactin, and α-tubulin.1-3,6,7 Consequently, treatment with HA-HDIs induces lysine acetylation of hsp90, cortactin, and α-tubulin.7-11 Acetylation of hsp90 reduces its binding to ATP and cochaperones, as well as inhibits the chaperone function of hsp90.9,10 This directs hsp90 client proteins (eg, Bcr-Abl, mutant FLT-3, AKT, and c-Raf) to polyubiquitylation and degradation by the 20S proteasome.9,12 These findings indicated that inhibition of HDAC activity may not only affect gene transcription but also deplete the levels of progrowth and prosurvival proteins in human leukemia cells.
It is now well established that treatment with HA-HDIs induces the CDK inhibitor p21WAF1 in a p53-independent manner, causing G1 arrest and differentiation of leukemia cells.4,13 HA-HDIs have also been shown to induce p16 (CDKN2 or INK4) and p27, but attenuate cyclin A and D levels leading to decreased activity of CDK4 and CDK2.14 Treatment with HA-HDI also triggers both the intrinsic pathway and sensitizes tumor cells to the death ligand–induced extrinsic pathway of apoptosis.1,14,15 For example, treatment with HA-HDIs induces the death receptors DR4 and DR5 for Apo-2L/TRAIL, down-regulates c-FLIP, and enhances Apo-2L/TRAIL-induced death signaling complex and apoptosis.15 HA-HDI treatment has also been shown to deplete the levels of several antiapoptotic proteins, including Bcl-xL, Bcl-2, XIAP, and survivin, but induce levels of prodeath proteins (eg, Bax, Bak, and Bim).15,16 In addition, HA-HDIs increase intracellular levels of ROSs, as well as up-regulate thioredoxin-binding protein-2 associated with a decrease in thioredoxin levels, which may also be responsible for the relative antitumor selectivity of HA-HDIs.17 In animal studies, both vorinostat and LAQ824 were shown to exert potent antitumor and antileukemia effects at relatively nontoxic concentrations.18,19 Recently, early trials have shown that as a single agent, HA-HDIs exhibit modest clinical activity against human leukemia, although significant activity of HA-HDIs was observed against cutaneous or peripheral T-cell lymphoma (cutaneous T-cell lymphoma [CTCL] or peripheral T-cell lymphoma [PTCL]).18,20,21 Notwithstanding this, de novo and acquired resistance to HA-HDIs is the uniform clinical outcome, and the potential intracellular mechanisms underlying HA-HDI resistance remain to be determined, partly due to the unavailability of a cellular model of HA-HDI resistance.
In the present studies, we have generated and characterized a stable, HA-HDI–resistant HL-60/LR cell line, by culturing the AML HL-60 cells under the continuous selection pressure of increasing concentrations of LAQ824. Compared with the parental HL-60, HL-60/LR cells are capable of growth in the continuous presence of 200 nM LAQ824, show significantly increased in vitro and in vivo growth potential, as well as exhibit various degrees of cross-resistance to other HDIs and antileukemia agents. However, notably, HL-60/LR cells demonstrate loss of HDAC6 expression with hyperacetylation of hsp90, and exhibit cross-sensitivity to hsp90 inhibitor 17-AAG. These findings provide the rationale to determine the activity of hsp90 inhibitors in overcoming in vivo resistance to HA-HDIs.
Methods
Cell culture
HL-60 cells were passaged twice a week as described previously.22 Logarithmically growing cells were used for all experiments.
Reagents and antibodies
17-AAG was obtained from Developmental Therapeutics Branch of Cancer Therapy Evaluation Program (CTEP)/National Cancer Institute (NCI)/National Institutes of Health (NIH, Bethesda, MD). LAQ824 and LBH589 were obtained from Novartis (Cambridge, MA), and vorinostat was provided by Merck (Boston, MA). Trichostatin A, Ara-C, and etoposide were purchased from Sigma-Aldrich (St Louis, MO). Antibodies for the immunoprecipitation and/or immunoblot analyses of hsp90 and hsp70 were obtained as previously described.9,22 Apo-2L/TRAIL was produced in Escherichia coli and was a gift from Genentech (San Francisco, CA).15 Affinity-purified polyclonal antibody against Ac-K69-hsp90 was generated by Alpha Diagnostic (San Antonio, TX) based on the synthetic 12–amino acid peptide flanking K69 (acetylated and unacetylated) ETLTDPSKLDSGK. Monoclonal anti–acetyl lysine, anti-HDAC3, and anti-HDAC4 were purchased from Cell Signaling Technology (Beverly, MA). Monoclonal anti–acetyl α-tubulin and monoclonal β-actin were purchased from Sigma-Aldrich, and the anti-HDAC1, polyclonal anti-HDAC2, pan-STAT5, anti–Hsf-1, p15, CEBPα, anti-HDAC6, and anti-TRX (3A1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal HDAC10 antibody was purchased from Biovision (Mountain View, CA), and other antibodies against p21, p27, c-Raf, pSTAT5, Bcl-xL, Bax, Bak, Bim, Mcl-1, XIAP, DR4, DR5, caspase-8, C-Flip, and FADD were procured as previously described.14,23-25 Monoclonal anti-MDR1 (4E3) was obtained from Abcam (Cambridge, MA). Anti-MRP1 was obtained from R&D systems (Minneapolis, MN). Anti-BCRP was obtained from Millipore (Billerica, MA). Anti-LRP (clone LRP-56) was obtained from Kamiya Biomedical (Seattle, WA). Polyclonal anti–thioredoxin-binding protein 2 (TBP2) was obtained from Zymed Laboratories (San Francisco, CA). Monoclonal anti-p53 (Ab-6) was purchased from EMD Biosciences (San Diego, CA).
Establishment of LAQ824-resistant cell line, HL-60/LR
HL-60/LR cells were obtained using a modification of a method for obtaining anthracycline-resistant cells.26 HL-60 parental cells were cultured in increasing concentrations of LAQ824 starting at 10 nM LAQ824. Viable cells were then passaged into a higher concentration of LAQ824 in 10-nM increments until a concentration of 200 nM LAQ824 was reached. HL-60/LR cells were then maintained in complete RPMI 1640 medium containing 200 nM LAQ824.
Immunoprecipitation of hsp90 and immunoblot analyses
Western analyses of proteins
Western analyses were performed using specific antisera or monoclonal antibodies according to previously reported protocols, and the horizontal scanning densitometry was performed on Western blots, as previously described.22-25
Flow cytometric analysis of cell-cycle status and membrane transporters
The flow cytometric evaluation of the cell-cycle status and the percentage of cells in the G1, S, and G2-M phases was calculated according to a previously described method.22-25 For the evaluation of membrane transporters MDR-1, BCRP1, LRP, and MRP1 cells were blocked with 3% BSA/PBS, then stained with specific antibodies for 30 minutes at room temperature according to the manufacturer's protocol, and then analyzed by flow cytometry. To assess CD11b expression on the surface of cells after LAQ treatment, cells were washed with PBS and then resuspended in 3% BSA containing PBS for 10 minutes. FITC-conjugated CD11b antibody was added, and the cells were incubated on ice in the dark for 20 minutes. Cells were washed and resuspended in 1× PBS and then analyzed by flow cytometry.
