Abstract
Although studies have demonstrated that androgen withdrawal increases thymic size, molecular mechanisms underlying this expansion remain largely unknown. We show that decreased androgen signaling leads to enhanced immigration of bone marrow T-cell precursors, as manifested by both an early increase of early thymic progenitors (ETP) and improved uptake of adoptively transferred quantified precursors into congenic castrated hosts. We provide evidence that the ETP niche is enhanced after androgen withdrawal by proliferation of UEA+ thymic epithelial cells (TEC) and increased TEC production of CCL25, a ligand critical for ETP entry. Moreover, the greatest increase in CCL25 production is by UEA+ TEC, linking function of this subset with the increase in ETP immigration. Furthermore, blockade of CCL25 abrogated the effects of castration by impairing ETP entry, retarding immature thymocyte development, limiting increase of thymic size, and impairing increase of thymopoiesis. Taken together, these findings describe a cohesive mechanism underlying increased thymic productivity after androgen withdrawal.
Introduction
Impaired thymopoiesis contributes to the immune deficiency after lymphopenia or allogeneic hematopoietic stem cell transplantation (HSCT).1,2 The thymus is the primary site of new naive T-cell generation and the site of central tolerance induction where potentially autoreactive T cells are deleted.3 Without thymic renewal during recovery from lymphopenia, an oligoclonal repertoire of T cells persists, leading to impaired clearance of infectious diseases and tumors, and increased rates of auto- or alloimmunity (graft-versus-host disease).4-8 Thus, an understanding of the mechanisms underlying renewed thymopoiesis may be of critical importance to correcting thymic deficits after HSCT, AIDS therapies, and chemotherapeutic treatment for cancer.
Androgen withdrawal presents a potential therapy to renew thymic function. Multiple studies have demonstrated that androgen withdrawal increases thymic size and thymocyte numbers in male mice.9-13 These studies suggested that this enlargement represents enhanced thymic activity as evidenced by subsequent increases in naive T cells in peripheral organs.12,14 Furthermore, transplant studies in mice and man have shown enhanced thymic recovery and increased peripheral recent thymic emigrants (RTE) after androgen withdrawal, consistent with overall augmented thymopoiesis.12,15,16
However, the mechanism by which androgen withdrawal induces thymic recovery remains incompletely understood. Because thymic epithelial cells (TEC) and thymocytes possess functional androgen receptors, studies have focused on these cells as mediators of thymic enlargement associated with androgen withdrawal.17,18 Initial studies into the thymic effects of androgen revealed rapid thymocyte apoptosis associated with thymic involution after androgen administration.19,20 Conversely, androgen withdrawal has been shown to enhance proliferation of thymocyte subsets concomitant with thymic expansion.13,21 Significantly, these effects of androgen on thymocytes were abrogated when the androgen receptor on TEC was dysfunctional,18,20 suggesting that TEC functions may underlie the thymic changes observed after androgen manipulation. Furthermore, there is evidence that these epithelia proliferate, perhaps increasing thymic size via proliferative expansion.22 Thus, these studies highlight that TEC and intrathymic alterations play an essential part in the mechanism of thymic renewal after androgen withdrawal and suggest that further investigation could appropriately include characterization of distinct TEC subsets.
In addition, emerging data reveal a key role for bone marrow precursors in thymic regulation. Recent publications demonstrate that T precursors arise from marrow LSK (defined phenotypically as mature Lineage-negative cells that are also Sca-1–positive and c-Kit–high).23 A subset of Flt-3+CCR9+ LSKs has been shown to home efficiently to the thymus and give rise to early T-lineage progenitors (ETP) upon thymic entry.24,25 After androgen withdrawal in male mice, Heng et al13 showed that ETP numbers were significantly increased. Although these data were the first to provide evidence that increased ETP numbers may be involved in the mechanism of enhanced thymopoeisis after castration, they did not identify whether the increase in ETPs was due to increased immigration, increased intrathymic proliferation, or altered transitions to more mature stages of T-cell development.
Other work revealing the mechanism of ETP uptake makes it possible to address these questions. Recent data demonstrate that the CCR9 receptor on ETPs is critical for postnatal thymic uptake.25 CCL25, the ligand for CCR9, is known to be produced by thymic medullary dendritic cells and by TEC in 10-fold higher concentration than any other tissue investigated to date.26,27 CCR9 was shown to be essential for postnatal ETP immigration because thymocyte renewal was significantly impaired when irradiated hosts were given marrow from CCR9−/− mice.28 In contrast to this critical role, CCR9-CCL25 interactions are not essential for intrathymic T-cell development because CCR9−/− mice exhibit normal thymopoiesis, albeit with reduced ETP and double negative 2 (DN2) populations.26,29 Thus, this new appreciation of the role of CCR9 and CCL25 in ETP uptake provides a means to investigate ETP immigration in thymic renewal after androgen withdrawal.
In this report, we present data that support a cohesive mechanism to explain enhanced thymopoiesis after androgen withdrawal. We confirm that castration enhances thymic activity, as defined by increasing splenic RTE number. Second, we provide evidence that increases in ETP number within the thymus by 8 days after castration is due to improved ETP immigration by using adoptively transferred marrow with enumerated LSK in unirradiated, castrated, congenic hosts. We then demonstrate that ETP uptake and acceleration of DN1 maturation is important for thymic expansion after castration and that the CCL25-CCR9 interaction is essential for androgen withdrawal-enhanced thymopoiesis. Finally, we show not only a proliferation of UEA+ TEC after castration, but that the greatest increase in CCL25 production is by these TEC, presenting an essential role for UEA+ TEC in this mechanism. These data reveal a cohesive mechanism of augmented thymopoiesis after androgen withdrawal: (1) proliferation of UEA+ TEC that increase production of CCL25, (2) a boost in ETP entry and immature thymocyte development secondary to this increase in niche size and CCL25 expression by UEA+ TEC, ultimately leading to (3) increased thymopoiesis as reflected by greater number of RTE in the periphery.
Methods
Animals
Age-matched postpubertal C57BL/6(B6)-Ly5.1 and B6 (Ly5.2) (congenic) male mice were purchased from the Animal Production Unit, National Cancer Institute. Animal care and experimental procedures were carried out under NCI-approved protocols.
Castration procedure
Animals were anesthetized using ketamine/xylazine, and scrotal incision made under sterile conditions. The testicles were removed; the spermatic cord was clamped and cauterized. Sutures or surgical clips were placed at incision site. Sham castration was performed using the same procedure without removal of the testes.
