Abstract
NK cell anti-tumor reactivity is governed by a balance of activating and inhibitory receptors including various TNF receptor (TNFR) family members. Here we report that human tumor cells release a soluble form of the TNF family member Glucocorticoid-Induced TNFR-Related Protein (GITR) ligand (sGITRL), which can be detected in cell culture supernatants. Tumor-derived sGITRL concentration-dependently reduced NK cell cytotoxicity and IFN-γ production, which could be overcome by neutralization of sGITRL using a GITR-Ig fusion protein. Although sGITRL did not induce apoptosis in NK cells, it diminished nuclear localized RelB, indicating that sGITRL negatively modulates NK cell NF-κB activity. Furthermore, we detected substantial levels of sGITRL in sera of patients with various malignancies, but not in healthy controls. Presence of sGITRL-containing patient serum in cocultures with tumor cells significantly reduced NK cell cytotoxicity and IFN-γ production, which could again be restored by neutralization of sGITRL. The strong correlation of tumor incidence and elevated sGITRL levels indicates that sGITRL is released from cancers in vivo, leading to impaired NK cell immunosurveillance of human tumors. Our data suggest that determination of sGITRL levels might be implemented as a tumor marker in patients, and GITRL neutralization may be used to improve immunotherapeutic strategies relying on NK cell reactivity.
Introduction
Since their discovery in 1975, natural killer (NK) cells are known as a central component of the cytotoxic lymphocyte compartment capable of lysing tumor cells without prior immune sensitization of the host.1,2 NK cell reactivity is guided by the principles of “missing-self” and “induced-self,” which implies that cells with a low or absent expression of major histocompatibility complex (MHC) class I (“missing-self”) and/or a stress-induced expression of ligands of activating NK receptors, such as, for example, NKG2D (“induced-self”), are preferentially recognized and eliminated by NK cells.3–6 Thus, the balance of various activating and inhibitory signals determines whether NK cell responses are initiated or not. The interaction of NK cells and tumor cells is further substantially influenced by the fact that various tumor-expressed ligands for NK cell receptors, such as NKG2D, are released as soluble forms from cancer cells.7–10 These mechanisms affect NK cell anti-tumor reactivity by reduction of ligand densities and distally modulating NK cell anti-tumor reactivity. Release in soluble form especially is a common characteristic of various members of the tumor necrosis factor (TNF) family with the intriguing feature that some of the soluble forms act as agonists whereas others act as antagonists.11 Because the transmembrane expression of most of the TNF family members indicates that they are meant to act locally, they may prove harmful when released under certain pathophysiologic conditions.11 In line, soluble forms of various TNF ligands have been shown to play an important role in the pathophysiology of autoimmune or malignant diseases by modulating differentiation, proliferation, activation, and death of immune effector cells, including NK cells.12
Over the recent years, the role of the TNF receptor (TNFR) family member Glucocorticoid-Induced TNF-Related Protein (GITR) and its ligand (GITRL) in the regulation of immune responses has received considerable interest: In mouse models, GITR has been implicated in the development of autoimmune diseases, graft-versus-host disease, and in the immune response against infectious pathogens.13,14 Furthermore, in murine tumor models, GITR modulation has been reported to influence animal survival and even lead to the eradication of tumors.15–19 However, available data suggest that GITR, like other immunoregulatory molecules including various NK cell receptors,3 may mediate different effects in mice and men,20 necessitating the study of GITR functions specifically in human anti-tumor immunity. In this study, we report that GITRL is released by tumor cells as a soluble form and address the effects of soluble GITRL (sGITRL) on NK cell–mediated anti-tumor immunity.
Methods
Patient sera
Blood samples from patients were obtained before therapy. Sera were frozen at −80°C until further analysis. All patients gave their written informed consent in accordance with the Declaration of Helsinki and the study was performed according to the guidelines of the local Ethics Committee.
Reagents
Recombinant human GITRL (rGITRL), anti-GITRL monoclonal antibody (mAb) clone 109101, mouse IgG1, human IgG1, and the polyclonal anti-GITRL antibody were from R&D Systems (Wiesbaden, Germany). The anti–mouse phycoerythrin (PE)-conjugate was from Jackson ImmunoResearch Laboratories (West Grove, PA). The anti-RelB antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were obtained from Sigma-Aldrich (St Louis, MO). The GITR fusion protein (human GITR with human IgG1 tail) was generated as previously described.21
Transfectants and cell lines
C1R transfectants were generated as previously described by electroporation (250 V/500 μF) using 15 μg of the vector RSV.5neo or RSV.5neo containing the GITRL open reading frame.21 The tumor cell lines NCCIT (germ cell), HCT116 (colon), SKMel (melanoma), and PC3 (prostate) were obtained internally at Eberhard Karls-University Tuebingen.
For the production of sGITRL-containing and control culture supernatants, C1R-GITRL transfectants and C1R-neo mock transfectants as control were grown in Iscove modified Dulbecco medium without any additives for 72 hours, then supernatants were collected, concentrated, and sGITRL levels were determined by enzyme-linked immunosorbent assay (ELISA) before addition in functional assays.
Preparation of NK cells
Polyclonal NK cells were generated by incubation of non–plastic-adherent peripheral blood mononuclear cells with irradiated RPMI 8866 feeder cells over 10 days as previously described.21 Alternatively, NK cells were isolated from peripheral blood by negative selection using NK cell Isolation Kit II and MACS columns (Miltenyi Biotec, Auburn, CA). Experiments were performed when purity of NK cells was more than 90% as determined by flow cytometry.
Flow cytometry
Tumor cells were incubated with anti-GITRL mAb or isotype control (both 10 μg/mL) followed by anti–mouse PE (1:100) as secondary reagent. Samples were analyzed on a FACScan (BD Biosciences, San Jose, CA).
Detection of sGITRL by ELISA
For the detection of GITRL in culture supernatants and sera, 96-well plates were coated with 100 μL of the polyclonal antibody at 2 μg/mL in phosphate-buffered saline (PBS), then blocked by addition of 100 μL 15% bovine serum albumin (BSA)–PBS and washed. Afterward, rGITRL as standard, culture supernatants (in some cases after concentration) or sera (after dilution 1:3 in 7.5% BSA-PBS) were added. After incubation, plates were washed, and 100 μL of the anti-GITRL mAb was added at 1 μg/mL in 7.5% BSA-PBS. Subsequently, plates were washed and anti–mouse IgG1-horseradish peroxidase (1:8000 in 3.5% BSA-PBS) was added. Then plates were washed and developed using the trimethoxybenzoate hydrochloride peroxidase substrate system (KPL, Gaithersburg, MD). The absorbance was measured at 450 nm.