Apoptosis assessment by annexin V staining
After drug treatment, cells were washed once with 1× PBS and resuspended in 100 μL staining solution (containing annexin V fluorescein and propidium iodide in a HEPES buffer, annexin V–FITC; BD Pharmingen, San Diego, CA). After incubation at room temperature for 15 minutes, cells were diluted in 1× annexin V–binding buffer and the percentages of apoptotic cells were analyzed by flow cytometry.22-25
Measurement of ROSs by flow cytometry
Cells were incubated with 10 μM 3′-(p-hydroxyphenyl) fluorescein (HPF) (Invitrogen, Carlsbad, CA) for 30 minutes at 37°C. After this, cells were washed to remove excess probe and fluorescence intensity was determined by flow cytometry on the FL-1 channel.27
Calculation of doubling time for HL-60/LR cells
To determine the doubling time for HL-60 and HL-60/LR cells, 250 000 cells were plated in complete RPMI 1640 medium. Cells were removed every 24 hours for 72 hours and counted with a Z-series Coulter counter (Beckman Coulter, Fullerton, CA).
Reverse-transcription–PCR analysis of HDAC6
HL-60 and HL-60/LR cells were harvested and total RNA was isolated using Trizol reagent (Invitrogen). Total RNA (5 μg) was reverse transcribed using a Superscript First-strand synthesis kit (Invitrogen). HL-60 and HL-60/LR cDNA was used for subsequent polymerase chain reaction (PCR) analysis of HDAC6 and β-actin. Oligonucleotide primers were designed to amplify a fragment of HDAC6 to establish its mRNA expression levels in HL-60/LR cells. Primers were also designed against β-actin to serve as an internal loading control for mRNA expression. PCR reactions were performed using the following parameters: 94°C for 5 minutes followed by 30 cycles of 94°C (1 minute), 55°C (1 minute), and 72°C (1 minute). Amplified products were separated on a 2% agarose gel.24
Confocal immunofluorescence microscopy to evaluate acetylated hsp90 expression
HL-60 and HL-60/LR cells were cultured in a chamber slide in RPMI medium with 10% FBS with or without LBH589 for 8 hours and stained with anti–acetyl-K69 (Ac-K69) antibody. Next, cells were washed with PBS and fixed with 4% paraformaldehyde for 10 minutes. After this, the slides were blocked with 3% BSA for 30 minutes and incubated with primary antibody at a dilution of 1:100 in blocking buffer for 2 hours. After 3 washes with PBS, the slides were incubated in Alexa Fluor 488 antirabbit secondary antibody (Invitrogen) for one hour at 1:3000 dilution. After 3 washes with PBS, the cells were counterstained with DAPI using Vectashield (Vector Labs, Burlingame, CA) mountant with DAPI and imaged using a Zeiss LSM510 confocal microscope (Carl Zeiss, Heidelberg, Germany).
In vivo leukemogenesis model
Nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (The Jackson Laboratory, Bar Harbor, ME) were used for leukemogenesis experiments pursuant to a protocol approved by Animal Use and Care Committee of Medical College of Georgia. Mice were irradiated with 250 Gy gamma radiation. The following day, mice were injected with 10 million viable HL-60 or HL-60/LR cells. Mice were monitored until they died or were humanely killed due to disease progression, according to the study protocol. The day of death was noted and plotted on a Kaplan-Meier plot.
Statistical analysis
Significant differences between values obtained in a population of leukemia cells treated with different experimental conditions were determined using the Student t test.
Results
HL-60/LR cells are highly resistant to LAQ824-, vorinostat-, and LBH589-mediated growth inhibition and apoptosis
First, we compared the apoptotic effects of various concentrations of LAQ824, vorinostat, and LBH589 in HL-60 (Figure 1A) versus HL-60/LR (Figure 1B) cells to determine the concentration of each agent that induced apoptosis in 50% of the treated cell population (IC50 values). The IC50 values are shown in the top left corner of each graph in Figure 1A,B). Compared with HL-60, HL-60/LR cells showed markedly higher (30- to 100-fold) IC50 values for apoptosis induced by each of the HA-HDIs tested. We also compared the morphologic effects of LAQ824 on HL-60 versus HL-60/LR cells by Wright-Giemsa staining followed by light microscopy. As shown in Figure 1C, treatment with 500 nM LAQ824 induced morphologic evidence of apoptosis in 90% of HL-60 but in less than 10% of HL-60/LR cells, without morphologic evidence for differentiation in the latter cells. We also determined differentiation effects of LAQ824 by assessing the induction of CD11b expression in HL-60 versus HL-60/LR cells. After a 24-hour exposure to 500 nM LAQ824, HL-60 but not HL-60/LR cells showed marked induction of CD11b (Figure 1D).
Compared with HL-60, HL-60/LR cells are highly resistant to apoptosis induced by LAQ824, SAHA, and LBH589. HL-60 (A) or HL-60/LR cells (B) were treated with the indicated concentrations of LAQ824 (top panel), SAHA (middle panel), and LBH589 (bottom panel) for 48 hours. After this, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. Values on the curves represent the mean of 2 experiments performed in duplicate. IC50 values were determined and indicated in the top left of each graph. Error bars indicate SE. (C) After treatment of cells with 500 nM LAQ824 for 24 hours, cells were Wright stained and the morphology of the untreated and LAQ824-treated cells was determined by light microscopy with a Nikon Eclipse 50i microscope (Nikon, Melville, NY) using a PLAN 40×/0.65 NA objective and a Nikon CCD camera. (D) HL-60 and HL-60/LR cells were treated with 500 nM LAQ824 for 24 hours. After treatment, CD11b expression on the surface of the cells was measured by flow cytometry.
Compared with HL-60, HL-60/LR cells are highly resistant to apoptosis induced by LAQ824, SAHA, and LBH589. HL-60 (A) or HL-60/LR cells (B) were treated with the indicated concentrations of LAQ824 (top panel), SAHA (middle panel), and LBH589 (bottom panel) for 48 hours. After this, the percentage of annexin V–stained apoptotic cells was determined by flow cytometry. Values on the curves represent the mean of 2 experiments performed in duplicate. IC50 values were determined and indicated in the top left of each graph. Error bars indicate SE. (C) After treatment of cells with 500 nM LAQ824 for 24 hours, cells were Wright stained and the morphology of the untreated and LAQ824-treated cells was determined by light microscopy with a Nikon Eclipse 50i microscope (Nikon, Melville, NY) using a PLAN 40×/0.65 NA objective and a Nikon CCD camera. (D) HL-60 and HL-60/LR cells were treated with 500 nM LAQ824 for 24 hours. After treatment, CD11b expression on the surface of the cells was measured by flow cytometry.