Flow cytometry
Single cell suspensions of thymus, spleen, lymph node, and bone marrow were harvested and counted at various time points after surgery (days 8, 30 [range, 28-41], 90 [range, 89-95], and 180 [range, 182-210]). Cells from the spleen, lymph node, and bone marrow were subjected to ammonium chloride potassium (ACK) lysis to remove red blood cells. All flow cytometry specimens were incubated with 2.4G2 blocking antibody before staining. Samples were labeled using combinations of the following antibodies: CD4, CD8, CD3, CD44, CD25, B220, AA4.1, CD45.2, UEA (Vector Laboratories, Burlingame, CA), Ly-51, CD45, Sca-1, c-kit (allophycocyanin [APC; eBioscience, San Diego, CA] or phycoerythrin [PE]), and interleukin-7Rα (IL7Rα; eBioscience). For DN and ETP/LSK subset determination, mature cells were labeled with biotinylated lineage markers: TER, CD8α, H57, Gr1, Mac1, NK1.1, IgM, CD19, B220, CD3, and CD11c. Biotinylated antibodies were developed with streptavidin PE-Cy5 or pacific blue (Invitrogen, Carlsbad, CA). All antibodies were purchased from BD Biosciences (San Jose, CA) unless indicated otherwise. Isotype controls were used for all rare populations, included in Full Minus One controls. Four-color flow cytometry was performed on a FACScalibur using CellQuest software. Four-, five-, and six-color panels were acquired on a LSR II flow cytometer (BD Biosciences). For thymic LSK/ETP studies, 5 to 7 × 106 cells were stained and greater than 1 million total events collected. All data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Cell sorting
For TEC sorting, cells were bulk-labeled using antibodies described above sorted on a FACSVantage DiVa cell sorter (BD Biosciences). Cells were reanalyzed on the same sorter to verify purities, which typically exceeded 95%.
BrdU incorporation
For thymic epithelial cell studies, sham-castrated and castrated age-matched C57BL/6 male mice were injected with 2 mg BrdU intraperitonially 1 day after the procedure. Oral 0.08% BrdU in water was administered for the next 7 days. Both groups were killed 1 week after castration. For all BrdU studies, C57BL/6 male mice not exposed to BrdU were used to define the BrdU negative population. Cells were processed and stained using labeling and detection kits BrdU (BD Biosciences) using either fluorescein isothiocyanate (FITC) or APC-conjugated anti-BrdU antibodies.
TREC analysis
Adoptive transfer of congenic T cell–depleted bone marrow
Bone marrow from C57BL/6 male mice was T cell–depleted (TCD) using CD8- and Thy1.2-conjugated paramagnetic beads and Midi separation columns (Miltenyi Biotec, Auburn, CA). LSKs quantified using flow cytometry as c-kit–high (eBioscience), Lineage-negative (as above), IL7Rα-negative (eBioscience), Sca-1–positive (BD Biosciences). TCD bone marrow with 105 LSK was injected intravenously into congenic (Ly5.2) sham- or castrated male mice.
Thymic epithelial cell studies
Thymi from three 6-week-old male mice were minced with razor blade and dispersed by stirring vigorously for 30 minutes at 4°C in RPMI 1640 medium (Invitrogen).32 Thymi were digested at 37°C for 4 digestions of 10 mL digestion cocktail (0.027 Liberase Blendzyme 4; Roche Diagnostics, Indianapolis, IN) and 624 mg of DNaseI (tissue culture grade in 50 mL RPMI 1640 medium) with 15 minutes per digestion and agitation every 5 minutes. Digested fractions were removed and placed at 4°C, and 2 mL fetal calf serum was added to halt the enzymatic reaction. Sorting experiments followed the same procedure, with 6 thymi per group, and each reagent increased 2-fold. Digested solution was drawn through a 25-gauge needle.
Immunofluorescent staining and analysis of tissue sections
Thymi were frozen in OCT freezing medium (Tissue-Tek, Torrance, CA). Six-micrometer cryosections were cut on a cryotome and stored at −80°C. Sections were first labeled with blocking serum (donkey serum and 2.4G2) followed by rabbit anti-mouse polyclonal antibody against K5 (Covance Research Products, Princeton, NJ) and a FITC-conjugated anti–rabbit IgG secondary (Jackson ImmunoResearch Laboratories, West Grove, PA) and a rat anti–mouse polyclonal antibody against K8 (Institut Pasteur, Paris, France), followed by a Texas Red–conjugated anti–rat IgG secondary (Jackson ImmunoResearch Laboratories). Hoechst DNA dye (Lonza Walkersville, Walkersville, MD) was used for tissue gate. Sections were mounted with VectaShield mounting (Vector Laboratories). Analysis was done using an iCys laser scanning cytometer (Compucyte, Cambridge, MA) equipped with lasers emitting at 488, 594, and 405 nm (for excitation of FITC, Texas red, and Hoechst 33342, respectively). Sections were scanned in 746 × 100-μm sections at a 0.5-μm resolution, and the resulting images were assembled into a montage of the entire section. The resulting image data were expressed cytometrically as dot plots for FITC-K5 versus Texas Red-K8 fluorescence, and gates were drawn to determine the percentage area of K5 versus K8 in square micrometers (using Hoechst 33342 area to define the total thymus area).
Semiquantitative and quantitative reverse transcriptase-PCR
TEC were spun down for 5 minutes at 1000g and resuspended in RNAlater (Qiagen, Valencia, CA). Total RNA was prepared using an RNeasy mini RNA isolation kit (Qiagen). cDNA was prepared using the SuperScript III reverse transcriptase kit (Invitrogen). Semiquantitative PCR was performed as described previously,33 except that conditions were optimized to generate PCR products in a linear range. DNA imaging and quantitation were performed using a Gel Logic 2200 imaging system with Kodak molecular imaging software (Carestream Health, Rochester, NY). Quantitative PCR was performed on the LightCycler 480 using the monocolor hydrolysis probe protocol (Roche).
