Abstract
Interferon-α (IFN-α) is a nonleukemogenic treatment of polycythemia vera (PV) able to induce cytogenetic remissions. Its use is limited by toxicity, leading to treatment discontinuation in approximately 20% of patients. We completed a phase 2 multicenter study of pegylated IFN-α-2a in 40 PV patients. Objectives included evaluation of efficacy, safety, and monitoring of residual disease using JAK2V617F quantification (%V617F). Median follow-up was 31.4 months. At 12 months, all 37 evaluable patients had hematologic response, including 94.6% complete responses (CRs). Only 3 patients (8%) had stopped treatment. After the first year, 35 patients remained in hematologic CR, including 5 who had stopped pegylated IFN-α-2a. Sequential samples for %V617F monitoring, available in 29 patients, showed %V617F decrease in 26 (89.6%). Median %V617F decreased from 45% before pegylated IFN-α-2a to 22.5%, 17.5%, 5%, and 3% after 12, 18, 24, and 36 months, respectively. Molecular CR (JAK2V617F undetectable) was achieved in 7 patients, lasting from 6+ to 18+ months, and persisted after pegylated IFN-α-2a discontinuation in 5. No vascular event was recorded. These results show that pegylated IFN-α-2a yields high rates of hematologic and molecular response in PV with limited toxicity, and could even eliminate the JAK2 mutated clone in selected cases. Available at www.clinicaltrials.gov as #NCT00241241.
Introduction
Polycythemia vera (PV) is a myeloproliferative disorder (MPD) marked by a risk of thrombotic and hemorrhagic complications, and by a long-term risk of evolution to myelofibrosis (MF), myelodysplastic syndrome (MDS), and acute leukemia (AL). In addition to its role in pathophysiology and diagnosis,1,2 the constitutively active JAK2V617F mutation3-6 may be a prognostic marker because the percentage of circulating mutated alleles (%V617F) appears to be significantly higher in patients who develop vascular complications.7 Finally, %V617F appears to be a good tool to monitor minimal residual disease and to evaluate treatment efficacy.8,9
Reducing the vascular risk and preventing evolution to MF, MDS, and AL are major purposes of PV treatment.10,11 Hydroxyurea (HU) is commonly used to treat PV patients requiring cytoreductive therapy, although it has never been shown in prospective clinical trials to be more effective and less toxic than other agents in PV. In addition, HU is unable to eradicate the malignant clone in PV. Finally, HU in combination with other therapies may increase the risk of AL, and nonleukemogenic drugs are more and more often advocated in younger patients requiring cytoreductive therapy.12-15
Interferon-α (IFN-α) is a nonleukemogenic drug that has shown efficacy in the treatment of PV for more than 20 years.16 In addition, IFN-α can induce cytogenetic remissions or reversion from monoclonal to polyclonal patterns of hematopoiesis in some cases, and may have an impact on the malignant clone.17-19 Its widespread use was offset by its parenteral administration, cost, and toxicity although, in the largest study of IFN-α with long-term follow-up reported to date,20 the frequency of treatment discontinuation resulting from toxicity was only 15% when low doses were used. The recent development of pegylated (peg) forms increased tolerance and efficacy in IFN-α-treated hepatitis patients. Several phase 2 studies with peg-IFN-α-2b have been performed in ET and PV patients,21-24 but peg-IFN-α-2a, another allelic form of IFN-α-2, has never been tested so far in MPD.
We performed a multicenter phase 2 trial of peg-IFN-α-2a (Pegasys, Roche Pharmaceuticals, Basel, Switzerland), in PV patients (PVN1 study). A preliminary report of this study, with limited follow-up, found significant molecular responses (reduction of %V617F) in the first 27 enrolled patients.9 We now report results of the completed PVN1 study, including clinical, molecular responses, and tolerance in the whole cohort of 40 patients included, with a median follow-up of 31.4 months.
Methods
Study design
The PVN1 study was approved by the Institutional Review Board of Hôpital R. Ballanger/Paris 13 University (Aulnay-Sous-Bois, France) and by the French Health Products Safety Agency. Informed consent was obtained in accordance with the Declaration of Helsinki. The trial was designed, conducted, and analyzed by the French academic PV-Nord group. Roche Diagnostics (Meylan, France), the manufacturer of peg-IFN-α-2a, provided the drug for patients but was not otherwise involved in the trial.