Cytotoxicity assay
Cytotoxicity of NK cells was analyzed by a standard chromium release assay as previously described.21 Concentrated supernatants from C1R-neo or C1R-GITRL cells were added to NK cells at the indicated final concentrations of sGITRL before addition of target cells. Alternatively, GITRL-containing patient sera were used in the cytotoxicity assays. Where indicated, 10 ng/mL GITR-Ig or human IgG1 as control was added to sera/supernatants 30 minutes before addition to the NK cells.
Determination of IFN-γ
IFN-γ determination was performed by ELISA according to the manufacturer's instructions using OptEIA sets from BD PharMingen (San Diego, CA). Cytokine concentrations in supernatants are expressed as mean plus or minus SD of triplicates.
Determination of NF-κB modulation
Nuclear extracts of NK cells were prepared as described previously.21 Twenty-microgram nuclear extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membrane (Whatman Schleicher and Schuell, Kassel, Germany), and probed with RelB antibody. Bands were visualized by enhanced chemiluminescence staining (GE Healthcare, Little Chalfont, United Kingdom). Densitometric analysis of bands was performed using ImageJ v3.91 software (http://rsb.info.nih.gov/ij).
Results
Tumor cells spontaneously release a soluble form of GITRL
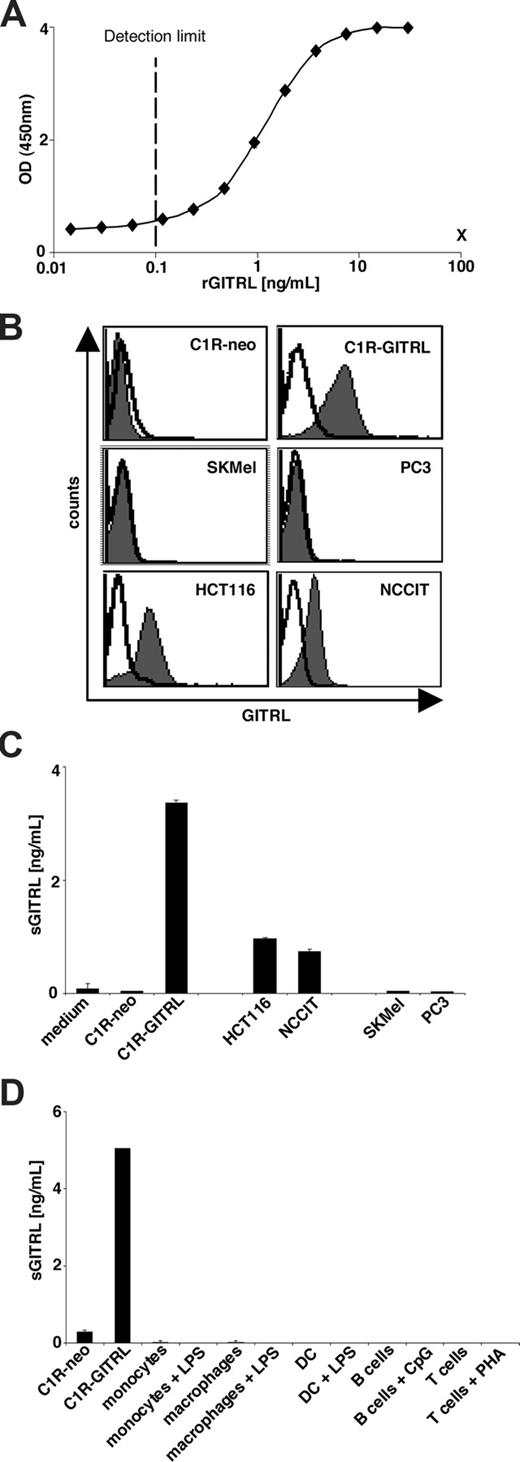
To study whether GITRL is released by tumor cells in soluble form, we established a highly sensitive sandwich ELISA using a polyclonal and a monoclonal GITRL antibody with rGITRL as standard. This ELISA detected rGITRL with a detection limit of approximately 0.1 ng/mL (Figure 1A). In contrast, only background signals were obtained with recombinant soluble MHC class I chain–related protein A at 100 ng/mL as control (Figure 1A). With this ELISA we analyzed culture supernatants from C1R-GITRL– and C1R–mock-transfectants (C1R-neo) as well as supernatants from GITRL-positive and -negative tumor cell lines (Figure 1B). After 48 hours of culture, substantial levels (3.37 ng/mL) of sGITRL were detected in supernatants of C1R-GITRL cells, while no sGITRL was found in supernatants of C1R-neo cells as control (Figure 1C). Furthermore, significant levels of sGITRL were detectable in supernatants of the GITRL-expressing tumor cell lines HCT116 and NCCIT (1.0 and 0.7 ng/mL, respectively), while no sGITRL was detected in supernatants of the GITRL-negative cell lines SKMel and PC3 (Figure 1C). Furthermore, we did not detect sGITRL in supernatants of resting or activated monocytes, macrophages, dendritic cells (DCs), B cells, and T cells (Figure 1D). Taken together, these results demonstrate that GITRL is spontaneously released as a soluble form from tumor cell lines expressing endogenous or transfected GITRL.
Tumor cells release a soluble form of GITRL. (A) Sandwich ELISA. Serial dilutions of rGITRL were analyzed using a polyclonal and a monoclonal anti-GITRL antibody followed by anti–mouse IgG1-horseradish peroxidase. X indicates 100 ng/mL MHC class I chain-related protein A as negative control. The means of 4 replicates are shown. (B) Cell surface expression of GITRL on the indicated tumor cell lines and transfectants was determined by FACS using anti-GITRL mAb (shaded peaks) with mouse IgG1 (open peaks) as isotype control followed by anti–mouse PE. (C) C1R-GITRL and C1R-neo transfectants and the indicated GITRL-positive or -negative tumor cell lines were cultured for 48 hours; then the supernatants were analyzed for the presence of sGITRL by ELISA. (D) In addition, supernatants from monocytes, macrophages, DCs, B cells, and T cells cultured in the absence or presence of LPS, CpG oligodeoxynucleotide, and PHA, with C1R transfectant supernatants as control were investigated. The data are means of triplicates with SD. One representative experiment each from a total of at least 3 with similar results is shown.
