Abstract
The transcobalamin (TC, TCII) receptor (TCblR) on the plasma membrane binds TC- cobalamin (Cbl) and internalizes the complex by endocytosis. This receptor was purified from human placental membranes by affinity chromatography. Tryptic digest of the protein extracted from a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and subjected to liquid chromatography/mass spectrometry identified 4 peptides that matched with a membrane protein in the data bank. TCblR belongs to the low-density lipoprotein receptor family, with 2 low-density lipoprotein receptor type A domains separated by a complement-like cysteine-rich region. The 282-amino acid sequence includes a signal peptide of 31 residues, extracellular domain of 198 residues, a transmembrane region of 21 residues, and a cytoplasmic domain of 32 residues. The binding of TC-Cbl does not require the cytoplasmic domain or its orientation in the plasma membrane because the recombinant extracellular domain binds TC-Cbl with high affinity and specificity. The protein is heavily glycosylated and accounts for the 58-kDa size by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The human gene first identified as 8D6A and more recently as CD 320 encoding TCblR is located at p13.2 on the short arm of chromosome 19, spans a length of 6.224 kb, and is composed of 5 exons and 4 introns.
Introduction
Cellular uptake of cobalamin (Cbl, vitamin B12) in mammalian cells is mediated by receptors expressed on the cell surface.1 Transcobalamin (TC, TCII), a plasma protein secreted by the endothelial cells, binds the Cbl absorbed in the distal ileum and carries between 10% and 30% of the total circulating Cbl.2,3 TC saturated with Cbl specifically binds to the receptor (TCblR) and is internalized by endocytosis. The TC is degraded in the lysosome, and the free Cbl released is converted into Cbl cofactors.4,5 Methylcobalamin is a cofactor for the cytosolic enzyme methionine synthase in the conversion of homocysteine to methionine using N5-methyltetrahydrofolate6 ; therefore, homocysteine is elevated in both Cbl and folate deficiency.7 The mitochondrial enzyme methylmalonyl CoA mutase converts methylmalonyl CoA to succinyl CoA and requires 5′deoxyadenosylcobalamin as a cofactor. The elevated methylmalonic acid in Cbl deficiency is a direct consequence of a block in this pathway.8 The definitive purification of TC9 followed by the identification of vascular endothelium as the source of TC in blood10 ultimately led to the cloning of the cDNA and the gene encoding this protein.11,12 Attempts to purify the receptor have yielded ambiguous results13,14 ; however, the functional properties of TCblR have been well characterized in cell culture models.15,16 We have previously described the functional and structural properties of TCblR based on binding of TC-Cbl to TCblR from human placenta and by crosslinking studies.17,18 Our data on the properties and structure of this receptor differ from 2 other reports describing the purification of this protein.13,14 The report by Bose et al14 described a receptor with different structural constituents. Since their first report, numerous publications by this group have described the structural and functional characterization of a putative receptor from human placenta.19-25 However, they did not establish the functional specificity of their receptor for TC-Cbl and have not identified the primary structure and the gene encoding the receptor. This report describes the purification and definitive identification of the primary structure and the gene encoding a receptor for the cellular uptake of TC-Cbl. This unique receptor has the specificity and affinity required for the cellular uptake of holo TC and differs from the 72/144 kDa monomer/dimer protein reported to be the receptor for TC-Cbl.14
Methods
Actigel ALD agarose (Sterogene Bioseparations, Carlsbad, CA), Ultralink matrix (Pierce Chemical, Rockford, IL), wheat germ agglutinin (WGA)-agarose and concanavalin A (Con A) agarose (Vector Laboratories, Burlingame, CA), [57Co]cyanocobalamin (MP Biomedicals, Irvine, CA), Empigen BB (Huntsman, Dayton, TX), recombinant 8D6 antigen and anti 8D6A antibody (R&D Systems, Minneapolis, MN), CD320 knockout mouse embryonic stem (ES) cells CC0426/129Ola and parental 129 ES cells (MMRC, University of California, Davis), HEK293 cells (ATCC CRL-1573), CD320 cDNA (Open Biosystems, Huntsville, AL), 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxy-1-propane-sulfonate (CHAPSO, Sigma-Aldrich, St Louis, MO), phenylmethylsulfonyl fluoride (Sigma-Aldrich), ethylenediaminetetraacetic acid (EDTA), and ethyleneglycoltetraacetic acid (Sigma-Aldrich).