Resistance of HL-60/LR cells to LAQ824-mediated growth inhibition, with higher growth rates and percentage of cells in S-phase of the cell cycle
The effect of exposure to LAQ824 for 24 hours followed by suspension of culture growth of HL-60 versus HL-60/LR cells in drug-free medium was determined. Figure 2A demonstrates that exposure to 250 or 500 nM LAQ824 caused more growth inhibition of HL-60/LR cells. We also assessed the cell-cycle phase–specific status and the doubling time of HL-60 and HL-60/LR cells in suspension culture. A significantly higher percentage of HL-60/LR cells were in S phase (62.4% ± 3.1%), compared with HL-60 cells (40.0% ± 1.5%; P < .01). To assess the consequences of higher growth fraction on the population doubling time of HL-60/LR cells in suspension culture, we observed the growth rate of HL-60 and HL-60/LR cells over 72 hours (Figure 2C). Notably, HL-60 cells exhibited a doubling time of approximately 24 hours, whereas HL-60/LR cells exhibited a markedly shorter doubling time of 12 hours. These findings appear consistent with the increase in S phase population between HL-60 and HL-60/LR cells. Notably, in vivo studies confirmed the higher growth fraction and aggressive growth of the HL-60/LR versus HL-60 cells. Intravenous administration led to a more rapid rate of engraftment of HL-60/LR than of parental HL-60 cells in the bone marrow of irradiated NOD/SCID mice. Survival of mice injected with HL-60/LR cells was also significantly shorter than of mice injected with HL-60 cells (P < .001; Figure 2C).
Compared with HL-60, a higher percentage of HL-60/LR cells are in S phase of the cell cycle, have shorter doubling time, and are resistant to LAQ824-mediated growth inhibition. (A) Untreated and LAQ824-treated HL-60 versus HL-60/LR cells (at the indicated concentrations of LAQ824 for 24 hours) were counted, and the percentage of growth inhibition of the treated cells was calculated and depicted as the curves in the panel. Values on the curves represent the mean of 2 experiments performed in duplicate. (B) Equal numbers of the log-phase HL-60 versus HL-60/LR cells were started in culture, and aliquots of cells were withdrawn at the indicated intervals and counted. The values on the curves represent the mean numbers (of 2 experiments performed in duplicate) of cells at the indicated time interval. Error bars indicate SD. (Inset) The table in the panel shows the percentage of cells in the various phases of cell cycle, as determined by PI staining and DNA content analysis by flow cytometry. The values represent the means plus or minus SE of 3 experiments. (C) Groups of 6 NOD/SCID mice were injected with HL-60 or HL-60/LR cells and monitored. Survival of the mice was plotted, and median survival time was calculated. (D) HL-60 and HL-60/LR cells were stained with isotype controls or specific antibodies for MDR1, MRP1, LRP/MVP, and BCRP1 and then analyzed by flow cytometry. HL-60/VCR cells that express MDR1, LRP/MVP, and BCRP1 were used as a positive control. Open black-lined histograms represent the isotype control; filled gray histograms, specific antibody staining.
Compared with HL-60, a higher percentage of HL-60/LR cells are in S phase of the cell cycle, have shorter doubling time, and are resistant to LAQ824-mediated growth inhibition. (A) Untreated and LAQ824-treated HL-60 versus HL-60/LR cells (at the indicated concentrations of LAQ824 for 24 hours) were counted, and the percentage of growth inhibition of the treated cells was calculated and depicted as the curves in the panel. Values on the curves represent the mean of 2 experiments performed in duplicate. (B) Equal numbers of the log-phase HL-60 versus HL-60/LR cells were started in culture, and aliquots of cells were withdrawn at the indicated intervals and counted. The values on the curves represent the mean numbers (of 2 experiments performed in duplicate) of cells at the indicated time interval. Error bars indicate SD. (Inset) The table in the panel shows the percentage of cells in the various phases of cell cycle, as determined by PI staining and DNA content analysis by flow cytometry. The values represent the means plus or minus SE of 3 experiments. (C) Groups of 6 NOD/SCID mice were injected with HL-60 or HL-60/LR cells and monitored. Survival of the mice was plotted, and median survival time was calculated. (D) HL-60 and HL-60/LR cells were stained with isotype controls or specific antibodies for MDR1, MRP1, LRP/MVP, and BCRP1 and then analyzed by flow cytometry. HL-60/VCR cells that express MDR1, LRP/MVP, and BCRP1 were used as a positive control. Open black-lined histograms represent the isotype control; filled gray histograms, specific antibody staining.
HL-60/LR cells exhibit greater resistance to chemotherapeutic agents, but are collaterally sensitive to the hsp90 inhibitor, 17-AAG
We next compared the sensitivity to apoptosis due to a variety of conventional and novel antileukemia drugs in HL-60 versus HL-60/LR cells. Table 1 shows the IC50 values for apoptosis of various drugs in HL-60 and HL-60/LR cells. First, HL-60/LR cells were cross-resistant to other HDAC inhibitors (eg, TSA and sodium butyrate), showing at least 10-fold greater resistance to each drug. HL-60/LR cells were also more resistant to Ara-C and etoposide, although the resistance index was modest (ie, 3.3 and 5.0, respectively). Next, we determined whether high cell surface expression of the main membrane drug transporters (ie, PGP, MRP1, LRP, or BCRP1) could be responsible for reduced intracellular accumulation and resistance to HA-HDIs in HL-60/LR versus HL-60 cells.28-30 HL-60/VCR cells were used as a positive control for expression of the resistance proteins. Figure 2D demonstrates that HL-60 and HL-60/LR cells have similar intracellular expression of LRP. But unlike HL-60/VCR, HL-60/LR cells do not express MDR1 or MRP1. HL-60 cells express low levels of BCRP; however, this expression is not observed in HL-60/LR cells. Notably, compared with HL-60, HL-60/LR cells demonstrated collateral sensitivity to 17-AAG and 17-DMAG (3-fold and 5-fold greater sensitivity, respectively; Table 1).