Tumor cells release a soluble form of GITRL. (A) Sandwich ELISA. Serial dilutions of rGITRL were analyzed using a polyclonal and a monoclonal anti-GITRL antibody followed by anti–mouse IgG1-horseradish peroxidase. X indicates 100 ng/mL MHC class I chain-related protein A as negative control. The means of 4 replicates are shown. (B) Cell surface expression of GITRL on the indicated tumor cell lines and transfectants was determined by FACS using anti-GITRL mAb (shaded peaks) with mouse IgG1 (open peaks) as isotype control followed by anti–mouse PE. (C) C1R-GITRL and C1R-neo transfectants and the indicated GITRL-positive or -negative tumor cell lines were cultured for 48 hours; then the supernatants were analyzed for the presence of sGITRL by ELISA. (D) In addition, supernatants from monocytes, macrophages, DCs, B cells, and T cells cultured in the absence or presence of LPS, CpG oligodeoxynucleotide, and PHA, with C1R transfectant supernatants as control were investigated. The data are means of triplicates with SD. One representative experiment each from a total of at least 3 with similar results is shown.
Tumor-derived sGITRL diminishes NK cell cytotoxicity
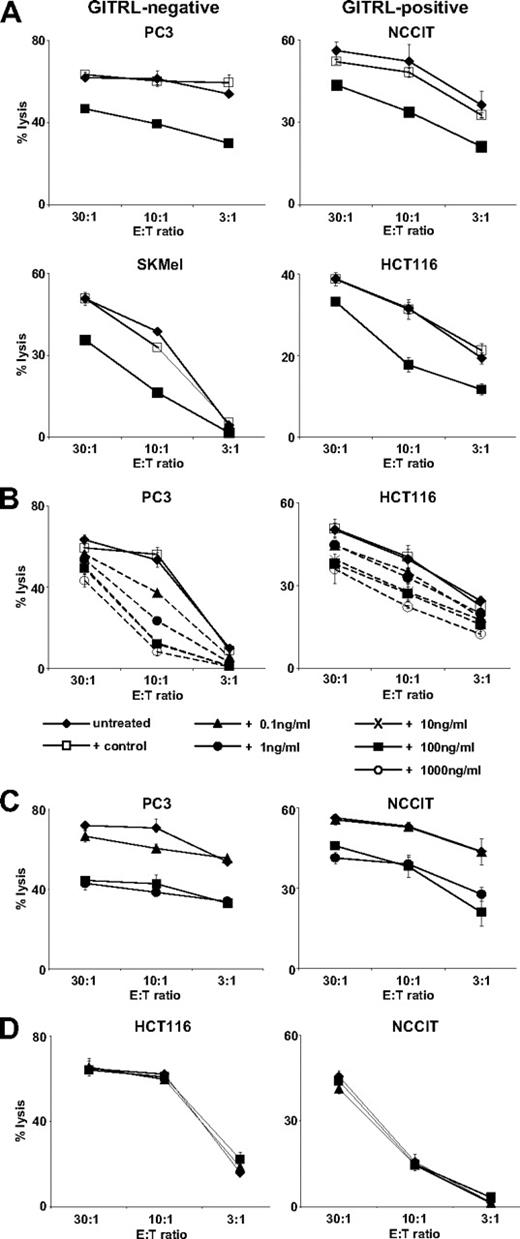
Next we wanted to determine how sGITRL influenced the lytic capacity of NK cells, which, as demonstrated recently, express GITR.21,22 Thus, we performed chromium release assays with various tumor cell lines in the absence or presence of 10 ng/mL sGITRL derived from C1R-GITRL transfectants using an equal volume of supernatant from C1R mock-transfectants as control. The presence of sGITRL caused a statistically significant (both P < .05, Mann-Whitney U test) reduction of NK cell cytotoxicity against the GITRL-negative tumor cell lines PC3 and SKMel (35% and 58%, respectively, at an effector-to-target (E:T) ratio of 10:1), while the control supernatant derived from C1R-neo cells did not substantially alter NK cell cytotoxicity (Figure 2A). A significant (both P < .05, Mann-Whitney U test) reduction of NK cell cytotoxicity in the presence of sGITRL was also observed when the tumor cell lines NCCIT and HCT116, which constitutively express GITRL on the cell surface, were used as targets (22% and 44% reduction by sGITRL, respectively, at an E:T ratio 10:1). To determine in which concentration sGITRL affected NK cell cytotoxicity, we added different amounts of sGITRL to cultures of tumor cell lines and NK cells before cytotoxicity assays. A concentration-dependent inhibition of the lysis of the GITRL-negative cell lines PC3 and SKMel as well as the cell-surface GITRL-positive cell lines HCT116 and NCCIT was observed, which in all cases reached statistical significance (all P < .05, Mann-Whitney U test, E:T ratio 10:1) with a concentration of 1 ng/mL or more. Again, the control supernatant corresponding in volume to the highest added amount of C1R-GITRL supernatant did not substantially alter NK cell cytotoxicity (Figure 2B; Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). To confirm that the reduction of NK cell cytotoxicity was indeed the result of sGITRL, we incubated sGITRL-containing (10 ng/mL) supernatants for 30 minutes in the absence or presence of 10 ng/mL of a GITR-Ig fusion protein with human IgG1 as isotype control before addition to the cocultures of NK cells with the tumor cells. The neutralization of sGITRL by GITR-Ig significantly (all P < .05, Mann-Whitney U test) diminished the inhibitory effect of the sGITRL-containing supernatants on NK cell cytotoxicity using both the GITRL-expressing and the GITRL-negative tumor cell lines, while addition of isotype control had no effect (Figures 2C, S1B). Taken together, these results demonstrate that tumor-derived sGITRL impairs cytotoxicity of NK cells.