Inclusion criteria
Summary of the PVN1 study was registered at www.clinicaltrials.gov as #NCT00241241. Inclusion criteria were as follows: PV diagnosis according to Polycythemia Vera Study Group (PVSG) criteria, age 18 to 65 years, and no previous treatment or only phlebotomies or cytoreductive treatment for less than 2 years. PV diagnosis workup included red cell mass measurement (performed in all cases), exclusion of secondary causes, spleen size assessment (clinically and by ultrasound, performed in all cases), serum erythropoietin (Epo) level measurement (performed in 37 cases), and search for endogenous erythroid colony formation (performed in 35 cases). Bone marrow biopsy and cytogenetic analyses (not required by the PVSG criteria used for inclusion in this study) were performed in 11 (confirming PV diagnosis in all cases) and in 14 (all normal) patients, respectively. Pretreatment evaluation also included patient history, physical examination, complete blood count and differential, serum chemistries, including liver and renal function studies, thyroid function tests, and molecular study for JAK2V617F mutation.
Exclusion criteria included usual contraindications to peg-IFN-α-2a (significant hepatic and renal dysfunction, history of psychiatric disorder, in particular depression, autoimmune hepatitis, severe and uncontrolled cardiac dysfunction, known hypersensitivity to IFN-α).
End points
The primary end point was hematologic response to peg-IFN-α-2a at 12 months. Complete hematologic response (CR) was defined by a hematocrit (Hct) level lower than 45% in males and 42% in females without phlebotomy, absence of splenomegaly, and normal white blood cell (WBC) (< 10 × 109/L) and platelet counts (< 400 × 109/L). Partial hematologic response (PR) was defined by Hct less than 45% in males and 42% in females, but with persistent splenomegaly or elevated (> 400 × 109/L) platelet count, or reduction of phlebotomy requirements by at least 50%. Failure was defined by persistent phlebotomies to maintain Hct in the target range without reduction of phlebotomy requirements by at least 50%.
Secondary end points included cumulative toxicity during the first 12 months of the study and evolution of %V617F during peg-IFN-α-2a therapy. Toxicity, also assessed beyond the first year of treatment, was evaluated using the International Common Toxicity Criteria (version 2.0, http://ctep.cancer.gov/reporting/ctc.html). Molecular response (MR) was defined as “complete” when JAK2V617F became undetectable with the technique used (ie, %V617F < 1%), “partial” when more than 50% decrease of baseline %V617F was obtained, and “minor” when %V617F decrease was between 20% and 49%.
Evaluations
Follow-up evaluations included adverse event (AE) assessment monthly for 3 months, then every third month until the 12th month, and every 6 months thereafter. Biologic follow-up included complete blood count and serum chemistries weekly during the first month and monthly thereafter. Thyroid function tests and abdominal ultrasound in patients with splenomegaly were repeated at 6 and 12 months. Blood samples for JAK2 mutation studies were taken every 6 months.
Treatment
After inclusion, all previously untreated patients were phlebotomized until Hct reached 45% (males) or 42% (females). In patients treated with HU before study entry, HU was stopped 2 weeks before Peg-IFN-α-2a initiation, and patients were also phlebotomized to reach the target Hct defined in “End points” when needed. In the absence of contraindication, all patients were treated with 100 mg of aspirin daily.
Peg-IFN-α-2a was started subcutaneously at 90 μg weekly for 2 weeks, with a dose escalation (every 2 weeks, and in the absence of toxicity) to 135 μg/wk and to 180 μg/wk in the absence of hematologic response.
Molecular analyses
JAK2 mutation.
V617F mutation was detected by a single nucleotide polymorphism genotyping assay performed in DNA samples extracted from purified granulocytes using real-time polymerase chain reaction (PCR)-based mutation detection (Taqman ABI Prism 7700).9 This PCR assay allows quantitative evaluation of %V617F with a sensitivity of approximately 1%. Patients lacking JAK2V617F were screened for exon 12 mutation using allele-specific PCR, as described.25
9p LOH analyses.
In 15 of the patients, loss of heterozygosity (LOH) on the short arm of chromosome 9 (9p) was screened using chromosome 9p markers (D9S1813, D9S288, D9S1810).26 Microsatellite analysis, carried out in duplicate on sequential samples, allowed recognition of 9p LOH when significant changes in allelic ratios occurred between samples (difference of allelic ratio > 15% between samples for one or more markers).
Statistical analyses
For continuous variables, median (Q1-Q3) values are given. Distributions of time to response and time to treatment discontinuation were estimated by the Kaplan-Meier method and compared across groups using the log-rank test. Comparisons of Eastern Cooperative Oncology Group staging and %V617F at 12 months vs baseline were based on the MacNemar χ2 test and the Wilcoxon sign test, respectively.