IC50 values of HDIs and other agents in HL-60 versus HL-60/LR cells
| Agents . | HL-60, IC50 dose . | HL-60/LR, IC50 dose . | Resistance index . |
|---|---|---|---|
| Sodium butyrate, μM | 2 | 20 | 10.0 |
| TSA, nM | 200 | 3000 | 15.0 |
| Ara-C, μM | 1.5 | 5 | 3.3 |
| Etopside, μM | 1 | 5 | 5.0 |
| 17-AAG, μM | 10 | 2 | −5.0 |
| 17-DMAG, nM | 600 | 150 | −4.0 |
| Apo-2L/TRAIL, ng/mL | 150 | > 2000 | > 13.3 |
| Agents . | HL-60, IC50 dose . | HL-60/LR, IC50 dose . | Resistance index . |
|---|---|---|---|
| Sodium butyrate, μM | 2 | 20 | 10.0 |
| TSA, nM | 200 | 3000 | 15.0 |
| Ara-C, μM | 1.5 | 5 | 3.3 |
| Etopside, μM | 1 | 5 | 5.0 |
| 17-AAG, μM | 10 | 2 | −5.0 |
| 17-DMAG, nM | 600 | 150 | −4.0 |
| Apo-2L/TRAIL, ng/mL | 150 | > 2000 | > 13.3 |
Levels of prodeath and prosurvival proteins in HL-60/LR versus HL-60 cells
To determine the molecular mechanism(s) underlying resistance to multiple antileukemia agents with diverse mechanisms of action, we next compared the levels of the main members of the Bcl-2 family and other prodeath and prosurvival proteins in HL-60 and HL-60/LR cells. Figure 3A demonstrates that, compared with HL-60 cells, HL-60/LR cells expressed higher levels of the antiapoptotic Bcl-xL and XIAP, but lower levels of Mcl-1 protein. In addition, HL-60/LR versus HL-60 cells expressed lower levels of proapoptotic BimEL, BimL, and BimS, but higher levels of Bax protein (Figure 3A). The levels of Bcl-2 and Bak were not different in the 2 cell types. Treatment with LAQ824 for 8 or 24 hours did not much alter the levels of the various Bcl-2 family members examined. We also compared the levels of the various determinants of the TRAIL-induced extrinsic pathway of apoptosis in HL-60/LR versus HL-60 cells. Compared with HL-60, HL-60/LR cells expressed higher levels of DR4 and DR5, as well as showed lower levels of c-FLIP. But, HL-60/LR versus HL-60 cells lacked expression of caspase-8 and showed markedly lower levels of the adaptor FADD (Figure 3B), which together could explain resistance to TRAIL. However, it is difficult to assign relative importance to the difference in the levels of the perturbed Bcl-2 family of proteins in altering the apoptotic threshold of HL60/LR cells for the HA-HDIs and other antileukemia drugs. We also compared the levels of other prosurvival signaling proteins in HL-60 and HL-60/LR cells. Notably, the levels of AKT, c-RAF, STAT5, and p-STAT5 were markedly higher in HL-60/LR versus HL-60 cells (Figure 3C). In addition, although treatment with LAQ824 further lowered AKT and c-RAF levels in HL-60 cells, it had no effect in HL-60/LR cells. Taken together, the higher levels of the prosurvival signaling proteins correlated with the drug resistance of HL-60/LR cells. Finally, we compared LAQ824-mediated induction of ROSs in HL-60 versus HL60/LR cells. As shown in Figure 3D, whereas endogenous ROS generation was low and not significantly different in HL-60 versus HL60/LR cells, significantly higher levels of ROSs were induced by LAQ824 treatment in HL-60 versus HL60/LR cells (P < .001). In addition, compared with HL-60, HL-60/LR cells express considerably lower levels of thioredoxin (TRX), as well as fail to show LAQ824-mediated increase in the levels of TRX-binding protein 2 (TBP2).17
Treatment with LAQ824 treatment failed to induce Bak, Bim, and Bax, but attenuated Bcl-xL and XIAP levels in HL-60/LR versus HL-60 cells. (A) After treatment of HL-60 and HL-60/LR cells with the indicated concentrations of LAQ824 for 8 and 24 hours, total cell lysates were harvested and immunoblot analyses were done for Bcl-2, Bcl-xL, XIAP, Mcl-1, Bak, Bax, and Bim. β-actin expression served as the control for protein loading. (B) HL-60 and HL-60/LR cells were treated with the indicated concentrations of Apo-2L/TRAIL for 24 hours. After this, cell lysates were obtained and immunoblot analyses were done for DR4, DR5, caspase-8, c-FLIP, and FADD. β-actin expression in the lysates served as the loading control. (C) HL-60 and HL-60/LR cells were treated with the indicated concentrations of LAQ824 for 24 hours. Then, immunoblot analyses were done for p-AKT, AKT, p-GSK3β, p-ERK1/2, ERK1/2, c-Raf, cyclin D1, p-STAT5, STAT5, TBP-2, and TRX. The levels of β-actin expression in the lysates served as the loading control. (D) HL-60 and HL-60/LR cells were treated for 16 hours with 250 nM LAQ824. Then cells were stained with HPF and ROS levels were determined by flow cytometry. Values represent the means plus or minus SE of 3 experiments.
Treatment with LAQ824 treatment failed to induce Bak, Bim, and Bax, but attenuated Bcl-xL and XIAP levels in HL-60/LR versus HL-60 cells. (A) After treatment of HL-60 and HL-60/LR cells with the indicated concentrations of LAQ824 for 8 and 24 hours, total cell lysates were harvested and immunoblot analyses were done for Bcl-2, Bcl-xL, XIAP, Mcl-1, Bak, Bax, and Bim. β-actin expression served as the control for protein loading. (B) HL-60 and HL-60/LR cells were treated with the indicated concentrations of Apo-2L/TRAIL for 24 hours. After this, cell lysates were obtained and immunoblot analyses were done for DR4, DR5, caspase-8, c-FLIP, and FADD. β-actin expression in the lysates served as the loading control. (C) HL-60 and HL-60/LR cells were treated with the indicated concentrations of LAQ824 for 24 hours. Then, immunoblot analyses were done for p-AKT, AKT, p-GSK3β, p-ERK1/2, ERK1/2, c-Raf, cyclin D1, p-STAT5, STAT5, TBP-2, and TRX. The levels of β-actin expression in the lysates served as the loading control. (D) HL-60 and HL-60/LR cells were treated for 16 hours with 250 nM LAQ824. Then cells were stained with HPF and ROS levels were determined by flow cytometry. Values represent the means plus or minus SE of 3 experiments.