Tumor-derived sGITRL down-regulates NK cell cytotoxicity. Cytotoxicity of NK cells was evaluated by chromium release assays with the indicated GITRL-positive or -negative tumor cells. (A) Cytotoxicity in the absence (diamonds) or presence of 10 ng/mL sGITRL derived from C1R-GITRL supernatants (filled squares) with an equal volume of C1R-neo supernatant as control (open squares). (B) Cytotoxicity in the absence or presence of the indicated concentrations of sGITRL derived from C1R-GITRL supernatants. As control, C1R-neo supernatant corresponding in volume to the highest concentration of C1R-GITRL supernatant was included. (C) Cytotoxicity assays in control medium (diamonds) or with 10 ng/mL sGITRL derived from C1R-GITRL supernatants in the absence (filled squares) or presence of 10 ng/mL human IgG1 (circles) or GITR-Ig fusion protein (triangles). (D) HCT116 and NCCIT tumor cells that express GITRL were cultured for 24 hours alone (diamonds) or on immobilized GITR-Ig (triangles) with human IgG1 (squares) as control to induce GITRL signaling. Subsequently cytotoxicity of NK cells was determined. Means of triplicates with SD of one representative experiment each of a total of at least 3 experiments with similar results are shown.
Tumor-derived sGITRL down-regulates NK cell cytotoxicity. Cytotoxicity of NK cells was evaluated by chromium release assays with the indicated GITRL-positive or -negative tumor cells. (A) Cytotoxicity in the absence (diamonds) or presence of 10 ng/mL sGITRL derived from C1R-GITRL supernatants (filled squares) with an equal volume of C1R-neo supernatant as control (open squares). (B) Cytotoxicity in the absence or presence of the indicated concentrations of sGITRL derived from C1R-GITRL supernatants. As control, C1R-neo supernatant corresponding in volume to the highest concentration of C1R-GITRL supernatant was included. (C) Cytotoxicity assays in control medium (diamonds) or with 10 ng/mL sGITRL derived from C1R-GITRL supernatants in the absence (filled squares) or presence of 10 ng/mL human IgG1 (circles) or GITR-Ig fusion protein (triangles). (D) HCT116 and NCCIT tumor cells that express GITRL were cultured for 24 hours alone (diamonds) or on immobilized GITR-Ig (triangles) with human IgG1 (squares) as control to induce GITRL signaling. Subsequently cytotoxicity of NK cells was determined. Means of triplicates with SD of one representative experiment each of a total of at least 3 experiments with similar results are shown.
Because GITRL signaling can induce alterations of adhesion and immunoregulatory molecules in tumor cells,21 we determined whether presence of GITR-Ig per se affected tumor cell susceptibility to NK cell lysis. The GITRL-expressing tumor cells HCT116 and NCCIT were cultured for 24 hours alone, on immobilized GITR-Ig or human IgG1 as control to induce GITRL signaling before cytotoxicity assays. No significant influence of this pretreatment on NK cell reactivity was observed, which indicates that alterations caused by GITR-Ig, which was used for neutralization of sGITRL, do not affect molecules critically determining NK cell cytotoxicity (Figure 2D).
Tumor-derived sGITRL impairs IFN-γ production of NK cells
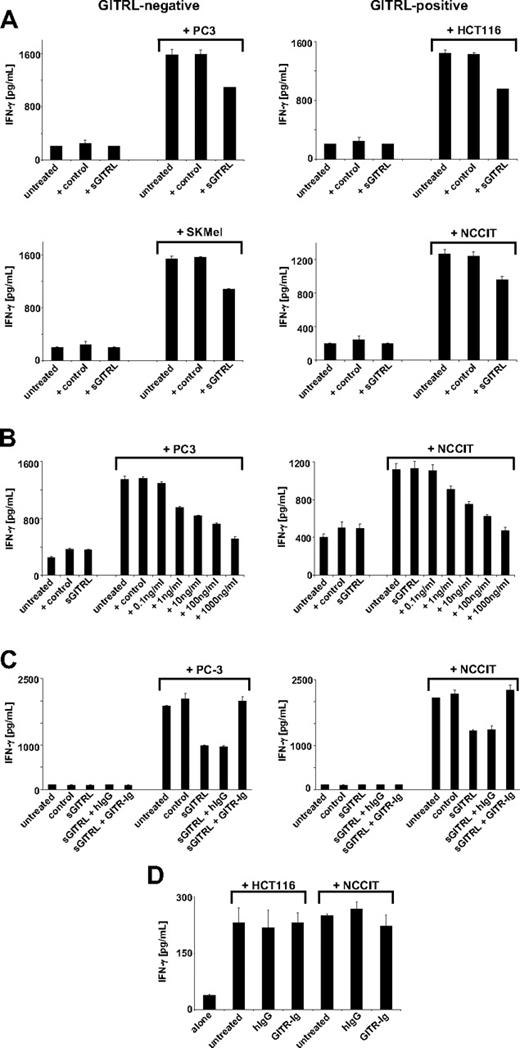
Release of IFN-γ is a second major mechanism by which NK cells participate in anti-tumor immunity. To analyze whether sGITRL also altered NK cell IFN-γ production, we cultured NK cells alone or together with GITRL-negative (PC3 and SKMel) as well as GITRL-positive (HCT116 and NCCIT) tumor cells for 24 hours in the presence of 10 ng/mL sGITRL derived from C1R-GITRL transfectants with an equal volume of C1R-neo supernatant as control. In the absence of tumor cells, NK cells produced low levels of IFN-γ, which were not markedly altered by the presence of sGITRL. The presence of tumor cells potently stimulated NK cell IFN-γ production, and this was significantly (all P < .05, Mann-Whitney U test) reduced by sGITRL. In line with the results obtained for cytotoxicity, the presence of sGITRL reduced NK cell reactivity in cultures with both GITRL-negative and -positive tumor cells (up to 34%), whereas the control supernatant had no effect (Figure 3A). Again, the inhibitory effect of sGITRL occurred in a concentration-dependent manner. The reduction of NK cell IFN-γ production in cocultures with both the GITRL-negative cell lines PC3 and SKMel as well as the GITRL-expressing cell lines NCCIT and HCT116 reached statistical significance (all P < .05, Mann-Whitney U test) with concentrations of 1 ng/mL upward. Again, no marked effect was observed with the control supernatant corresponding in volume to the highest amount of C1R-GITRL supernatant (Figures 3B, S1C).