Mean decrease of %V617F over time was tested using a mixed effect model, accounting for intrapatient correlation of serial measures. This allowed further testing for predictive factors of response.
All statistical tests were 2-sided, with P values of .05 or less denoting statistical significance. Analysis was performed using SAS (SAS Institute, Cary, NC) and R (http://www.r-project.org/) software packages.
Results
Patient characteristics
The 40 planned patients were enrolled between September 2004 and October 2005. Three patients were excluded from analysis because PV diagnostic criteria were not fulfilled in one, of consent withdrawal before starting peg-IFN-α-2a in one, and severe allergic reaction at first peg-IFN-α-2a injection in the last patient, who had a history of allergy to several drugs. Thus, analysis was performed at the reference date of January 2008 in 37 patients. Median follow-up was 31.4 months (Q1-Q3, 26.4-35.1 months).
Baseline characteristics of the patients are summarized in Table 1. Median age was 49 years (Q1-Q3, 42-53 years), and 16 (43%) patients were males. Thirteen patients (35%) had splenomegaly (including 7 with palpable splenomegaly and 6 detected on ultrasound). Mean spleen size on ultrasound was 14 cm (range, 11-18 cm), and mean spleen enlargement below costal margin in patients with clinical splenomegaly was 2 cm (range, 1-4 cm). Five patients (14%) had a history of major thrombotic events. Treatment before inclusion included phlebotomies alone in 20 (54%) patients, with a median number of phlebotomies during the 3 months before study entry of 3 (range, 2-11; Q1-Q3, 3-5), HU in 12 (32%) patients, and no treatment in the remaining 5 (14%) patients, all recently diagnosed. Thirty-one (86%) patients were already on low-dose aspirin at study entry. Median time between PV diagnosis and inclusion was 5 months (Q1-Q3, 1-11 months).
Initial blood samples for JAK2 analysis were available in 35 of 37 patients. JAK2V617F was found in 29 of 35 (83%), with a median %V617F at study entry of 45% (Q1-Q3, 35%-60%). JAK2 exon 12 mutation (N542-E543del) was found in one JAK2V617F-negative patient, so that a JAK2 mutation was found overall in 30 of 35 patients (85.7%). The 5 JAK2 nonmutated patients, however, fulfilled PVSG inclusion criteria, in particular with presence of endogenous erythroid colony and low Epo levels in all cases.
Hematologic response to Peg-IFN-α-2a
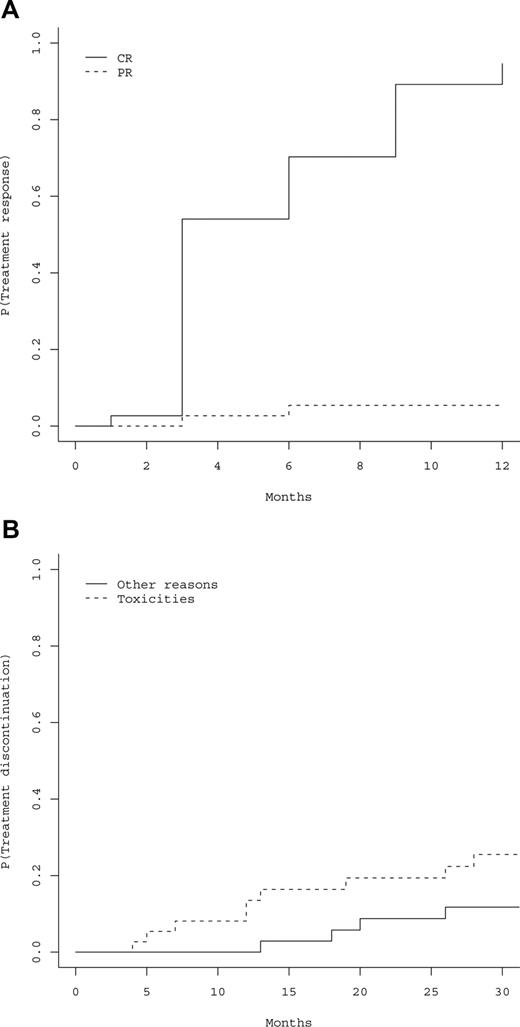
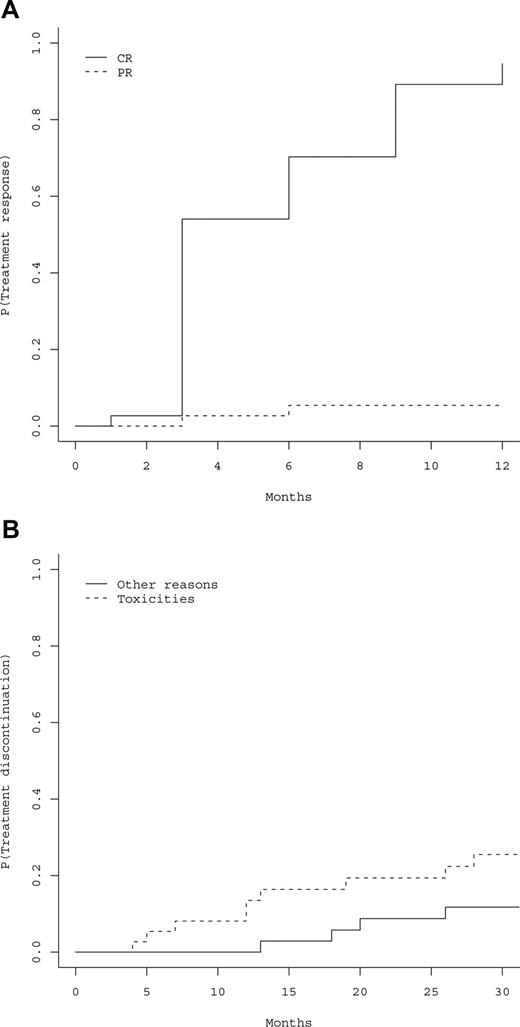