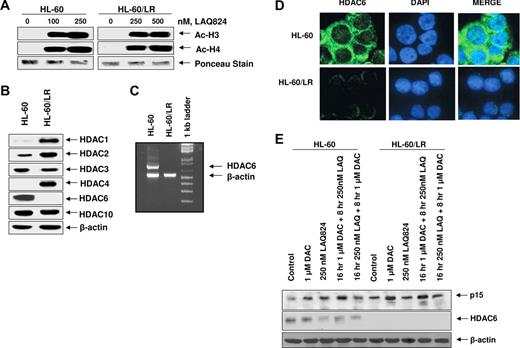
HL-60/LR cells lack HDAC6, but express higher levels of HDAC1, HDAC2, and HDAC4
To elucidate the mechanism underlying resistance to HDIs, we determined the ability of HA-HDIs to induce histone H3 and H4 acetylation, as well compared the levels of the main class I and II HDACs in the HL-60 versus HL-60/LR cells. Figure 4A,B demonstrate that HL-60/LR cells possess significantly higher levels of HDAC1, HDAC2, and HDAC4 than HL-60 cells. Treatment with LAQ824 induced similar levels of histone acetylation in HL-60 and HL-60/LR cells, especially at the 500-nM level of LAQ824 (Figure 4A and data not shown). This was also the case after treatment with 50 to 100 nM LBH589 (data not shown). Notably, HL-60/LR versus HL-60 cells showed lack of expression of HDAC6 mRNA by RT-PCR analysis (Figure 4C), and of HDAC6 protein by immunoblot analysis (Figure 4B), although a faint expression of HDAC6 protein could be detected in the cytoplasm by confocal immunofluorescence microscopy (Figure 4D). The expression level of HDAC10, another member of the class IIB family of HDACs, was similar in HL-60 and HL-60/LR cells (Figure 4B).
Compared with HL-60, HL-60/LR cells lack HDAC6 expression and LAQ824 induces acetylation of histones H3 and H4 in both HL-60 as well as HL-60/LR cells. (A) After treatment of HL-60 and HL-60/LR cells with the indicated concentrations of LAQ824 for 24 hours, histones were isolated and immunoblot analyses of acetylated histones H3 and H4 were performed. Ponceau staining was used to compare equal protein loading in each lane. (B) Total cell lysates from HL-60 and HL-60/LR cells were used to perform Western blot analyses with specific antibodies against HDAC1, HDAC2, HDAC3, HDAC4, HDAC6, HDAC10, and acetylated α-tubulin. The levels of β-actin served as the loading control. (C) Total RNA from HL-60 and HL-60/LR cells was analyzed by reverse-transcription (RT)–PCR using HDAC6 and β-actin primers. (D) HL-60 and HL-60/LR were fixed on slides, stained with FITC-conjugated anti-HDAC6 antibody (green) and/or DAPI (blue), and imaged with a Zeiss LSM510 confocal microscope with a 63×/1.2 NA water-immersion objective lens. (E) HL-60 and HL-60/LR cells were treated with decitabine and/or LAQ824 as indicated. Then, immunoblot analyses were done for p15 and HDAC6 on the total cell lysates. The levels of β-actin in the lysates served as the loading control.
Compared with HL-60, HL-60/LR cells lack HDAC6 expression and LAQ824 induces acetylation of histones H3 and H4 in both HL-60 as well as HL-60/LR cells. (A) After treatment of HL-60 and HL-60/LR cells with the indicated concentrations of LAQ824 for 24 hours, histones were isolated and immunoblot analyses of acetylated histones H3 and H4 were performed. Ponceau staining was used to compare equal protein loading in each lane. (B) Total cell lysates from HL-60 and HL-60/LR cells were used to perform Western blot analyses with specific antibodies against HDAC1, HDAC2, HDAC3, HDAC4, HDAC6, HDAC10, and acetylated α-tubulin. The levels of β-actin served as the loading control. (C) Total RNA from HL-60 and HL-60/LR cells was analyzed by reverse-transcription (RT)–PCR using HDAC6 and β-actin primers. (D) HL-60 and HL-60/LR were fixed on slides, stained with FITC-conjugated anti-HDAC6 antibody (green) and/or DAPI (blue), and imaged with a Zeiss LSM510 confocal microscope with a 63×/1.2 NA water-immersion objective lens. (E) HL-60 and HL-60/LR cells were treated with decitabine and/or LAQ824 as indicated. Then, immunoblot analyses were done for p15 and HDAC6 on the total cell lysates. The levels of β-actin in the lysates served as the loading control.
Treatment with decitabine and/or LAQ824 does not derepress HDAC6 in HL-60 or HL-60/LR cells
We next determined the effects of the DNA-demethylating agent decitabine (5-aza-2′-deoxycytidine [DAC]) and/or LAQ824 on HDAC6 and p15 in HL-60 and HL-60/LR cells. Compared with HL-60, HL-60/LR cells showed higher expression of p15 (Figure 4E). The biologic significance of this is unclear. Without affecting HDAC6 levels in HL-60, treatment with decitabine did not derepress HDAC6 in HL-60/LR cells. In HL-60 cells, treatment with either decitabine or LAQ824 induced p15 levels (Figure 4E). LAQ824 treatment partially depleted HDAC6 levels in HL-60 cells, without inducing HDAC6 levels in HL-60/LR cells. In contrast, decitabine, but not LAQ824, induced p15 expression in HL-60/LR cells (Figure 4E). Compared with either agent alone, sequential treatment with decitabine followed by LAQ824 (but not the reverse sequence of treatment) augmented p15 induction in HL-60 but not in HL-60/LR cells (Figure 4E).
HL-60/LR cells lack p-HSF1 and hsp70, and LAQ824 treatment does not induce differentiation-associated proteins p15, p21, or CEBPα nor acetylation of α-tubulin and hsp90 in HL-60/LR cells
We had previously shown that HDAC6 is the deacetylase for hsp90, and knockdown or inhibition of HDAC6 induces acetylation of α-tubulin and hsp90.9 This was shown to inhibit the chaperone function of hsp90, resulting in activation of HSF1 and up-regulation of hsp70.9,15,25 As shown in Figure 5A, HL-60/LR cells have a higher expression of hsp90, but markedly reduced expressions of p-HSF and hsp70. In addition, although treatment with LAQ824 increased p-HSF1 and hsp70 levels in HL-60 cells, this was not seen in HL-60/LR cells (Figure 5A). LAQ824 did not alter hsp90 levels in either cell types. Consistent with marked perturbations in class I and IIB HDAC levels noted (Figure 4B), treatment with LAQ824 induced p21 and p15 expression while depleting CEBPα levels,31,32 as well as increased α-tubulin acetylation in HL-60 but not in HL-60/LR cells (Figure 5B). LAQ824 did not induce p53 expression in either HL-60 or HL-60/LR cells (data not shown). This suggests that LAQ824 induces p21 and p15 in HL-60 cells in a non–p53-dependent manner. Consistent with lack of HDAC6 expression in HL-60/LR cells, immunoprecipitation of hsp90 followed by immunoblotting with anti–acetyl lysine antibody demonstrated hyperacetylation of the endogenous hsp90 in HL-60/LR but not HL-60 cells (Figure 5C). These findings were confirmed by immunoblot analysis using an anti–acetyl-K69 hsp90 antibody that detects lysine acetylation on the lysine 69 residue on hsp90 (Figure 5D). In addition, although treatment with LAQ824 induced hyperacetylation of hsp90 in HL-60 cells, it did not further augment hsp90 acetylation in HL-60/LR cells (Figure 5C,D). Confocal immunofluorescence microscopy performed after staining with anti–acetyl-K69 hsp90 antibody demonstrated that untreated HL-60/LR cells showed higher cell surface expression of hyperacetylated hsp90 than HL-60 cells. Notably, treatment with LAQ824 increased the cell-surface expression of hyperacetylated hsp90 in HL-60 cells (Figure 5E). These findings suggest that loss of HDAC6 induces hyperacetylation and localization of hyperacetylated hsp90 on the surface of cells.