Tumor-derived sGITRL diminishes IFN-γ production of NK cells. NK cells were incubated for 24 hours with or without the indicated GITRL-positive or -negative tumor cell lines; afterward, supernatants were harvested and analyzed for IFN-γ by ELISA. (A) Cultures in the absence or presence of 10 ng/mL sGITRL derived from C1R-GITRL supernatants with an equal volume of C1R-neo supernatant as control. (B) Cultures in the absence or presence of the indicated concentrations of sGITRL with C1R-neo supernatant corresponding in volume to the highest concentration of C1R-GITRL supernatant as control. (C) Cultures with or without 10 ng/mL sGITRL derived from C1R-GITRL supernatants in the absence or presence of 10 ng/mL human IgG1 or GITR-Ig fusion protein. (D) GITRL-positive HCT116 and NCCIT tumor cells were cultured for 24 hours alone or on immobilized GITR-Ig with human IgG1 as control to induce GITRL signaling before addition of NK cells. Means of triplicates and SD of one representative experiment each of a total of 4 experiments with similar results are shown.
Tumor-derived sGITRL diminishes IFN-γ production of NK cells. NK cells were incubated for 24 hours with or without the indicated GITRL-positive or -negative tumor cell lines; afterward, supernatants were harvested and analyzed for IFN-γ by ELISA. (A) Cultures in the absence or presence of 10 ng/mL sGITRL derived from C1R-GITRL supernatants with an equal volume of C1R-neo supernatant as control. (B) Cultures in the absence or presence of the indicated concentrations of sGITRL with C1R-neo supernatant corresponding in volume to the highest concentration of C1R-GITRL supernatant as control. (C) Cultures with or without 10 ng/mL sGITRL derived from C1R-GITRL supernatants in the absence or presence of 10 ng/mL human IgG1 or GITR-Ig fusion protein. (D) GITRL-positive HCT116 and NCCIT tumor cells were cultured for 24 hours alone or on immobilized GITR-Ig with human IgG1 as control to induce GITRL signaling before addition of NK cells. Means of triplicates and SD of one representative experiment each of a total of 4 experiments with similar results are shown.
To ascertain that neutralization of sGITRL also restored NK cell IFN-γ production, we used our approach of adding 10 ng/mL GITR-Ig or isotype control to the C1R-GITRL culture supernatant for 30 minutes before cocultures with the tumor cells. As observed for NK cell cytotoxicity, the GITR-Ig fusion protein neutralized the inhibitory effect of sGITRL on NK cell IFN-γ production (all P < .05, Mann-Whitney U test), whereas addition of isotype control had no effect (Figures 3C, S1D). This was again observed with both GITRL-expressing and GITRL-negative tumor cells. Thus, in addition to cytotoxicity, tumor-derived sGITRL also diminishes IFN-γ production of NK cells.
Again, it was important to ascertain that treatment of GITRL-expressing tumor cells with GITR-Ig did not cause cellular alterations affecting NK cell IFN-γ production. Thus, we cultured HCT116 and NCCIT cells for 24 hours alone, on immobilized GITR-Ig or on human IgG1 as control to induce GITRL signaling and subsequently performed cocultures with NK cells for 24 hours before determination of IFN-γ in culture supernatants by ELISA (Figure 3D). No marked effect of GITRL signaling in tumor cells on NK cell reactivity was observed, which is in line with the results obtained for cytotoxicity and indicates that GITR-Ig–induced alterations in tumor cells do not substantially affect NK cell reactivity.
sGITRL reduces NF-κB activity of NK cells
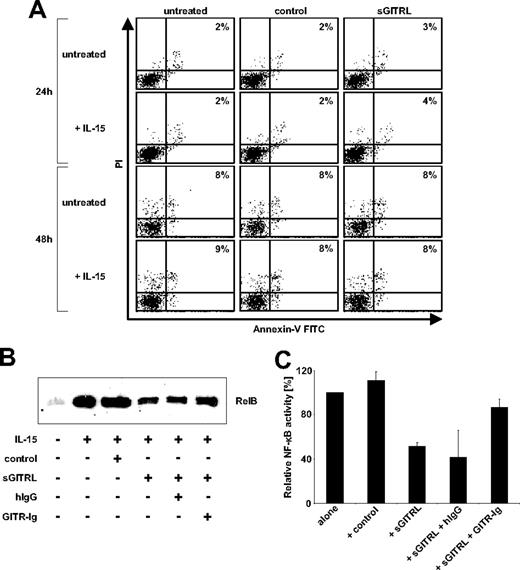
Because proapoptotic effects have been reported after GITR stimulation,23,24 we investigated whether induction of apoptosis by sGITRL was responsible for reduced NK cell reactivity. Unstimulated and IL-15 stimulated NK cells were incubated alone, in the presence of sGITRL (10 ng/mL) or the corresponding volume of control culture supernatant for 24 hours and 48 hours and subsequently the percentage of apoptotic NK cells was determined. Fluorescence-activated cell sorter (FACS) analysis using annexin V–fluorescein isothiocyanate/propidium iodide staining revealed that sGITRL did not induce substantial changes in the percentage of apoptotic NK cells (Figure 4A). In addition, NK cells were counted at the end of the assay. At neither time point did we observe relevant differences in the total cell number, thus excluding that apoptotic cells had disappeared during the culture (data not shown). These results indicate that the inhibitory effects of sGITRL are not a consequence of apoptosis induction in GITR-expressing NK cells.
Functional characterization of the effects of sGITRL on NK cell reactivity. (A) NK cells were cultured for 24 hours in the absence or presence of 10 ng/mL IL-15 and afterward incubated for an additional 24 hours or 48 hours with 10 ng/mL sGITRL derived from C1R-GITRL supernatants with an equal volume of C1R-neo supernatant as control. Subsequently, determination of apoptosis was performed by FACS using propidium iodide (PI) and annexin V–fluorescein isothiocyanate. The percentage of dead cells is indicated. One representative experiment each of a total of 3 experiments with similar results is shown. (B) NK cells were cultured for 24 hours with or without of 10 ng/mL IL-15 in the presence or absence of control supernatant or 10 ng/mL sGITRL. Where indicated, 10 ng/mL GITR-Ig or IgG1 had been added to sGITRL-containing supernatant. Subsequently, nuclear extracts were prepared and RelB protein was analyzed by Western blot. (C) Intensity of bands was densitometrically determined using ImageJ software, and results obtained with IL-15–stimulated NK cells were defined as 100%. Columns represent means with SD of 4 independent experiments.