All the 37 patients had responded to peg-IFN-α-2a at the 12-month evaluation (primary study end point), including 35 hematologic CRs (94.6%), and 2 PRs (5.4%). Cumulative incidences of hematologic CRs and PRs are represented in Figure 1A, showing that 100% of responses were achieved within 12 months. Thirty-six patients became phlebotomy-free after 3 months, the last patient after 6 months, and 36 (97.3%) patients remained phlebotomy-free during all the follow-up period (Table 2). Time to response was not influenced by age (P = .24), gender (P = .54), baseline values of hemoglobin (P = .45), Hct (P = .32), platelet count (P = .26), absolute neutrophil count (ANC) (P = .86), presence of splenomegaly (P = .18), presence of JAK2 mutation (P = .7), %V617F (P = .71), presence of 9p LOH (P = .92), and cumulative dose of peg-IFN-α-2a (P = .33). Median cumulative dose of peg-IFN-α-2a received during the first 12 months was 109 μg/wk (Q1-Q3, 90-135 μg/wk).
Hematologic responses and treatment discontinuations. (A) Estimated cumulative incidence of treatment response, including hematologic complete response (CR) or partial response (PR). P indicates probability of response to treatment. (B) Cumulative incidence of treatment discontinuation according to the cause of discontinuation. P indicates probability of treatment discontinuation.
Hematologic responses and treatment discontinuations. (A) Estimated cumulative incidence of treatment response, including hematologic complete response (CR) or partial response (PR). P indicates probability of response to treatment. (B) Cumulative incidence of treatment discontinuation according to the cause of discontinuation. P indicates probability of treatment discontinuation.
Hematologic responses were sustained beyond the first year (Table 2). At the reference date of analysis (January 2008; median follow-up of 31.4 months), 29 of the 37 patients (78.4%) had received only peg-IFN-α-2a since inclusion, whereas 8 had switched to another treatment, including HU (n = 7), or a phlebotomy regimen (n = 1). Reasons for switching to another treatment were toxicity of peg-IFN-α-2a in 6 and achievement of only hematologic PR with peg-IFN-α-2a in 2.
The 29 patients treated with peg-IFN-α-2a alone were in hematologic CR, still on treatment (n = 24) or off peg-IFN-α-2a therapy (n = 5) Those last 5 patients had stopped peg-IFN-α-2a after 12 to 24 months of treatment because of sustained CR with very low doses (n = 3) or to toxicity (n = 2), and were still in hematologic CR 3, 3, 6, 10, and 18 months, respectively, after peg-IFN-α-2a discontinuation, without requirement for any additional cytoreductive treatment.
The 8 patients who required treatments other than peg-IFN-α-2a were still in CR (n = 6, after switch to HU) or PR (n = 2, after HU in 1, and phlebotomies only in 1).
Overall, after 31.4 months of median follow-up, all patients were still in hematologic response, including 35 (94.6%) CRs and 2 PRs.
No patient experienced signs or symptoms of thrombosis or hemorrhage during the whole study period.