The procurement of fresh human placentas, preparation of membranes, measuring TC-Cbl binding in membranes, solubilization of receptor activity, and determination of TC-Cbl binding activity in the soluble fraction are described in our previous publications.17,18
For large-scale solubilization of membrane proteins, 400 to 800 g of membranes was homogenized in 2.5 volumes of buffer (20 mM Tris/150 mM NaCl/1 mM phenylmethylsulfonyl fluoride, pH 7.5), and Empigen BB, a nonionic detergent, was added to a final concentration of 0.5% in 3 volumes of buffer. The membranes were mixed overnight at 4°C, pelleted at 15 000g for 20 minutes, and the supernatant fraction subjected to 100 000g centrifugation to obtain the soluble protein fraction.
Binding of TC-Cbl to purified TCblR
A facile assay to monitor the TC-Cbl binding activity of TCblR proved very useful to follow the functional activity during multiple purification steps. This assay uses either WGA or Con A-agarose to bind TCblR18 and takes advantage of the carbohydrate content of TCblR that binds to these lectins and TC, a nonglycosylated protein that does not. [57Co]Cbl-TC (10 000 cpm/0.015 pmol) is incubated with 1 to 2 μL of each fraction in 500 μL of buffer 1 (20 mM of Tris/150 mM of NaCl/0.5% Empigen, pH 7.5) at 4°C and after 1 hour, 100 μL of a 50% suspension of lectin-agarose is added and the incubation continued for an additional hour with constant mixing; 1 mL of buffer 1 is added to each tube, and the agarose pelleted at 1000g for 5 minutes, washed once with 1 mL of buffer 1, and the radioactivity in the pellet determined. Less than 2% of the radioactivity is associated with the lectin-agarose in 10 mM EDTA or when the TCblR is excluded from the reaction.
Affinity purification of the receptor protein
Our previous experience with the application of conventional as well as ligand-specific affinity purification techniques for the isolation of the soluble receptor protein from human placental membranes17 resulted in low recovery and lack of purity. This was attributed to loss of functional activity and multiple proteins nonspecifically binding to the Cbl-TC affinity matrix and eluting with EDTA. With the primary objective of obtaining a homogeneous protein band by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) for definitive identification of the protein by liquid chromatography-mass spectrometry (LC-MS), a 3-step affinity purification strategy was applied.