Western blot
Cells in suspension were centrifuged, homogenized through a 23-gauge needle, and lysed at 20 × 106 cells/mL for 30 minutes on ice in radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA) and clarified by centrifugation at 13 000 rpm for 15 minutes at 4°C. Whole thymi were disrupted with a razor blade, homogenized through a 23 gauge needle, lysed, and clarified at 1 mL/thymus as suspension cells. Supernatant was harvested and diluted 1:1 with 2× SDS protein gel loading solution (Quality Biological, Gaithersburg, MD). Samples were normalized to thymus weight and electrophoresed (Protean II; Bio-Rad Laboratories, Hercules, CA) on a 12% SDS-polyacrylamide gel and transferred using a Trans-blot semi-dry transfer cell (Bio-Rad) to polyvinylidene difluoride membrane (GE Healthcare, Chalfont St Giles, United Kingdom). Membranes were blocked with 10% nonfat dry milk (Bio-Rad) for 1 hour at room temperature and incubated with primary antibodies (actin C11-horseradish peroxidase [HRP; Santa Cruz Biotechnology]; mouse anti–human CCR ligand 25 (CCL25)/thymus-expressed chemokine (TECK) antibody (R&D Systems, Minneapolis, MN); K8-rat anti–mouse polyclonal antibody (Institut Pasteur) overnight at 4°C. Membranes were washed in Tris-buffered saline-Tween 20 and incubated with appropriate HRP-labeled secondary antibodies (rabbit anti–goat-HRP [R&D Systems], donkey anti–rat HRP [Jackson ImmunoResearch], anti-rabbit HRP, Cell Signaling Technology, Danvers, MA). After additional washing, membranes were incubated in Supersignal West Pico chemiluminescent substrate (Pierce, Rockford, IL) and exposed to Enhanced ChemiLuminescence (ECL)–sensitive film (Hyperfilm; GE Healthcare).
Administration of CCL25 neutralizing antibody
C57BL/6 male mice, 2-5 months old, were injected intraperitoneally with 100 μg of CCL25-Teck (goat; R&D Systems) or a control antibody of affinity-purified anti-Human IgG (goat; Rockland Immunochemicals, Gilbertsville, PA) on the day of castration or sham castration and 50 μg of the same antibody on postoperative days 3 and 5 for the early blockade of CCL25 with animals killed on day 8. Late blockade of CCL25 was accomplished using 100 μg of TECK (goat; R&D Systems) or control antibody (Rockland) on postoperative days 4 and 6 with sacrifice on day 8. Near-continuous blockade of CCL25 was accomplished by giving 100 μg of TECK (goat or rat; R&D Systems) or control antibody (Rockland) postoperative days −1, 5, and 6 with sacrifice on day 8.
Statistical analysis
Statistical analysis was performed using Statview 5.0.1 software (SAS Institute, Cary, NC). All studies were analyzed using unpaired Student t test.
Results
Androgen withdrawal increases thymic size and major thymic subsets
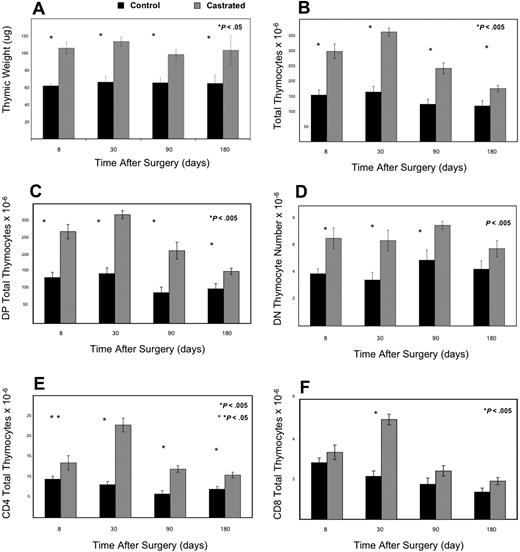
First, we investigated whether thymic enlargement in castrated male mice represented enhanced thymopoiesis in our model, consistent with prior studies. Comparing castrated mice with age-matched control littermates, there was a statistically significant doubling of thymic weight, thymocyte number, (DN) CD4−CD8−, and double-positive (DP) CD4+CD8+ thymocytes as early as 8 days after orchiectomy (Figure 1A-D). By 1 month, single positive thymocytes CD4+ and CD8+ were also significantly increased (Figure 1E,F).
Androgen withdrawal enhances thymopoeisis as evidenced by increased thymic size, weight, and thymocyte subsets with initial increases in DN and DP and subsequent increases in mature SP populations. Panel A demonstrates that thymic weight increased significantly by 1 week after castration and was maintained for 6 months postoperatively (> 8 mice/group). Panels B-E represent absolute thymocyte counts from 6- to 8-week-old C57BL/6 male mice at various time points after castration or sham castration (6-12 mice per group). (B) Total thymocyte number dramatically rose as well within 1 week after castration and persisted for 3 months before signs of involution. Double-positive (C) and double-negative (D) thymocyte total number were significantly increased by 1 week after castration (P < .005). CD4 (E) and CD8 (F) total thymocyte number rose dramatically by one month (later than the dramatic increase in immature subsets; P < .005).
Androgen withdrawal enhances thymopoeisis as evidenced by increased thymic size, weight, and thymocyte subsets with initial increases in DN and DP and subsequent increases in mature SP populations. Panel A demonstrates that thymic weight increased significantly by 1 week after castration and was maintained for 6 months postoperatively (> 8 mice/group). Panels B-E represent absolute thymocyte counts from 6- to 8-week-old C57BL/6 male mice at various time points after castration or sham castration (6-12 mice per group). (B) Total thymocyte number dramatically rose as well within 1 week after castration and persisted for 3 months before signs of involution. Double-positive (C) and double-negative (D) thymocyte total number were significantly increased by 1 week after castration (P < .005). CD4 (E) and CD8 (F) total thymocyte number rose dramatically by one month (later than the dramatic increase in immature subsets; P < .005).
Androgen withdrawal enhances thymic activity
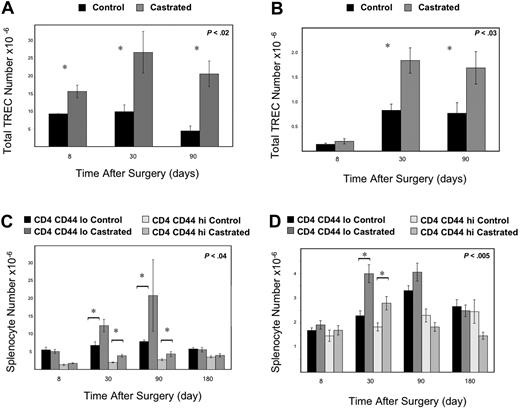
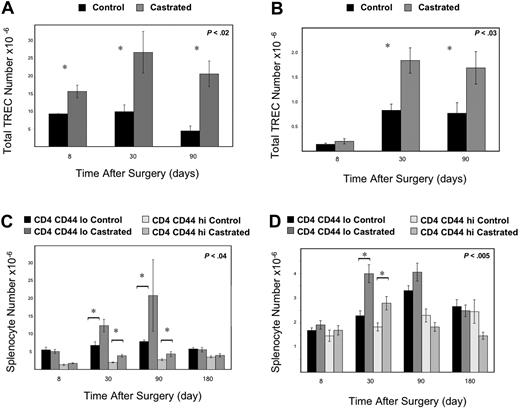
To assess whether increased thymic size and thymocyte number indicated increased thymic activity and productivity, we enumerated TREC (a molecular marker of thymic function) in the thymus and periphery. Augmented thymopoiesis was confirmed by increases in thymic TREC by 1.7-fold at 1 week, 2.7-fold at 1 month, and 4.6-fold at 3 months (Figure 2A). This increase in thymic activity led to a subsequent increase in peripheral cell populations, with the greatest proportional increase in naive CD44loCD4+, and CD8+ T cells in the spleen (Figure 2C,D). Splenic RTE were also increased as enumerated by TREC (Figure 2B).