Compared with HL-60, HL-60/LR cells lack p-HSF1 and hsp70, and LAQ824 treatment did not increase acetylation of hsp90 or induce p21 levels in HL-60/LR cells. (A) HL-60 and HL-60/LR cells were treated with the indicated concentrations of LAQ824 for 8 hours. After this, Western blot analyses were done for hsp70, hsp90, and HSF-1. (B) HL-60 and HL-60/LR cells were treated with the indicated concentrations of LAQ824 for 24 hours. Then total cell lysates were harvested and immunoblot analyses were done for p21, p15, CEBPα, and acetylated α-tubulin. The levels of β-actin expression in the lysates served as the loading control. (C) After treatment with the indicated concentrations of LAQ824, immunoprecipitates of hsp90 were immunoblotted with anti–acetyl lysine or anti-hsp90 antibody. (D) HL-60 and HL-60/LR cells were treated with 250 nM LAQ824 for 8 hours. Then, cell lysates were harvested and immunoblot analyses were done with anti–acetylated lysine 69 hsp90 or anti-hsp90 antibody. The levels of β-actin in the lysates served as the loading control. (E) HL-60 and HL-60/LR cells were treated with 250 nM LAQ824 for 8 hours. Then, cells were cytospun and fixed on slides, and stained with FITC-conjugated anti-acetylated lysine 69 hsp90 antibody (green) and/or DAPI (blue), and imaged with a Zeiss LSM510 confocal microscope with a 63×/1.2 NA water-immersion objective lens.
Compared with HL-60, HL-60/LR cells lack p-HSF1 and hsp70, and LAQ824 treatment did not increase acetylation of hsp90 or induce p21 levels in HL-60/LR cells. (A) HL-60 and HL-60/LR cells were treated with the indicated concentrations of LAQ824 for 8 hours. After this, Western blot analyses were done for hsp70, hsp90, and HSF-1. (B) HL-60 and HL-60/LR cells were treated with the indicated concentrations of LAQ824 for 24 hours. Then total cell lysates were harvested and immunoblot analyses were done for p21, p15, CEBPα, and acetylated α-tubulin. The levels of β-actin expression in the lysates served as the loading control. (C) After treatment with the indicated concentrations of LAQ824, immunoprecipitates of hsp90 were immunoblotted with anti–acetyl lysine or anti-hsp90 antibody. (D) HL-60 and HL-60/LR cells were treated with 250 nM LAQ824 for 8 hours. Then, cell lysates were harvested and immunoblot analyses were done with anti–acetylated lysine 69 hsp90 or anti-hsp90 antibody. The levels of β-actin in the lysates served as the loading control. (E) HL-60 and HL-60/LR cells were treated with 250 nM LAQ824 for 8 hours. Then, cells were cytospun and fixed on slides, and stained with FITC-conjugated anti-acetylated lysine 69 hsp90 antibody (green) and/or DAPI (blue), and imaged with a Zeiss LSM510 confocal microscope with a 63×/1.2 NA water-immersion objective lens.
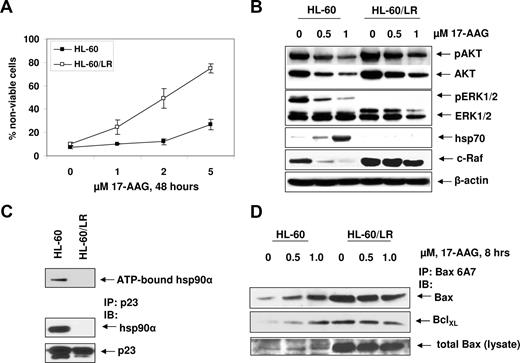
HL-60/LR cells are cross-sensitive to 17-AAG, and treatment with 17-AAG fails to induce hsp70 levels in HL-60/LR but not HL-60 cells
Although resistant to HA-HDIs, conventional antileukemia drugs, and TRAIL, HL-60/LR cells showed increased loss of cell viability compared with HL-60 cells, after exposure to 17-AAG (Figure 6A) and 17-DMAG (data not shown). A 4- and 5-fold increased sensitivity of HL-60/LR versus HL-60 cells to 17-AAG and 17-DMAG, respectively, was observed (Table 1). Compared with HL-60, HL-60/LR cells demonstrated higher expression of p-AKT, AKT, and c-Raf (Figure 6B), but barely detectable levels of p-ERK1/2 and hsp70. After treatment with 17-AAG, p-AKT, AKT, c-Raf, and p-ERK1/2, levels were markedly reduced in HL-60 cells (Figure 6B). In HL-60/LR cells, although treatment with 17-AAG reduced p-AKT, AKT, and c-Raf, levels of these proteins remained considerably higher in HL-60/LR versus HL-60 cells (Figure 6B). Notably, although treatment with 17-AAG caused a marked increase in hsp70 levels in HL-60 cells, there was no 17-AAG–mediated induction of hsp70 in HL-60/LR cells (Figure 6B). Consistent with this, hsp90 from HL-60/LR versus HL-60 cells demonstrated reduced binding to ATP and the cochaperone p23 (Figure 6C). Previously, we have reported that induction of hsp70 confers resistance to 17-AAG–mediated cell death by binding and inhibiting Bax conformation change and its localization to the mitochondria.25 Although untreated HL-60/LR cells showed higher Bax and conformationally changed Bax, it was bound to Bcl-xL, levels of which were higher in HL-60/LR cells (Figure 6D). After treatment with 17-AAG, levels of conformationally changed Bax, its ratio to Bcl-xL, and total Bax were higher in HL-60/LR versus HL-60 cells (Figure 6D). Taken together, these findings are consistent with collateral sensitivity of HL-60/LR cells to 17-AAG.
HL-60/LR cells are collaterally sensitive to 17-AAG. (A) HL-60 and HL-60/LR cells were treated with the indicated concentrations of 17-AAG for 48 hours. After this, the cells were stained with trypan blue and the percentage of positively stained nonviable cells was determined by light microscopy. Values on the curves represent the mean plus or minus SE of 2 experiments performed in duplicate. (B) HL-60 and HL-60/LR cells were treated with the indicated concentrations of 17-AAG for 24 hours. After this Western blot analyses were done for p-AKT, AKT, p-ERK1/2, ERK1/2, c-Raf, and hsp70 on the cell lysates. The levels of β-actin expression served as the loading control. (C) Reduced binding of ATP and p23 to hyperacetylated hsp90 in HL-60/LR versus HL-60 cells. After incubation of cell lysates containing hsp90 with ATP-sepharose, ATP-sepharose was pelleted and Western blot analysis was done for hsp90. Alternatively, immunoprecipitates were probed with anti-p23 and anti-hsp90 antibody. (D) After treatment of HL-60 and HL-60/LR cells with 17-AAG, lysates were immunoprecipitated with anti-Bax (6A7) antibody and immunoblotted with anti-BclXL or anti–total Bax antibody.