Peg-IFN-α-2a tolerance
AEs (secondary study end point) were reported in 33 (89%) patients during the first 12 months of the study, whereas the other 4 (11%) patients remained free of AEs (Table 3). A total of 239 AEs were reported (median number per patient, 4 AEs; Q1-Q3, 2-10 AEs). All reported AEs were of grade 1 or 2, except one grade 3 skin toxicity (see next paragraph). The percentage of patients having AEs slightly decreased over time, from 65% during the first month to 50% at 12 months. The toxicity profile for the entire cohort is summarized in Table 3. Most common toxicities during the first month included musculoskeletal pain in 13 patients, skin toxicity in 6, asthenia in 6, and gastrointestinal symptoms in 4. At 12 months, those symptoms were still present in 7, 5, 7, and 2 patients, respectively. The Eastern Cooperative Oncology Group score at baseline and at 12 months was 0 in 90.3% and 83.3% patients, and 1 in 9.7% and 16.7% patients, respectively (P = .56).
During the whole study period, treatment was stopped in 13 patients, including 9 (24.3%) for toxicity (3%-8.1% during the first year). Figure 1B shows time to treatment discontinuation, distinguishing between discontinuation because of AEs and other causes. Causes of treatment discontinuation for toxicity during the first year (n = 3) were: the only observed case of recurring grade 2 neutropenia that impeded weekly peg-IFN-α-2a administration resulting in nonsatisfactory control of Hct in this JAK2 unmutated patient, grade 3 skin toxicity after 6 months (n = 1), and grade 2 fatigue and headaches after 9 months (n = 1). After the first year, toxicity led to treatment discontinuation in 6 additional patients, including liver toxicity (n = 1), occurrence of biologic markers of autoimmunity (n = 1), muscular pain (n = 2), peripheral neurologic toxicity (n = 1), and fatigue (n = 1). Discontinuation in the 4 remaining patients was the result of investigator's decision because of sustained hematologic CR (n = 2), end of the predefined treatment period (n = 1), and discovery of colon cancer (n = 1).
Molecular response
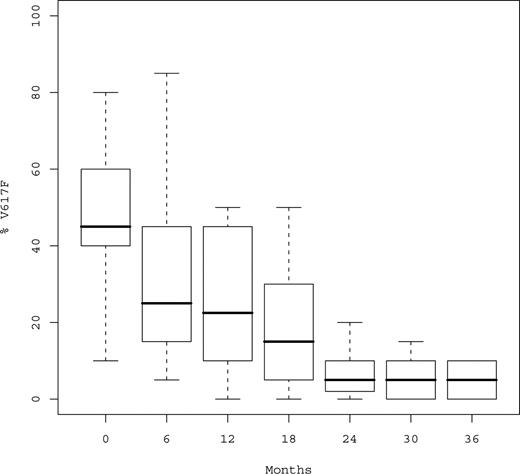
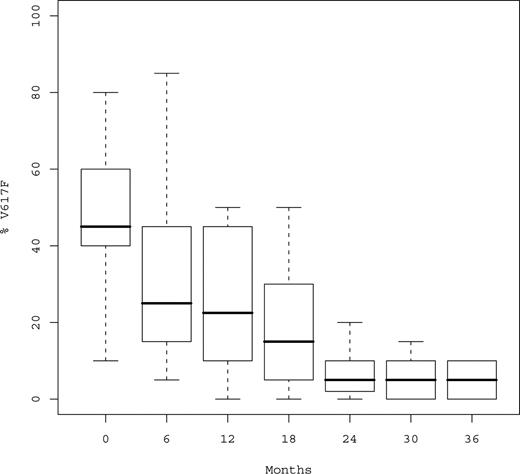
At time of analysis, a total of 152 serial samples were available for JAK2V617F monitoring in the 29 mutated patients (median number of samples per patient, 5; Q1-Q3, 4-6; Table 4). Median %V617F was 45% (Q1-Q3, 35%-60%) before peg-IFN-α-2a, 30% after 6 months, and 22.5% after 12 months (P < .001; Figure 2). Median relative decrease of %V617F during the first year was 50% (Q1-Q3, 33%-60%). %V617F decreased in 26 of 29 (89.6%) patients, and only 3 patients experienced no variation in %V617F, all of whom however achieved hematologic response (including 2 CRs after 3 months and 1 PR).
Boxplots of %V617F over time. %V617F indicates percentage of JAK2V617F circulating alleles. Median %V617F was 45%, 22%, 5%, and 3% at baseline, 12, 24, and 36 months, respectively.