Androgen withdrawal leads to enhanced thymic activity with increased thymic TREC, splenic subsets, and splenic TREC. Mice were killed at 8 days, 1 month, and 3 months after castration, and thymic (A) and splenic TREC (B) was enumerated by real-time PCR (6-12 mice/group). (A) By 1 week after castration, thymic TREC was increased (P < .02) and persisted for 3 months compared with sham-castrated control mice. (B) One month after androgen withdrawal, enhanced thymic activity was reflected in increased splenic TREC (P < .03). Splenic subsets demonstrated increased CD4+ (C) and CD8+ (D) subsets 1 month after androgen withdrawal compared with control mice (6-12 mice/group). Persistent increase in CD4+ splenocytes was seen at 3 months after castration as well.
Androgen withdrawal leads to enhanced thymic activity with increased thymic TREC, splenic subsets, and splenic TREC. Mice were killed at 8 days, 1 month, and 3 months after castration, and thymic (A) and splenic TREC (B) was enumerated by real-time PCR (6-12 mice/group). (A) By 1 week after castration, thymic TREC was increased (P < .02) and persisted for 3 months compared with sham-castrated control mice. (B) One month after androgen withdrawal, enhanced thymic activity was reflected in increased splenic TREC (P < .03). Splenic subsets demonstrated increased CD4+ (C) and CD8+ (D) subsets 1 month after androgen withdrawal compared with control mice (6-12 mice/group). Persistent increase in CD4+ splenocytes was seen at 3 months after castration as well.
Androgen withdrawal increases thymic epithelial cell proliferation
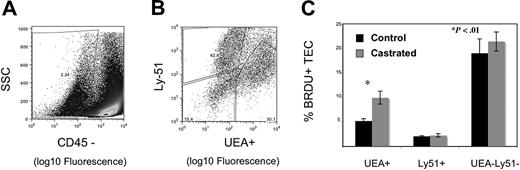
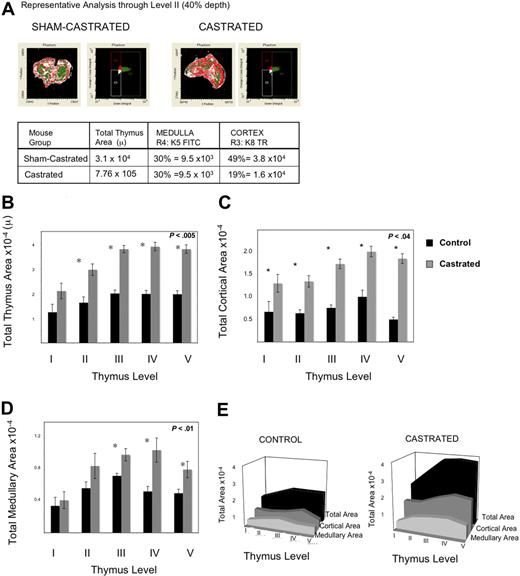
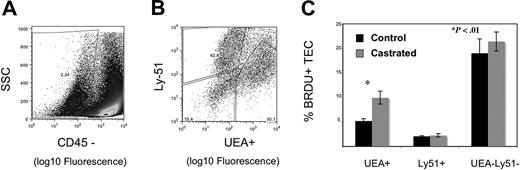
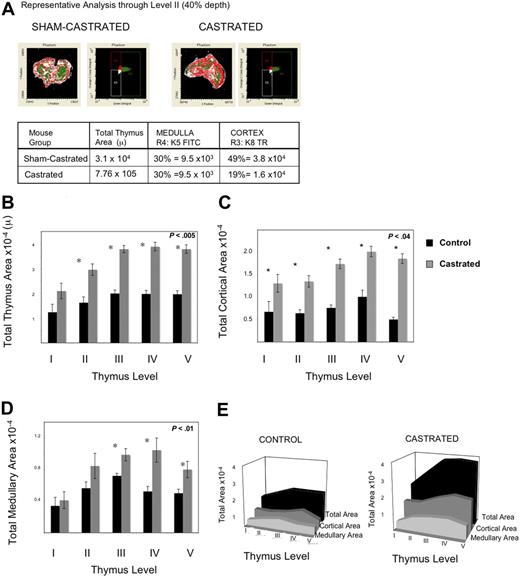
Having observed enhanced thymic activity, we postulated that TECs were proliferating, thereby providing a larger niche for developing immature thymocytes. We explored this hypothesis in 2 ways: by measuring BrdU incorporation by TEC and by quantifying cortical and medullary epithelial cell area using laser scanning cytometry (LSC) of frozen thymic sections. Because TEC proliferate slowly, mice were injected and then fed continuous BrdU to permit enough incorporation for reproducible measurements. By 1 week after castration, UEA+ medullary epithelial cell proliferation was significantly greater, as measured by the frequency of BrdU incorporation (Figure 3C). Because we observed an increase in proliferation of TEC, the total TEC were evaluated for evidence of increased cell number by LSC. Because of the variable quantity of medullary or cortical area at different depths through the frozen thymi (eg, anterior or posterior aspects), the thymi were frozen in the same orientation for each experiment, and slides were cut from the anterior aspect to the point at which 2 separate lobes were visible to the naked eye. The total number of slides for each thymus was divided into 5 levels (1, 20%; 2, 40%; 3, 60%; 4, 80%; and 5, 100%); 100% depth was the point at which the 2 lobes separated. Two slides around the midpoint of each level were stained; 4 were from level 3 (because this spanned 2 experimental days) to verify consistency. Epithelial cell area quantified histologically was used to assess cortical and medullary TEC cell number and niche size after castration. Beyond 3 weeks after castration, epithelial cell area was significantly increased, consistent with geographic TEC expansion after androgen withdrawal (Figure 4B-E). Thus, these data reveal that (1) UEA+ medullary TEC are proliferating at a much higher rate than other TEC populations examined after castration and (2) both cortical and medullary TEC number and thymic size are subsequently increased.