Functional characterization of the effects of sGITRL on NK cell reactivity. (A) NK cells were cultured for 24 hours in the absence or presence of 10 ng/mL IL-15 and afterward incubated for an additional 24 hours or 48 hours with 10 ng/mL sGITRL derived from C1R-GITRL supernatants with an equal volume of C1R-neo supernatant as control. Subsequently, determination of apoptosis was performed by FACS using propidium iodide (PI) and annexin V–fluorescein isothiocyanate. The percentage of dead cells is indicated. One representative experiment each of a total of 3 experiments with similar results is shown. (B) NK cells were cultured for 24 hours with or without of 10 ng/mL IL-15 in the presence or absence of control supernatant or 10 ng/mL sGITRL. Where indicated, 10 ng/mL GITR-Ig or IgG1 had been added to sGITRL-containing supernatant. Subsequently, nuclear extracts were prepared and RelB protein was analyzed by Western blot. (C) Intensity of bands was densitometrically determined using ImageJ software, and results obtained with IL-15–stimulated NK cells were defined as 100%. Columns represent means with SD of 4 independent experiments.
Because GITR has been reported to alter the activation of the transcription factor NF-κB,25,26 we next investigated how sGITRL modulated NF-κB activity in NK cells. Resting NK cells were stimulated with IL-15 for 24 hours alone or in the presence of sGITRL-positive and -negative culture supernatant. In addition, where indicated, 10 ng/mL of GITR-Ig or isotype control was added to the cultures. Subsequently, Western blot analysis for RelB was performed in nuclear extracts and compared with the levels in resting NK cells. IL-15 potently stimulated NF-κB activity of NK cells, and a marked reduction of nuclear localized RelB was observed in the presence of sGITRL-positive, but not with sGITRL-negative culture supernatant. Presence of GITR-Ig, but not isotype control, partially restored the levels of RelB indicating that indeed sGITRL was responsible for the observed inhibition of NF-κB activity (Figure 4B). Densitometric analysis of the results of 4 independent experiments (Figure 4C) revealed that the inhibition of NK cell NF-κB activity by sGITRL was statistically significant, whereas the effect of GITR-Ig closely failed to reach statistical significance (Student t test, P < .05 and P = .065, respectively). Together, these results suggest that diminished NK cell reactivity in the presence of sGITRL might, at least in part, be mediated by inhibition of signals that activate NF-κB.
Next we wanted to confirm further that the effects of sGITRL resulted from triggering of GITR on NK cells and not to a potential influence on the tumor cells. Using FACS and quantitative polymerase chain reaction analysis, no relevant expression of GITR protein and mRNA was observed in PC3, SKMel, HCT116, and NCCIT tumor cells, whereas high levels of GITR mRNA were detectable in resting and activated NK cells as positive control (Figure S2A,B). Furthermore, we cultured all tumor cells used in our study alone, with sGITRL or control supernatant for 1 hour or 24 hours. Subsequently, the medium was exchanged and cytotoxicity assays with NK cells were performed. No significant differences in tumor cell lysis were observed, which further excludes that the effects of sGITRL and its neutralization depend on a modulation of tumor sensitivity to NK cell cytotoxicity (Figure S2C).
Elevated sGITRL levels are contained in sera of patients with cancers and hematologic malignancies
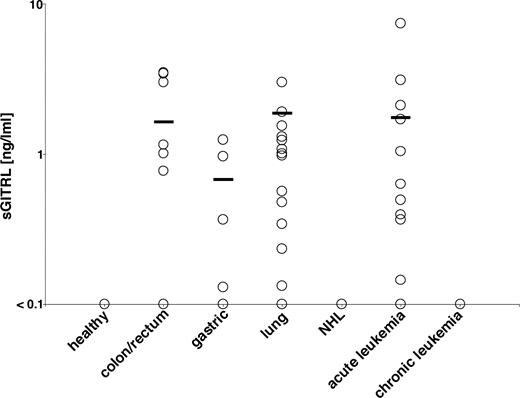
To determine whether release of GITRL by human tumors also occurred in vivo, we analyzed sGITRL levels in sera of patients with various malignancies with healthy volunteers as control. In the sera of the healthy volunteers (n = 8), no sGITRL levels above the detection limit of the ELISA were observed (Figure 5). In contrast, a significant proportion of a total of 60 investigated sera from patients with colon cancer, gastric cancer, and lung cancer contained elevated levels of sGITRL. Within the patients with colon cancers (n = 25), 56% revealed elevated levels of sGITRL above the detection limit of the ELISA with a mean of 1.62 ng/mL and a range from 0.77 to 3.47 ng/mL. Among the patients with gastric cancer (n = 8), 50% had elevated sGITRL serum levels with a mean of 0.67 ng/mL and a range from 0.13 to 1.24 ng/mL. In addition, 56% of the sera of the lung cancer patients (n = 27) contained substantial GITRL levels with a mean of 1.86 ng/mL and a range from 0.13 and 3.00 ng/mL. This strong correlation of tumor incidence and elevated sGITRL levels clearly suggest that GITRL is released at significant amounts from cancers in vivo. To extend our analyses to hematologic malignancies, we analyzed sera of patients with acute myeloid (AML) and lymphocytic leukemia (ALL), chronic myeloid leukemia, chronic lymphocytic leukemia, and non-Hodgkin lymphoma (NHL). We found that among the 25 investigated AML patients 40% had elevated levels of sGITRL (mean, 1.73 ng/mL; range, 0.15-7.37 ng/mL). Surprisingly, none of the patients with ALL (n = 6), chronic lymphocytic leukemia (n = 15), chronic myeloid leukemia (n = 1), low-grade (n = 7) or high-grade (n = 10) NHL displayed sGITRL levels above the detection limit of the ELISA. Although it remains unclear whether this is the result of a specific role of GITRL in AML pathophysiology or due to the relatively low number of cases with other hematologic malignancies, these data demonstrate that sGITRL may, at least in certain entities, also affect NK cell reactivity against hematologic malignancies.
sGITRL in sera of patients and healthy controls. Sera from healthy volunteers and from patients with the indicated cancers and hematologic malignancies were investigated by ELISA. The cutoff for sGITRL presence was at 0.1 ng/mL. Each symbol represents the mean of triplicates obtained by analysis of a single donor. The mean within a group is indicated by solid line.
sGITRL in sera of patients and healthy controls. Sera from healthy volunteers and from patients with the indicated cancers and hematologic malignancies were investigated by ELISA. The cutoff for sGITRL presence was at 0.1 ng/mL. Each symbol represents the mean of triplicates obtained by analysis of a single donor. The mean within a group is indicated by solid line.