HL-60/LR cells are collaterally sensitive to 17-AAG. (A) HL-60 and HL-60/LR cells were treated with the indicated concentrations of 17-AAG for 48 hours. After this, the cells were stained with trypan blue and the percentage of positively stained nonviable cells was determined by light microscopy. Values on the curves represent the mean plus or minus SE of 2 experiments performed in duplicate. (B) HL-60 and HL-60/LR cells were treated with the indicated concentrations of 17-AAG for 24 hours. After this Western blot analyses were done for p-AKT, AKT, p-ERK1/2, ERK1/2, c-Raf, and hsp70 on the cell lysates. The levels of β-actin expression served as the loading control. (C) Reduced binding of ATP and p23 to hyperacetylated hsp90 in HL-60/LR versus HL-60 cells. After incubation of cell lysates containing hsp90 with ATP-sepharose, ATP-sepharose was pelleted and Western blot analysis was done for hsp90. Alternatively, immunoprecipitates were probed with anti-p23 and anti-hsp90 antibody. (D) After treatment of HL-60 and HL-60/LR cells with 17-AAG, lysates were immunoprecipitated with anti-Bax (6A7) antibody and immunoblotted with anti-BclXL or anti–total Bax antibody.
Discussion
Several reports have now highlighted the preclinical activity of HA-HDIs against human leukemia and lymphoma cells.17-20 Early clinical trials have also suggested modest activity against human acute leukemia and non-Hodgkin lymphoma (NHL).33,34 Among the NHLs, CTCL and PTCL are particularly sensitive, leading to approval of vorinostat for the treatment of CTCL.19,20 As currently used in the clinic, continuous and protracted treatment with HA-HDI is likely to lead to selection and emergence of HA-HDI–resistant human leukemia and NHL cells, which most likely undermines the long-term efficacy of HA-HDIs in the therapy of human leukemia and NHL. Here we report that in vitro continuous exposure of the human AML HL-60 cells to the HA-HDI LAQ824 resulted in the emergence of HL-60/LR cells, which exhibit perturbations in the levels of several HDACs, as well as demonstrate cross-resistance to other HDIs and antileukemia agents. However, HL-60/LR cells showed collateral sensitivity to the geldanamycin analogues, 17-AAG and 17-DMAG. Compared with the parental HL-60, HL-60/LR cells have markedly increased in vitro growth fraction (percentage of cells in S phase) and shorter doubling time. Consistent with this, when introduced into the NOD/SCID mice, HL-60/LR cells caused significantly shorter time to the onset of acute leukemia. Rapid in vivo growth of HL-60/LR cells led to earlier mortality in the mouse model, with leukemia initiated by HL-60/LR cells and not by HL-60 cells. A shorter doubling time and rapid in vitro and in vivo growth of HL-60/LR cells are consistent with markedly reduced levels of p21 but increased levels of p-STAT5, STAT5, and cyclin D1 in HL-60/LR versus HL-60 cells. However, in the present studies, we did not establish mechanistic link to any of these molecular features to the aggressive growth rate of HL-60/LR cells. Compared with HL-60, in HL-60/LR cells, AKT and c-Raf levels are markedly elevated, yet the levels of p-GSK3β and p-ERK1/2, the phosphorylated substrates downstream of AKT and c-Raf signaling, respectively,35,36 are considerably depleted in HL-60/LR cells. Depleted levels of pGSK3β and p-ERK1/2 may be due to decreased specific activity of the kinases or increased activity of the specific phosphatases for the substrates that have not been evaluated in the present studies. This makes it unlikely that AKT and c-Raf elevations are associated with increased kinase activity of AKT or c-Raf, thereby ruling out the activity of these kinases as responsible for the high growth fraction and growth rate of HL-60/LR cells.37 High STAT5 activity and cyclin D1 levels, as well as markedly reduced p21 levels, remain as possible explanations.38 In addition, absence of LAQ824-mediated p21 induction or depletion of p-STAT5 and cyclin D1 may be responsible for lack of LAQ824-mediated growth arrest of HL-60/LR versus HL-60 cells.37,38 HL-60/LR cells show high levels of resistance not only to HA-HDIs other than LAQ824 (eg, SAHA, TSA, and LBH589), but also to other HDIs (eg, sodium butyrate, which is structurally different), suggesting a role for a mechanism(s) that would confer general resistance to pan-HDAC inhibitors. To probe this mechanism, we determined and compared the levels of HDACs in HL-60/LR versus HL-60 cells. HL-60/LR cells showed increased protein levels of HDAC1, HDAC2, and HDAC4. Although overexpression of HDAC1 has been reported previously to increase resistance to sodium butyrate in melanoma cells, presence of perturbations in the levels of multiple HDACs in HL-60/LR cells rules this mechanistic possibility out.39 Altered levels of HDACs in HL-60/LR cells did not appear to affect global histone acetylation in response to HA-HDI, because histones H3 and H4 were acetylated approximately to a similar level in HL60/LR and HL-60 cells, after treatment with LAQ824. This observation suggests that reduced intracellular accumulation of HA-HDI is unlikely to be the mechanism of resistance to HDIs in HL-60/LR cells, which has been noted as a potential mechanism of resistance to at least some of the HDIs in other cell types.40 Our results showing similar levels of histone acetylation induced by LAQ824 in HL-60 and HL-60/LR cells also suggest that, for the attenuated apoptosis response due to LAQ824 in HL-60/LR cells, LAQ824-mediated transcriptional perturbations in gene expression are less important than the loss of HDAC6-related deacetylation events and the absence of LAQ824-mediated hsp70 and ROS induction. Compared with HL-60, HL-60/LR cells show lack of increased expression of the cell surface drug transporters MDR, MRP, LRP, and BCRP.28-30 However, these findings also support the conclusion that the resistance of HL-60/LR cells to HA-HDIs is not likely to be due to decreased intracellular uptake of etoposide or HA-HDIs. The biologic significance of higher levels of HDAC2 and HDAC4 in HL-60 versus HL60/LR cells is unclear and remains to be determined. HL-60/LR versus HL-60 cells show higher levels of the antiapoptotic Bcl-xL and XIAP, as well as show markedly lower levels of the proapoptotic Bak and Bim.41 Overexpression of Bcl-2 or Bcl-xL has been reported to confer resistance in transformed cells to HDAC inhibitors and may in part contribute to the resistance of HL-60/LR cells to LAQ824.42 However, HL-60/LR cells also demonstrate higher levels of proapoptotic Bax and lower levels of antiapoptotic Mcl-1.41 It is noteworthy that, although