Boxplots of %V617F over time. %V617F indicates percentage of JAK2V617F circulating alleles. Median %V617F was 45%, 22%, 5%, and 3% at baseline, 12, 24, and 36 months, respectively.
After the first year, %V617F continued to decrease without evidence for a plateau (Figure 2), the proportion of mutant JAK2 being always similar or lower in the last sample compared with the previous one and none of the responding patient experienced an increase of %V617F during follow-up. Median %V617F was 17.5% at 18 months, 5% at 24 months, 5% at 30 months, and 3% at 36 months (P < .001). Complete or partial MR was observed in 21 (72.4%) patients, including 17 (58.6%) after 12 months and 4 beyond the first year.
Hematologic response was achieved in 89% of the patients within 3 months (Table 2), although reaching significant MR seemed to require longer exposure to IFN-α (Table 4). Indeed, the proportion of patients in hematologic response still having greater than 10% %V617F was 72%, 52%, 24%, and 6% at 12, 18, 24, and 30 months of treatment, respectively.
At time of last analysis, complete MR (ie, JAK2V617F becoming undetectable with the method used) was achieved in 7 of 29 (24.1%) patients: at 12 months in 2 patients, at 18 months in 1, 24 months in 1, and at 30 months in 3 (Table 4). Persistence of complete MR was confirmed in 6 of those 7 patients on one or several samples obtained after at least 6 months, the longest duration in complete MR being 18 months. In 3 additional patients, %V617F was very faintly positive at the 1% sensitivity limit of our technique at 24 months. Thus, reduction of the V617F clone to 1% or less was reached in as many as 10 of 29 (34.5%) patients. In the 5 patients who achieved complete MR and subsequently discontinued peg-IFN-α-2a, JAK2V617F was still undetectable 6 to 18 months after drug discontinuation (patients 9, 12, 22, 25, and 29; Table 4).
%V617F decrease at 12 months was not influenced by age (P = .85), sex (P = .43), Hct (P = .92), hemoglobin (P = .88), initial %V617F (P = .98), and cumulative dose of peg-IFN-α-2a (P = .64), but there was a strong trend for greater %V617F decrease in patients with lower baseline platelets count (P = .06) and lower baseline ANC (P = .05).
Thirteen of 29 patients (44.8%) had %V617F more than or equal to 50% in the baseline sample (Table 4) and were therefore presumed to carry homozygously mutated cells (mean baseline %V617F, 68.5% ± 20.2% in those patients). At 12 months, %V617F had decreased in 12 (92%) of them and was lower than 50% in 9 (69%), including 6 (46%) complete or partial MR. At last evaluation, complete or partial MR was finally observed in 9 of those 13 patients (70%), with a mean final %V617F of 20% plus or minus 19.4%. Complete MR was achieved in 3 (23%) of them.
In 7 of 15 (46.7%) tested patients, serial sampling during treatment using microsatellite analysis with 9p markers showed a clear-cut decrease in the allelic ratios in parallel with a decrease in %V617F, showing that the abnormal clone had 9p LOH (Table 4). Those 7 patients with 9p LOH had significantly higher median %V617F at baseline (65%; Q1-Q3, 50-80) than the 8 patients without 9p LOH (40%; Q1-Q3, 40%-50%; P = .03), significantly higher WBC counts at diagnosis (median, 14.3 × 109/L (Q1-Q3, 13.7-14.7 × 109/L) versus 8.05 × 109/L (Q1-Q3, 7-9.6 × 109/L); P = .03), and were slower molecular responders, median %V617F being 45% (Q1-Q3, 45%-55%) versus 20% (Q1-Q3, 10%-40%) at 6 months (P = .03), and 45% (Q1-Q3, 25%-45%) versus 20% (Q1-Q3, 5%-40%) at 12 months (P = .10). However, with longer follow-up, patients with 9p LOH had similar MRs as those without 9p LOH, %V617F at 18 months being 23.3% plus or minus 20.2% and 23.3% plus or minus 2.9%, respectively (P = .65). Furthermore, 1 patient with 9p LOH achieved complete MR at 24 months (patient 25, Table 3).
Discussion
Compared with previously reported PV studies using HU, standard IFN-α, or peg-IFN-α-2b, this first completed trial of peg-IFN-α-2a in PV showed some differences. First, all patients had hematologic response within the first year of treatment, including complete hematologic response in 94.6% cases. Second, 92% of patients could receive peg-IFN-α-2a for at least 12 months, resulting from moderate short-term toxicity. Finally, the high incidence of MR suggested in our preliminary report9 was confirmed, and complete MR could be achieved in 24% of the patients at time of last analysis.