Androgen withdrawal increases UEA+ thymic epithelial cell proliferation. Six-week-old male mice were treated with 2 mg of BrdU i.p. on day 1 and 0.08% BrdU p.o. continuously for 7 days after castration or sham castration. Data represent 5 experiments with 3 thymi pooled per group for digestion. TEC populations were enumerated by flow cytometry, with medullary TEC defined as CD45− UEA+, cortical TEC as CD45− Ly51+, and a double-negative population of CD45− UEA− Ly51−. Representative flow cytometry plots are shown for gating of CD45 negative population (A) and discrimination of medullary and cortical populations within the CD45− population (B). BrdU-negative populations were defined using a noncastrated control group that underwent TEC preparation and were stained with BrdU antibody but did not receive exogenous BrdU. (C) Graph represents the mean frequency of BrdU + populations with standard error bars.
Androgen withdrawal increases UEA+ thymic epithelial cell proliferation. Six-week-old male mice were treated with 2 mg of BrdU i.p. on day 1 and 0.08% BrdU p.o. continuously for 7 days after castration or sham castration. Data represent 5 experiments with 3 thymi pooled per group for digestion. TEC populations were enumerated by flow cytometry, with medullary TEC defined as CD45− UEA+, cortical TEC as CD45− Ly51+, and a double-negative population of CD45− UEA− Ly51−. Representative flow cytometry plots are shown for gating of CD45 negative population (A) and discrimination of medullary and cortical populations within the CD45− population (B). BrdU-negative populations were defined using a noncastrated control group that underwent TEC preparation and were stained with BrdU antibody but did not receive exogenous BrdU. (C) Graph represents the mean frequency of BrdU + populations with standard error bars.
Androgen withdrawal increases medullary and cortical epithelial cell area 3 weeks after castration. Thymi were removed 3 weeks after castration or sham castration from 6 to 7 mice per group. Immunohistochemistry analysis of frozen sections using laser scanning cytometry was performed on 5 levels for each thymus, 2 or more slides per level, a minimum of 10 sections per mouse, 6 mice per group, were analyzed. Thus, data represent average area of 12 to 21 slides per level. (A) Representative laser scanning cytometry pseudoplot of K8+ cortical area (Texas Red) and K5+ medullary area (FITC) for sham-castrated thymus (left) and castrated thymus (right). To the right of the pseudoimage, the corresponding dot-plot for FITC-K5 versus Texas Red-K8 fluorescence is shown. The table below summarizes the calculations of medullary and cortical areas using this method. (B) Graph of total thymus area for each level. (C) Graph of K8+ cortical epithelial area for each level. (D) Graph of K5+ medullary epithelial area per level. (E) Three-dimensional representation of area across levels for total area, K8+ cortical area, K5+ medullary area. All graphs include standard error bars.
Androgen withdrawal increases medullary and cortical epithelial cell area 3 weeks after castration. Thymi were removed 3 weeks after castration or sham castration from 6 to 7 mice per group. Immunohistochemistry analysis of frozen sections using laser scanning cytometry was performed on 5 levels for each thymus, 2 or more slides per level, a minimum of 10 sections per mouse, 6 mice per group, were analyzed. Thus, data represent average area of 12 to 21 slides per level. (A) Representative laser scanning cytometry pseudoplot of K8+ cortical area (Texas Red) and K5+ medullary area (FITC) for sham-castrated thymus (left) and castrated thymus (right). To the right of the pseudoimage, the corresponding dot-plot for FITC-K5 versus Texas Red-K8 fluorescence is shown. The table below summarizes the calculations of medullary and cortical areas using this method. (B) Graph of total thymus area for each level. (C) Graph of K8+ cortical epithelial area for each level. (D) Graph of K5+ medullary epithelial area per level. (E) Three-dimensional representation of area across levels for total area, K8+ cortical area, K5+ medullary area. All graphs include standard error bars.
Androgen withdrawal increases thymic ETP
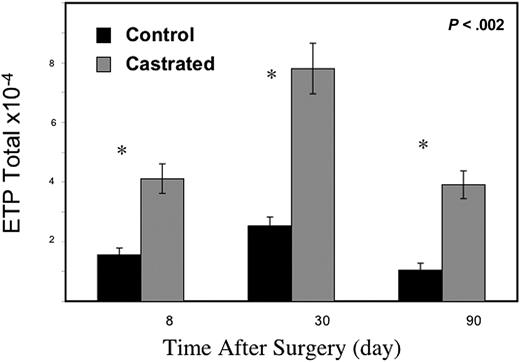
We next hypothesized that greater TEC niches would lead to enhanced uptake of ETP and enumerated this thymic subset to evaluate this hypothesis. As early as 8 days after castration, ETPs (Lin− c-KIThi CD44hi IL7Rα−) were increased in the thymus of the castrated cohort (Figure 5). Increased ETPs were evident for at least 3 months after androgen ablation.
Androgen withdrawal enriches ETP population within the thymus. By 1 week after castration, ETP numbers were significantly elevated within the thymus compared with sham-castrated control mice (6-12 mice/group) with standard error bars.
Androgen withdrawal enriches ETP population within the thymus. By 1 week after castration, ETP numbers were significantly elevated within the thymus compared with sham-castrated control mice (6-12 mice/group) with standard error bars.
Androgen withdrawal enhances ETP entry
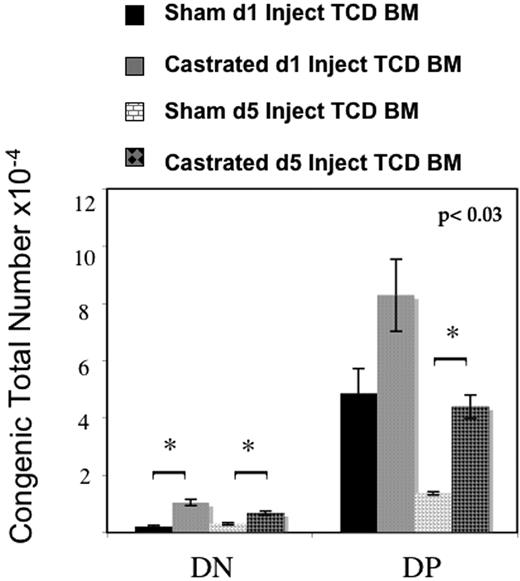
These findings raised the question of whether ETP entry was enhanced after androgen withdrawal or whether the thymic ETP pool was increased because of altered intrathymic ETP dynamics (either by diminished progression to DN1 or increased intrathymic ETP proliferation). To answer this question, congenic T cell–depleted bone marrow (TCD BM) was adoptively transferred into sham-castrated or castrated male mice. Congenic double negative thymocytes were significantly increased in castrated mice when TCD BM was administered on either the day of surgery (day 1) or 4 days later (day 5) (Figure 6). In addition, in mice injected on day 5 and killed 13 days after injection, with less time for intrathymic expansion, there was a significant increase in congenic double-positive thymocytes as well. These data support a mechanism of enhanced uptake of ETP into the thymus. Furthermore, these data suggest that androgen withdrawal may also accelerate development because of the significant and rapid increase in congenic DP in just 13 days, the minimum of time required for DP development. Because the number of rare congenic DP was consistent between mice, this increase was more likely to be due to improved precursor entry and accelerated development, rather than intrathymic expansion of few congenic ETP that should have resulted in greater variability as observed in the mice injected earlier with greater time for intrathymic expansion of subsets.