treatment with LAQ824 induced Bim levels in HL-60/LR cells, as has been reported for the treatment with other HDAC inhibitors SAHA and trichostatin A (TSA) leading to activation of Bax and apoptosis, high expression of Bcl-xL and XIAP may have negated the proapoptotic effects of the Bim induction in HL-60/LR cells.42,43 High levels of Bcl-xL and XIAP combined with low levels of Bim isoforms may also be responsible for setting a higher threshold for the mitochondria outer membrane permeability (MOMP) and apoptosis induced by HA-HDIs in HL-60/LR versus HL-60 cells.44 In addition, the lack of HA-HDI–mediated induction of Bak and Bax and depletion of AKT and c-Raf may collectively produce resistance to MOMP and apoptosis in HL-60/LR versus HL-60 cells induced by HA-HDIs and by the other commonly used antileukemia agents. This may also be the reason why HL-60/LR cells display resistance to Ara-C even though a significantly higher percentage of HL-60/LR cells are in the S phase of the cell cycle. Notably, treatment with LAQ824 induced high levels of intracellular ROSs in HL-60 but not in HL-60/LR cells. HA-HDI treatment is known to induce ROSs in human AML cells.45 In addition, treatment with LAQ824 markedly increased TBP2, while reducing the levels of TRX, which would facilitate ROS-induced cell death of HL-60 cells (Figure 3B,D). Conversely, absence of ROS induction, as well as any detectable influence of LAQ824 on TRX and TBP2 levels could explain resistance to MOMP and the lack of sensitivity to LAQ824 in HL-60/LR cells.17 Collectively, these findings also suggest that in HL-60/LR cells the redox mechanism involving TRX and TBP2 may not be functioning, and that during selection with LAQ824 other antioxidant mechanisms may have emerged and be functional in HL-60/LR cells. HL-60/LR cells are also more resistant to apoptosis induced by TRAIL. Resistance to TRAIL-induced apoptosis could be due not only to the collective inhibitory effects of the perturbations in the MOMP regulators (Figure 3), but also to the mechanisms that would explain poor assembly of TRAIL-induced death-inducing signaling complex (DISC).46 This consists of the recruitment of FADD and caspase-8 (and inhibitory FLIP) to the death domains of DR4 and DR5.47 Although HL60/LR versus HL-60 cells express higher levels of DR4 and DR5, as well as show low levels of FLIP, HL-60/LR cells also possess markedly reduced levels of FADD and caspase-8, which is likely to dampen TRAIL-induced DISC formation and signaling. This would explain reduced TRAIL-mediated caspase-8 processing and activity, as well as TRAIL-induced MOMP and apoptosis. The latter would also be facilitated by high levels of Bcl-xL and XIAP combined with low levels of Bim isoforms (Figure 3A). This is consistent with previous reports that have highlighted the loss of caspase-8 as an important mechanism underlying resistance to TRAIL-induced apoptosis in cancer cells.47,48 It is noteworthy that HL-60/LR cells do not express HDAC6 mRNA detectable by RT-PCR, and show only faint expression of HDAC6 protein by immunofluorescent microscopy. Loss of HDAC6 is consistent with markedly elevated levels of acetylated α-tubulin and hsp90 in HL-60/LR cells.7,9 Hsp90 acetylation was estimated by performing immunoblot and immunofluorescent microscopy, using for the first time a polyclonal anti–acetyl-K69 hsp90 antibody. This was also verified by immunoprecipitation of hsp90 and subsequently immunoblotting with anti–acetyl lysine antibody. In addition, because of HDAC6 depletion, treatment with LAQ824 did not increase acetylation of hsp90 or α-tubulin in HL-60/LR cells. In HL-60/LR cells, high level of the acetylated hsp90 is also mostly extracellular in location. Parenthetically, in breast cancer cells, the extracellular hsp90α was shown to induce maturation of matrix metalloproteinase and increase tumor cell invasion and metastasis.49,50 Along with possessing high levels of hyperacetylated hsp90, HL-60/LR cells were collaterally sensitive to treatment with the hsp90 inhibitor 17-AAG. Although, HL-60/LR cells express HSF-1, the transcriptional activator of hsp70,51 they express neither p-HSF-1 nor hsp70. In addition, unlike the parental HL-60, treatment with 17-AAG failed to induce hsp70 levels in HL-60/LR cells. This could be explained by absence of ATP and the cochaperone p23 bound hsp90 (which would be inactive as a chaperone), as well as lack of LAQ824-mediated induction of p-HSF1 in HL-60/LR versus HL-60 cells (Figure 6C). Because 17-AAG–mediated induction of hsp70 has been reported to inhibit 17-AAG–induced apoptosis,52 the absence of hsp70 induction may be responsible for the collateral sensitivity of HL-60/LR cells to 17-AAG. The differential role of lack of hsp70 induction in regulating sensitivity to HA-HDI versus 17-AAG is likely to be due to different downstream mechanisms that are involved in mediating cell death from HA-HDI, compared with 17-AAG, that are not affected by hsp70 levels. Lastly, results of treatment with decitabine and/or LAQ824 demonstrate that neither decitabine nor LAQ824 is able to derepress HDAC6 in HL-60/LR cells, making it unlikely that DNA methylation is the underlying mechanism. In contrast, both in HL-60 and HL-60/LR cells, treatment with decitabine induced p15 levels, whereas LAQ824 induced p15 only in HL-60 cells. These results indicate that, unlike HDAC6, DNA methylation was responsible for repressing p15 in HL-60/LR cells. Taken together, findings presented here highlight several molecular characteristics of HA-HDI–resistant human AML cells isolated by a clinically relevant continuous exposure to an HA-HDI. These findings also highlight that a geldanamycin analog hsp90 inhibitor may override resistance to HA-HDI.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.B. directed this research and was mainly responsible for paper preparation; W.F., R.R., B.H., J.C., R.K., K.E., and Y.Y. performed the in vitro studies and assisted with paper preparation; P.F. and P.L. performed the in vivo studies; Y.W., R.J., and A.M. helped maintain the cell cultures and performed in vitro studies; P.A. provided important reagents; and S.P. helped direct the studies and provided reagents.
Conflict-of-interest disclosure: P.A. is an employee of Novartis Pharmaceuticals and K.B. has received clinical and laboratory research grant from Novartis Institute for Biomedical Research Inc. The remaining authors declare no competing financial interests.
Correspondence: Kapil Bhalla, MCG Cancer Center, Medical College of Georgia, 1120 15th Street, CN-2101 Augusta, GA 30912; e-mail: kbhalla@mcg.edu.