Neutralization of sGITRL contained in cancer patient sera increases NK cell anti-tumor reactivity
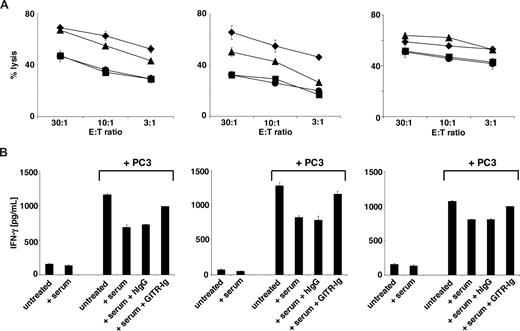
Because elevated levels of sGITRL were detectable in sera of a substantial proportion of cancer patients and sGITRL derived from tumor cell lines decreased NK cell anti-tumor reactivity, we next evaluated the effect of sGITRL-containing patient sera on NK cell effector functions. Thus, we performed cocultures of NK cells with PC3 tumor cells in the absence or presence of sGITRL-containing sera of patients with colon, lung, and gastric cancer (sGITRL levels 2.5, 3.0, and 1.0 ng/mL, respectively). The presence of GITRL-containing patient sera significantly (all P < .05, Mann-Whitney U test) diminished both cytotoxicity (up to ∼ 50% reduction) and IFN-γ production (up to ∼ 41% reduction) of NK cells. The levels of inhibition caused by the patient sera corresponded to the levels of sGITRL in the respective sera. Importantly, neutralization of sGITRL in patient sera with GITR-Ig before addition to the cocultures, in line with the results obtained with GITRL-containing cell culture supernatants, significantly increased and in some cases restored NK cell cytotoxicity and IFN-γ production (all P < .05, Mann-Whitney U test), whereas isotype control had no effect (Figure 6). This confirms the NK cell-suppressive effect of sGITRL derived from tumor cell culture supernatants and indicates that sGITRL-mediated reduction of NK cell cytotoxicity and IFN-γ production may be of relevance in cancer patients in vivo.
Effect of sGITRL contained in cancer patient sera on NK cell anti-tumor reactivity. (A) NK cells were incubated with PC3 tumor cells in the absence (diamonds) or presence of sGITRL-containing patient serum (squares). Where indicated, 10 ng/mL human IgG1 (circles) or GITR-Ig fusion protein (triangles) was added to patient serum 30 minutes before the cocultures. Cytotoxicity was analyzed by chromium release assays. (B) IFN-γ production of NK cells after 24 hours of culture alone or with PC3 cells in the absence or presence of patient serum and/or GITR-Ig with human IgG1 as control was determined by ELISA. (Left panels) Serum of a colon cancer patient. (Middle panels) Serum of lung cancer patient. (Right panels) Serum of a gastric cancer patient. Means of triplicates and SD of one representative experiment each of a total of at least 4 experiments with similar results are shown.
Effect of sGITRL contained in cancer patient sera on NK cell anti-tumor reactivity. (A) NK cells were incubated with PC3 tumor cells in the absence (diamonds) or presence of sGITRL-containing patient serum (squares). Where indicated, 10 ng/mL human IgG1 (circles) or GITR-Ig fusion protein (triangles) was added to patient serum 30 minutes before the cocultures. Cytotoxicity was analyzed by chromium release assays. (B) IFN-γ production of NK cells after 24 hours of culture alone or with PC3 cells in the absence or presence of patient serum and/or GITR-Ig with human IgG1 as control was determined by ELISA. (Left panels) Serum of a colon cancer patient. (Middle panels) Serum of lung cancer patient. (Right panels) Serum of a gastric cancer patient. Means of triplicates and SD of one representative experiment each of a total of at least 4 experiments with similar results are shown.
Discussion
Differentiation, proliferation, activation, and death of both tumor cells and immune effector cells including NK cells are substantially influenced by various members of the TNF/TNFR family.12 The TNFR family member GITR, also known as TNFRSF18, and its ligand (GITRL) have, in humans, independently been identified by 2 groups in 1999.27,28 Two years earlier, GITR was identified in mice.29 GITR is constitutively expressed at low levels on responder T cells and is up-regulated after activation and on regulatory T cells. In addition, GITR has been detected in B cells, mast cells, granulocytes, monocytes, macrophages, DCs, and more recently also on NK cells.13,21,22,24 Its cognate ligand has been detected in DCs, monocytes, macrophages, B cells, activated T cells, endothelial cells, osteoclasts, and various healthy nonlymphoid tissues.14 Very recently we demonstrated that GITRL is expressed by human cancers in vitro and in vivo.21
In this study, we demonstrate that GITRL-expressing tumor cell lines and GITRL-transfectants, but not GITRL-negative tumor cells, spontaneously release GITRL as a soluble form. Our observation that a high proportion of cancer patient sera, but not sera of healthy donors, contained significantly elevated levels of sGITRL clearly suggests that the release of sGITRL is not an artifact of in vitro tumor cell culture but rather a feature of human tumors. We also detected elevated levels of sGITRL in many sera of patients with AML, which is in line with our finding that AML cells can express GITRL (H.R.S. et al, unpublished data, 2008) and indicates that sGITRL may also affect NK cell reactivity against leukemia. Surprisingly, no sGITRL was detectable in the investigated sera of patients with other hematologic malignancies, such as ALL, chronic leukemia, and high- and low-grade NHL. Comprehensive analyses of larger numbers of patients are required to determine whether GITRL expression on tumor cells and presence in soluble form is relevant in the latter and other hematologic malignancies and solid tumors. Alternatively, it will be important to uncover whether and how GITRL plays a specific role in the pathophysiology of AML compared with other hematologic diseases. In this context, it is noteworthy that a recent study, in which sGITRL levels in healthy donors and atopic dermatitis patients were investigated, did not report increased sGITRL levels in the investigated patients.30 Because we did also not detect sGITRL in culture supernatants of resting and activated T cells, B cells, monocytes, macrophages, and DCs, GITRL may play a specific role in the pathophysiology of certain diseases. The mechanisms and differences underlying GITRL processing in healthy compared with malignant cells and/or between different tumor entities are yet unclear and are, together with the molecular characterization of sGITRL, the subject of a presently ongoing study.