ETP immigration increases after androgen withdrawal. Six- to 8-week old male mice were castrated or sham-castrated followed by intravenous injection of congenic T cell–depleted marrow on the day of operation or 4 days later. All mice were killed 3 weeks after procedure. Castrated mice displayed significantly more congenic DN cells at both time points and significantly more DP at the later adoptive transfer time point, demonstrating enhanced ETP uptake in castrated mice (6-8 mice per group per time point, standard error bars shown).
ETP immigration increases after androgen withdrawal. Six- to 8-week old male mice were castrated or sham-castrated followed by intravenous injection of congenic T cell–depleted marrow on the day of operation or 4 days later. All mice were killed 3 weeks after procedure. Castrated mice displayed significantly more congenic DN cells at both time points and significantly more DP at the later adoptive transfer time point, demonstrating enhanced ETP uptake in castrated mice (6-8 mice per group per time point, standard error bars shown).
Androgen withdrawal increases thymic niche and enhances thymopoiesis through CCL25 production and accelerated DN maturation
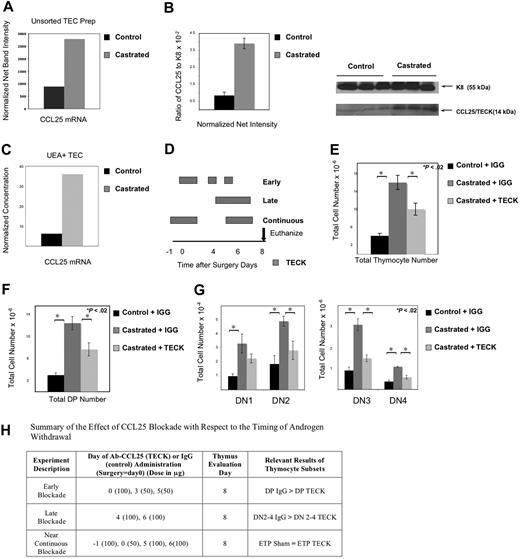
Having observed both an expansion of TEC and enhanced ETP entry, we then investigated whether TEC function might be modulated as well and might be involved in improved ETP entry and DN maturation. We therefore undertook studies to evaluate CCL25, a TEC-produced molecule important for postnatal ETP entry and DN migration. CCL25 mRNA expression was indeed increased in TEC digestions of castrated thymi (Figure 7A). Increases in CCL25 protein were observed by Western blot analysis of whole thymus lysates as well (Figure 7B). TEC subsets were subsequently isolated to determine which population was responsible for CCL25 production. Measurement of gene expression revealed the greatest increase in CCL25 mRNA production was by UEA+ medullary epithelial cells in castrated thymi (Figure 7C).
Androgen withdrawal increases CCL25 production from UEA+ medullary TEC; CCL25 production is critical for thymic expansion after castration. Thymi were removed from 3 mice per group and TEC digestion performed to isolate epithelial populations for mRNA analysis. Whole thymus lysates from 7 mice in each group was used for protein studies. (A) RT-PCR demonstrated increased CCL25 production in castrated mice 2 weeks after castration (data representative of 3 separate experiments with confirmation of similar TEC enrichment by flow cytometry). (B) Western blot confirmed enhanced CCL25 protein production after castration in whole thymus lysates using Keratin 8 to normalize epithelial cell numbers (graph includes normalized data from 3 mice in each group with standard error bars; CCL25 protein was also increased compared with actin, data not shown; data are representative of 2 separate experiments, 6-7 thymi per group total). These images were developed with a Kodak GelLogic 2200 camera using Kodak Molecular Imaging Software 4.5.1SE. (C) Sorted epithelial populations (from 6 thymi per group) confirmed the greatest increase in CCL25 production was in the UEA+ TEC of the castrated cohort (data representative of 2 sorted experiments) using quantitative RT-PCR. (D) Figure displays the timing of CCL25 antibody (TECK) or control IgG antibody with respect to the timing of surgery (day 0) or euthanasia (day 8) of the 3 experimental groups (early, late, and near-continuous). Small boxes (in the early group) represent the smaller (50 μg) dose, larger boxes represent the larger (100 μg) dose and may overlap days to reflect the anticipated increase in circulating antibody levels over this time frame. Thymocyte (E) and DP (F) increase after castration is abrogated in the presence of neutralizing antibody to CCL25 (summary of 4-5 mice/group with SE bars, mice received 100 μg of control IgG or CCL25 antibody (TECK) on the day of surgery, and 50 μg of antibody 3 and 5 days after surgery; killed 1 week after castration). (G) DN 2-4 increases after castration were abrogated in the presence of TECK antibody after administration of 100 μg on days 4 and 6 after surgery compared with IgG antibody with castration (summary of 5-6 mice/group with SE bars killed 1 week after castration). (H) Summary of the effect of the timing of CCL25 blockade with respect to the timing of androgen withdrawal.