After 1 year of treatment, all patients had hematologic response to peg-IFN-α-2a, including 94.6% hematologic CR, and all patients were phlebotomy-free (a result maintained in 97.3% of them after a median follow-up of 31.4 months). Of note, hematologic CR (for which there is no international consensus definition in PV) was defined in this study not only by control of Hct, but also by normalization of WBC and platelet counts, and disappearance of splenomegaly. In previously published studies, patients were sometimes considered in hematologic response when Hct was corrected, even if they kept platelet counts between 400 and 600 × 109/L or persistent splenomegaly, whereas normalization of WBC count had never been taken into account for response criteria.20,22 Although platelet count has not been shown to influence the vascular risk in PV,27 recent data suggest that leukocytosis has a major prognostic relevance, increasing the risk of thrombosis (and also of transformation to AL).28-30 Normalization of WBC counts, potentially able to reduce the risk of thrombosis, could be an important objective of PV treatment in future trials. In addition, although similar control of leukocytosis can be achieved with conventional treatments, such as HU, our MR data suggest that peg-IFN-α-2a preferentially decreases the proportion of clonal circulating granulocytes, an effect not reported with HU.8 This selective effect on mutated granulocytes may impact on the incidence of thrombosis, as it has been shown that leukocyte activation and hemostatic changes were correlated with the presence of JAK2V617F.31-33 Accordingly, no thrombosis was observed in this cohort after more than 30 months of median follow-up, although approximately 5 cases would have been expected, given the cumulative rate of 5.5 events per 100 patients per year reported in the literature.10,34 Silver also reported no vascular events in 55 PV patients treated with standard IFN-α, after an average follow-up of 53 months.20
The high clinical response rate we observed was not the result of a selection bias because characteristics of the patients included in this study indicated active disease with a median number of phlebotomies received during the 3 months preceding trial inclusion of 3, and median WBC and platelet counts of 10 × 109/L and 720 × 109/L, respectively. Efficacy in the control of myeloproliferation had been reported with other forms of IFN, but at lower rates, ranging from 45% to 70%.22-24,35
Another finding of this study was the relatively good short-term tolerance of peg-IFN-α-2a, indeed, almost similar to that reported with HU, because only 8% of patients had stopped treatment at 1 year. A preliminary report of another peg-IFN-α-2a study in 50 PV and ET patients, with 11 months of median follow-up, found 12% therapy withdrawal because of toxicity36 (by comparison, the dropout rate of HU treatment reported in large prospective randomized trials of PV and ET patients ranged from 10% to 19%37-39 ). We observed dose-dependent hematologic toxicity (grade 2 neutropenia) in only one patient, who was negative for JAK2 mutations. Such rare toxicity may be easier to avoid using IFN forms with shorter half-life.
The low limiting toxicity rate we observed during the first 12 months of PV therapy with IFN-α had not been reported in a multicenter study. However, the overall 24% withdrawal for toxicity seen after 31.4 months of median follow-up was comparable with that observed during the first year of treatment in other IFN-α studies, and to the 15% long-term dropout rate reported in a single investigator experience.20
A major finding of the present study was the high complete MR rate (24%), whereas in an additional 10% of the patients, %V617F was lowered to approximately 1%, ie, the detection limit. Such MR had never been obtained with any other treatment in PV, to our knowledge. Preliminary results obtained in the first patients included in this trial showed rapid decrease in JAK2V617F burden.9 They led to the hope that more prolonged treatment would lead to further reduction in %V617F. Current updated results of %V617F monitoring, with longer follow-up and larger patients numbers, confirm that hypothesis. Overall, 89.6% of patients had MR (including CR, PR, and minor MR) after 1 year of peg-IFN-α-2a, including 58.6% of the patients who had complete or partial MR (ie, a greater than 50% decrease in baseline %V617F). With longer follow-up, %V617F continued to decrease, and 72.4% of the patients achieved complete or partial MR at last evaluation. In none of the responding patients did %V617F increase during follow-up. Molecular response was achieved independently of initial %V617F, as shown by similar response rates observed in the whole cohort, in presumably homozygous patients (baseline %V617F > 50%), and in patients with 9p LOH (those 2 last patient categories partly overlapped as 6 of 7 of 9p LOH patients had %V617F ≥ 50%). In addition, initial %V617F did not influence kinetics of decrease or final level of %V617F. However, platelet count and ANC at diagnosis were negatively correlated to MR, suggesting that major MR is easier to achieve in patients without trilineage myeloproliferation.