The release of TNF family members as soluble forms can alter ligand densities on the cell surface and enables the soluble molecules to exert systemic functions. However, the soluble form of a certain TNF ligand may mediate similar, partially different, or quite the opposite effects compared with its surface-expressed counterpart. For example, removal of CD95/Fas ligand from the cell surface is thought to reduce its biologic activity by diminishing its ability to crosslink CD95.31 In contrast, cleavage of, for example, TNF, TRAIL, and 4-1BB ligand, the latter being closely related to GITRL, results in active soluble molecules that induce responses at sites distal to the producing cells.32–34 Because triggering GITR on NK cells by cell surface-expressed GITRL diminishes their effector functions,21 we investigated how GITRL in soluble form affected NK cell anti-tumor responses. We found that sGITRL concentration-dependently diminished both cytotoxicity and IFN-γ production of NK cells in cocultures with GITRL-negative as well as GITRL-positive cancer cells. Our data that the tumor cells used in this study did not express GITR and were not affected by pretreatment with sGITRL confirm that the effects of sGITRL on NK cell reactivity were indeed the result of triggering GITR on NK cells. The observed inhibitory effect of tumor-derived sGITRL on NK cell responses is in line with our results regarding the effect of its cell surface-expressed counterpart.21 Our data suggest that surface-expressed and soluble GITRL may mediate additive/synergistic effects and may cooperate to reduce NK cell reactivity, thus enabling the evasion of human tumor cells from NK cell–mediated immunosurveillance. Although our data are in line with another recent study, which described that GITR inhibits the reactivity of human NK cells,24 our findings are seemingly in contrast to available data from mouse models, where GITR mAb or injection of adenovirus expressing recombinant GITRL into tumors stimulated anti-tumor immunity. The effects of GITR modulation have, in these studies, been attributed to enhanced T-cell activity.15–19 However, GITR stimulation by agonistic antibody or using recombinant ligand might not reflect the consequences of GITR interaction with its natural, tumor-derived ligand in vivo. Furthermore, studies evaluating immune responses in GITR−/− mice have so far not led to a clear picture of the role of GITR in normal physiology. Although a costimulatory role for GITR in combination with anti-CD3 has been reported, other investigators describe that T cells from GITR−/− mice are hyperresponsive to immobilized anti-CD3 and that GITR abrogates suppression of murine regulatory T cells.35–37 In addition, GITR and GITRL are expressed by multiple cell types, and the functional relevance of murine GITR and its ligand for non-T cells, especially NK cells, is still largely unclear. This complicates the interpretation of available studies and makes it difficult to construe the accountable effect following a therapeutic GITR/GITRL modulation in mice.14,23 Our notion that sGITRL is a mediator of NK cell suppression is further supported by our results that sGITRL, without affecting viability, caused a significant reduction of NK cell NF-κB activity as revealed by analysis of nuclear localized RelB. These results confirm and extend our findings regarding the effect of cell-surface expressed GITRL and the results of Liu et al, who described that agonistic GITR mAb inhibited NK cell cytokine secretion and negatively modulated the NF-κB pathway21,24 and, at least in part, provide a molecular basis for the observed inhibition of NK cell reactivity. It should however be noted that Liu et al found that stimulation of GITR by agonistic antibody caused apoptosis in NK cells of a substantial proportion of donors analyzed in their study, which may be the result of the differences in experimental conditions and reagents applied. With regard to the GITR-mediated reduction of NK cell NF-κB activity, recent data suggest that GITR can both positively and negatively modulate NF-κB resulting from the association with different TRAF molecules.25,26 Taking into consideration our results obtained with cell surface–expressed GITRL21 and available data regarding other NK cell receptors, such as, for example, 2B4/CD244, where alternative negative or positive adaptors cause differential functional outcomes,3 it may well be that in contrast to the immunostimulatory effect of GITR in mice, in humans GITR diminishes NK cell NF-κB activity resulting from association with different TRAF molecules. This is even more because GITR also seems to mediate different effects in human and murine T cells because suppression of human regulatory T cells, in contrast to their counterparts in mice, is not inhibited by GITR.20
Further evidence for the inhibitory effect of sGITRL on NK cell–mediated tumor immunosurveillance arises from our observation that sGITRL-containing cancer patient sera reduced NK cell cytotoxicity and IFN-γ production, and neutralization of sGITRL contained in tumor cell supernatants and patient sera using a GITR-Ig fusion protein restored NK cell reactivity. This confirms that sGITRL is indeed responsible for the inhibition of NK cell functions and suggests that sGITRL contained in patient sera may substantially reduce NK cell reactivity in tumor patients. Cytotoxicity and production of IFN-γ are the 2 critical tasks that need to be performed by NK cells to eradicate tumors, the latter participating in cancer elimination by inhibiting cellular proliferation and angiogenesis, promoting apoptosis and stimulating the adaptive immune system.38 Our data indicate that, in the reciprocal interaction between human tumor cells and NK cells, tumor-derived sGITRL is a mediator of immunosubversion because it diminishes NK cell anti-tumor reactivity by transducing signals via GITR, which negatively modulate NF-κB. It will be important to determine whether the levels of sGITRL in patient sera correlate to progress and outcome of malignancies, can be used as an immunologic tumor marker and whether neutralization of sGITRL in patient sera may be implemented as a strategy to enhance immune responses in therapeutic approaches relying on NK cell reactivity, such as, for example, adoptive NK cell transfer or antibody-dependent cellular cytotoxicity of NK cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the German Research Foundation (SA 1360/2-2 and SA 1360/4-1), Deutsche Krebshilfe (107537), and Wilhelm Sander-Stiftung (2007.115.1).
Authorship
Contribution: K.M.B., M.K., and T.B. performed the majority of the experiments; B.J.S. and A.B. contributed some of the experiments; H.R.S. designed the project; P.B. designed some of the experiments and contributed to writing the paper; and M.K. and H.R.S. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Helmut R. Salih, Department of Hematology/Oncology, Eberhard-Karls University, Otfried-Mueller-Strasse 10, D-72076 Tuebingen, Germany; e-mail: Helmut.Salih@med.uni-tuebingen.de.
References
Author notes
*K.M.B., M.K., and T.B. contributed equally to this work.