Androgen withdrawal increases CCL25 production from UEA+ medullary TEC; CCL25 production is critical for thymic expansion after castration. Thymi were removed from 3 mice per group and TEC digestion performed to isolate epithelial populations for mRNA analysis. Whole thymus lysates from 7 mice in each group was used for protein studies. (A) RT-PCR demonstrated increased CCL25 production in castrated mice 2 weeks after castration (data representative of 3 separate experiments with confirmation of similar TEC enrichment by flow cytometry). (B) Western blot confirmed enhanced CCL25 protein production after castration in whole thymus lysates using Keratin 8 to normalize epithelial cell numbers (graph includes normalized data from 3 mice in each group with standard error bars; CCL25 protein was also increased compared with actin, data not shown; data are representative of 2 separate experiments, 6-7 thymi per group total). These images were developed with a Kodak GelLogic 2200 camera using Kodak Molecular Imaging Software 4.5.1SE. (C) Sorted epithelial populations (from 6 thymi per group) confirmed the greatest increase in CCL25 production was in the UEA+ TEC of the castrated cohort (data representative of 2 sorted experiments) using quantitative RT-PCR. (D) Figure displays the timing of CCL25 antibody (TECK) or control IgG antibody with respect to the timing of surgery (day 0) or euthanasia (day 8) of the 3 experimental groups (early, late, and near-continuous). Small boxes (in the early group) represent the smaller (50 μg) dose, larger boxes represent the larger (100 μg) dose and may overlap days to reflect the anticipated increase in circulating antibody levels over this time frame. Thymocyte (E) and DP (F) increase after castration is abrogated in the presence of neutralizing antibody to CCL25 (summary of 4-5 mice/group with SE bars, mice received 100 μg of control IgG or CCL25 antibody (TECK) on the day of surgery, and 50 μg of antibody 3 and 5 days after surgery; killed 1 week after castration). (G) DN 2-4 increases after castration were abrogated in the presence of TECK antibody after administration of 100 μg on days 4 and 6 after surgery compared with IgG antibody with castration (summary of 5-6 mice/group with SE bars killed 1 week after castration). (H) Summary of the effect of the timing of CCL25 blockade with respect to the timing of androgen withdrawal.
Having demonstrated that UEA+ TEC increased CCL25 production after castration, we undertook studies to investigate whether CCL25 activity was a critical step in thymic renewal after androgen withdrawal. The importance of CCL25 was confirmed by CCL25 blockade using anti-CCL25 antibodies. After CCL25 blockade, the thymi from castrated mice were significantly smaller than those from control castrated mice given control antibody (Figure 7E). To separate the effect of CCL25 blockade on ETP immigration and intrathymic movement and maturation of DN, we undertook experiments to evaluate the effect of the timing of anti-CCL25 antibody administration on thymic subsets (Figure 7H). For these studies, all mice were killed on postoperative day 8, the earliest day that statistical increases in ETP were observed. Three protocols of CCL25 blockade were developed, administering antibody early, late, or in a near-continuous way with respect to the day 8 evaluation time point (Figure 7D). To evaluate the effect of CCL25 neutralization on DNs after castration, late blockade and sacrifice shortly after were performed. To test the effect of CCL25 neutralization on DPs after castration, early blockade was used. As expected, early administration of anti-CCL25 antibody after castration led to a statistically significant decrease in DPs (Figure 7F) compared with the control antibody with castration. We hypothesized that this occurred because increased CCL25 accelerates DN maturation based on its known role in early thymocyte migration within the thymus; thus, when CCL25 was neutralized from the initial time of castration, fewer DPs developed in the interim. In contrast, late CCL25 blockade after surgery with sacrifice 2 days later led to a significant decrease in DN 2-4 populations compared with the castrated mice given the control antibody (Figure 7G), without alteration in DPs between the castrated groups (data not shown). Because these studies suggested that CCL25 blockade could delay DN development, we theorized that ETP entry was unchanged in these 2 initial experiments because of the delay in transition from ETP to DN1 in the late blockade and the ability to up-regulate CCL25 production in the last few days of the early blockade. We therefore administered a near-continuous blockade of CCL25, after which ETP entry was impaired. The ETP number of anti-CCL25 antibody-treated animals with castration was equal to the sham-castrated cohort (data not shown), in contrast to earlier experiments where ETP numbers were consistently higher in castrated versus sham-castrated cohorts (Figure 5). Thus, these data suggest that after androgen withdrawal, UEA+ epithelial cells produce CCL25, which increases ETP uptake, accelerates DN maturation, and effectively provides a larger niche, resulting in overall enhanced thymopoiesis.
Discussion
Mechanisms to enhance thymopoiesis may benefit patients for whom thymic function is diminished as a result of age, AIDS, or cytotoxic injury from therapeutic regimens. For these mechanisms to be clinically beneficial, increased thymic size must reflect an increase in overall thymopoiesis with export of a diverse repertoire of naive T cells to the periphery. Androgen withdrawal is a known positive thymic regulator, resulting in an increase in thymic size, thymocyte number, and proliferation of early thymocyte subsets in both middle-aged and elderly male mice.21 Recently, it has been shown that androgen withdrawal leads to an increase in ETP number as well.13 These data suggest that androgen withdrawal leads to enhanced thymopoiesis with increases precursor ETP entry or ETP proliferation but do not provide evidence that these changes in ETP parameters are necessary to mediate the effects of androgen signaling on thymopoeisis.
We first generated evidence of enhanced thymopoeisis after castration in our experiments, as shown by an increase in intrathymic TREC by 1 week after castration. Overall enhanced thymopoiesis with increases in mature subsets occurred shortly thereafter. Furthermore, this enhanced thymic function resulted in accumulation of splenic RTE by 1 month after castration.
Because androgen signaling by TEC is essential for thymic renewal after androgen ablation,18 we hypothesized that TEC proliferation occurred to increase thymic niches. Consistent with the concept that TEC signals influence thymic size, expansion of thymi has been linked with TEC proliferation after keratinocyte growth factor34,35 in vivo and growth hormone36,37 in vitro. Castration has been shown to increase the Ly51− MHC IIhi epithelial cell population in older mice (10 months) by Ki67, increasing the levels of these medullary cells to that observed in younger mice.22 In these experiments, the cortical proportion of TEC fell concomitant with this increase, altering the ratio of cortical to medullary epithelia. These data were limited by a negative bead depletion of thymocytes before flow cytometric analysis, the use of Ki-67 that is found in all cells not in resting phase, and the absence of a positive marker for medullary identification. Our data confirm and provide further insight on this work, demonstrating a subset specific effect. We demonstrate increased proliferation of UEA+ medullary TEC after castration using BrdU (a more dynamic marker of proliferation) to define the proliferating population. Furthermore, greater cells were stained and collected to obviate the need for thymocyte depletion with possible alteration of the final product of TEC analyzed. Our data reveal that the UEA+ medullary TEC subset proliferates rapidly and significantly after androgen withdrawal in relatively young postpubertal mice. These data were surprising because the most immature thymocyte subsets (DN and DP), with the greatest changes early after androgen withdrawal, develop in the cortex. However, UEA+ TEC seem to be essential for initial thymocyte development and decrease with age.38-40 Thus, it is likely that these UEA+ TEC play a role in early thymocyte development. Furthermore, our data suggest that cortical cells are likely proliferating at a higher rate postcastration as well, albeit slower than the UEA+ TEC. Three weeks after castration, immunohistochemistry studies showed an increase in medullary (K8+) and cortical (K5+) TECs without an altered ratio of subsets. Because cortical epithelial cells proliferate at a much lower rate, 8-fold less than medullary in sham-castrated mice, it is possible that our BrdU studies were unable to detect the incremental increase in cortical TEC between castrated and sham mice in a short period of BrdU exposure. Nonetheless, the TEC seem to increase after castration, directing thymic growth, with the earliest changes in the UEA+ subset.