The fact that 7 patients (24% of the whole cohort, 27% of the responding patients) achieved molecular CR, and 3 additional patients had %V617F at the lower limit of detection on last examination, suggests that peg-IFN-α-2a may greatly reduce the JAK2V617F clone in a substantial proportion of patients and perhaps eradicate it in selected cases. Because our real-time quantitative polymerase chain reaction assay had a sensitivity of approximately 1% and in the absence of bone marrow studies, we however cannot ascertain that the mutated clone was eliminated in our patients.40 In addition, studies comparing clonality based on X-chromosome inactivation patterns or coexisting cytogenetic abnormalities to JAK2 mutant allele frequency suggest that JAK2V617F could be a secondary genetic event in at least some PV patients.41-43 In those cases, measurement of JAK2V617F would only reflect the evolution of subclones. Achievement of complete MR with peg-IFN-α-2a had been previously reported in one patient after only 2 months of treatment.44 This patient discontinued peg-IFN-α-2a one month after complete MR was obtained, and JAK2V617F reappeared 2 months later. In our study, JAK2V617F was still undetectable 6 to 18 months after treatment discontinuation in 5 patients who had received 12 to 24 months of peg-IFN-α-2a and had achieved complete MR. Thus, achieving prolonged complete MR may require sufficient treatment duration, suggesting that peg-IFN-α-2a should not be discontinued as soon as molecular CR has been reached.
Possible mechanisms of action of interferon on JAK2 mutated clones are unknown. They probably involve direct effects of the cytokine on hematopoietic cells, including inhibition of the proliferation of erythroid and megakaryocytic progenitors, and modulation of the thrombopoietin receptor signaling.20 But they may also involve the pleiotropic immunologic properties of IFN, including increase of cytotoxicity of T and natural killer cells, and induction and amplification of cell-mediated and humoral immune responses to candidate tumor antigens.45,46 In this regard, the recently identified PV-associated tumor antigens may represent targets for immune effectors.47,48 The time interval between hematologic and MRs observed in the present study could suggest that direct cytotoxic and antiproliferative properties of IFN were responsible for the rapid hematologic response (within 3 months in ∼ 90% of the patients), whereas immune-mediated mechanisms could be involved in MR, achieved later.
The unexpectedly high rate of complete MRs we observed may open new perspectives in the management of PV, perhaps at the image of the chronic myeloid leukemia story. At least before JAK2 inhibitors are available in clinical practice,49 the place of peg-IFN-α-2a should be more precisely evaluated by comparison with HU. Our results also question the therapeutic attitude of limiting cytoreductive therapy to patients at high risk of vascular events according to current guidelines,10 not only with respect to vascular events, but also because of reduction in phlebotomy requirements and possible delay in developing myelofibrosis, first promulgated by others.20 Such limitation may no longer be justified if reduction of the malignant clone can be achieved using a nonleukemogenic drug with limited toxicity. If so, early peg-IFN-α-2a treatment could be proposed to PV patients to reduce the vascular risk by efficient control of all blood parameters and reduction of the mutated clone, an attitude possibly supported by the absence of vascular events in the present cohort of 37 patients followed during a median of 31.4 months.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank investigators of the PV-Nord group who enrolled patients in the study (Marie-Jose Grange, Jean-Luc Dutel, Kamel Ghomari, Philippe Rousselot, Yasmina Chait, Jean-Marc Zini, William Vainchenker, Nathalie Parquet, and Lina Abdelkader-Aljassem) and Jean-François Bernard for his help in study design and data collection.
This study was supported in part by a research grant from Roche France to the PV-Nord group.
Authorship
Contribution: J.-J.K., B.C., C.C., and P.F. designed research; J.-J.K. is the coordinator of the PVN1 study; J.-J.K., S.C., and P.F. analyzed data; J.-J.K. drafted the paper; B.C. performed molecular analyses; B.G. performed 9p LOH analyses; P.T., N.C., M.R., and S.B. enrolled patients; and S.C. performed statistical analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pierre Fenaux, Assistance Publique-Hôpitaux de Paris, Hopital Avicenne and Paris 13 University, Service d'Hematologie Clinique, 125 rue de Stalingrad, 93000 Bobigny, France; e-mail: pierre-fenaux@avc.aphp.fr.
References
Author notes
*J.-J.K. and B.C. contributed equally to this paper as first authors.