Having shown that TEC were increased consistent with enhanced thymic niche size, we hypothesized that ETP immigration was involved in thymic expansion after androgen withdrawal. By 1 week after castration, there was a statistically significant increase in thymic ETP, corroborating observations of others.13 However, it was previously unknown whether the increase in thymic ETP was due to intrathymic proliferation, decreased progression to DN1, or enhanced entry of circulating ETP. Using adoptively transferred congenic T cell–depleted BM into castrated and sham-castrated mice, we show that the accumulation of ETP is due to improved immigration. Castrated mice had an increase in congenic double negative thymocytes whether injected on the day of surgery or several days later. This suggests that enhanced uptake of ETP is a key regulatory point in the enhanced thymopoiesis after castration, and an early step in thymic renewal. Furthermore, castrated mice injected with congenic TCD BM at both time points had the same number of congenic double negative thymocytes evaluated 3 weeks after surgery, implicating ETP entry rather than intrathymic expansion of ETP. It is likely that the DPs injected on the same day as surgery are not increased significantly because there is great expansion occurring over these 21 days of development, variably incorporating the congenic cells to result in a broad range of total congenic DP at this time point. In contrast, the group injected later has less time for intrathymic expansion, leading to a more consistent level of congenic DP in this group. These data demonstrate that ETP immigration is an early regulatory point for thymic renewal after androgen withdrawal.
Our initial hypothesis was that proliferating TEC provided an anatomic niche for entering ETP. However, our new data suggest that CCL25 production by these expanding TEC is also important in controlling the ETP/DN developmental niche, expanding it after castration. We are the first to present data that CCL25 production is increased at the mRNA and protein levels after androgen withdrawal and that this is a critical regulatory step. Furthermore, using sorted TEC, we demonstrate that CCL25 production is increased most in the UEA+ medullary TEC, the same TEC population that rapidly proliferates after castration. Finally, we show that blockade of this molecule abrogates thymic expansion after castration, implicating this ligand in the mechanism of enhanced thymopoeisis after castration. We demonstrate that CCL25 production increases thymic niche after castration in 2 ways. First, blockade of this molecule blocks ETP uptake, leading to decreased ETP with near-continuous blockade. Second, however, CCL25 blockade reduces specific thymocyte populations in relation to when the blockade is begun, linking this molecule to the rate of thymocyte development. Our data suggest that CCL25 also accelerates DN maturation and that this role for CCL25 is critical to the mechanism of enhanced thymopoiesis after castration. This interpretation of the data also explains the apparent discordance between the published time line of thymocyte development kinetics, showing 13 days for DN to DP development, and our observed increase in the DP by 8 days after castration.41 When CCL25 is blocked early after castration, DP decreases profoundly at day 8 (Figure 7H). We hypothesize that this occurs because early DN and ETP entry were blocked diminishing cells available to progress to DP; however, expanding UEA+ TEC increased production of CCL25, restoring ETP and DN 2-4 to the elevated castrated numbers in the same experiment. In contrast, when CCL25 is blocked only near the time of sacrifice, DPs are unaffected (because early thymocytes have been accelerated into this developmental stage), but DN 2-4 are significantly decreased consistent with the notion that CCL25 blockade hinders their developmental advance just before sacrifice. Finally, with nearly continuous blockade of CCL25, ETP entrance is impaired, and the total number of ETP is equal to that of sham-castrated mice. Thus, our data suggest that CCL25 normally constrains thymopoiesis by restricting ETP uptake and limiting early thymocyte maturation thereby identifying CCL25 as a part of thymic regulation.
These data may have important clinical implications. Enriched donor LSK in the stem source may aid in thymic reconstitution by providing more ETP upon thymic renewal and increased CCL25 production. Studies have demonstrated enhanced thymic T cell reconstitution of irradiated hosts with increasing stem cells, supporting this hypothesis.30 In addition, an early expansion of UEA+ TEC may have potential clinical benefit in the setting of stem cell transplantation. If, as our data suggest, UEA+ medullary TEC proliferate more rapidly at baseline than Ly51+ cortical subsets, these medullary cells may be more susceptible to injury from cytoreductive regimens than the more quiescent cortical populations. This is supported by the observation that medullary TEC are preferentially reduced after radiation damage.42,43 Furthermore, because UEA+ TEC are important mediators of negative selection and tolerance through AIRE expression,44 loss of UEA + TEC may contribute to alloimmunity. In support of this hypothesis, emerging data also reveal that graft-versus-host disease is greater in the setting of impaired thymopoiesis.2,45-47 Studies also suggest that reduced intensity regimens may permit greater thymic renewal after transplant,48,49 presumably as a result of TEC preservation. In addition to a selective loss of UEA+ TEC, our data suggest that renewal of thymopoiesis may be impaired because of diminished CCL25 production and inability to mediate ETP uptake and augment early DN development, restricting niche availability. Prior data suggest that a TEC niche is critical for precursor entry and thymic renewal.50 Our data suggest that UEA+ TEC, through CCL25 production, may have an essential role in thymic recovery.
In summary, these data suggest a cohesive mechanism of enhanced thymopoiesis after androgen withdrawal and that CCL25 expression is essential in this mechanism and a critical point in regulation of thymopoiesis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank William Telford and Fran Hakim for their review of the manuscript.
Authorship
Contribution: K.M.W., P.J.L., C.V.B., J.W., E.Z., Y.C., and V.K. performed research; P.J.L., C.V.B., and K.M.W. analyzed the data; and K.M.W. and R.E.G. designed research and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kirsten Marie Williams, MD, Assistant Clinical Investigator, Pediatric Hematology-Oncology, Experimental Transplantation and Immunology Branch, National Cancer Institute, NIH, Building 10 CRC, Room 3-3288, 10 Center Drive, Bethesda, MD 20892; e-mail: williaki@mail.nih.gov.