First affinity purification on a Sepharose-mAb matrix
The immobilized affinity ligand for this step of the purification was a monoclonal antibody (mAb) to human TC covalently coupled to agarose. The mouse mAb IgG fraction was purified on a protein G column and was coupled to Actigel aldehyde agarose matrix as per instructions provided by the manufacturer. The mAb chosen for this matrix is a high affinity binding mAb that does not interfere with the receptor binding site or the Cbl binding site. This mAb efficiently captures the TC-Cbl in solution and, in doing so, captures the TCblR-TC-Cbl complex formed. Placental membranes prepared as described earlier were solubilized in 3 volumes of buffer 1. Each 300-mL aliquot of a 100,000g supernatant fraction was mixed with 10 μg of recombinant human TC (rhTC),26 representing a 6-fold molar excess of TC, presaturated with 10-fold molar excess B12. After, 4 to 6 hours at 4°C, the affinity matrix (2 mL) is added and mixed end-over-end overnight at 4°C. The matrix was collected by allowing it to settle for 2 hours or by centrifugation at 1000g for 10 minutes, washed batch-wise twice in 40 mL of buffer 1, resuspended in 10 mL of buffer 1, and transferred to a column. Typically, 600 g of placental membranes derived from 3 placentas was solubilized and processed as a single batch for receptor purification. The matrix was washed sequentially in buffer 1 (30 mL) followed by 250 mM NaCl (10 mL) and 500 mM NaCl (8 mL) in buffer 1. Additional washing was done with buffer 1 without any NaCl (8 mL) and with 0.1 M MgCl2 (8 mL) and 0.2 M MgCl2 (8 mL). The receptor bound to the matrix was eluted with 0.5 M MgCl2 and 2-mL fractions collected. This concentration of MgCl2 dissociates the TC-TCblR complex but does not affect the binding of Cbl with TC or the binding of TC-Cbl complex to the antibody. Eluting with MgCl2 provides a distinct advantage in that TC-Cbl binding activity could be restored by simply diluting or dialyzing the sample to less than 0.1 mM MgCl2. An aliquot of each fraction was assayed for TC-Cbl binding by the lectin binding assay. After elution of the receptor, the matrix could be reused or the bound TC-Cbl removed by washing with 3 M MgCl2 or glycine, pH 2.7, and the antibody-containing matrix reused.
Second affinity purification on an Ultralink-Cbl-TC matrix
We have previously reported the purification of human TC9 using Cbl that is covalently attached to a matrix whereby the cobalt of Cbl is linked to the matrix by an aminopropyl spacer.27 This matrix can be used to bind TC, and the TC-Cbl on the matrix will then serve as the affinity ligand to capture the receptor protein. Because the cobalt carbon bond is photo-labile, the Cbl-TC-TCblR complex formed can be released from the matrix by simply exposing the matrix to white light such as a 300-W lamp. One disadvantage of this procedure is that both TC and TCblR are released as a complex and the affinity matrix cannot be reused. However, the protocol eliminates many of the contaminant proteins that elute from the matrix with MgCl2 or EDTA.
The aminopropyl Cbl was synthesized and purified as previously described27 and was coupled to Ultralink matrix as per instructions provided by the manufacturer. For large-scale purification using this procedure, the affinity matrix was prepared by mixing 1 mL of the Cbl-aminopropyl-Ultralink matrix with 0.5 mg of partially purified apo rhTC. This provided approximately 10 to 11 nmol of TC on the matrix. The matrix was extensively washed with buffers as described previously for the purification of TC.9 The eluted fractions from the first affinity matrix were assayed for functional activity, pooled, dialyzed against buffer 1, and mixed with the affinity matrix overnight. The matrix was recovered, washed batch-wise twice with 50 mL buffer 1, transferred to a column, and washed with 15 mL of 20 mM Tris/150 mM NaCl/5 mM CHAPSO, pH 7.5. The column was clamped, 1 mL of 20 mM Tris/300 mM NaCl/5 mM CHAPSO, pH 7.5, was added, and the matrix was exposed to a 300-W tungsten lamp placed approximately 20 cm above the matrix; and the TCblR-TC-Cbl complex was released by photo-dissociation of the Cbl from the matrix as described for the purification of TC.9 The matrix was kept cold by placing the column in ice and by dissipating the heat from the lamp with a portable blower. After 1 hour, the buffer in the column was drained and the matrix washed with 1 mL buffer. The photolysis was repeated 2 additional times and the elutions were pooled.
Third affinity purification on an agarose-Con A matrix
The material from the aforementioned purification was diluted with an equal volume of 20 mM of Tris/5 mM of CHAPSO and was mixed with 200 μL of Con A agarose matrix. There were 2 objectives to this third purification step: (1) to remove the large excess of free TC-Cbl expected in this sample because all the TC on the matrix is released by the preceding photo dissociation procedure; and (2) it allowed us then to elute the TCblR from the lectin matrix, thus adding an additional purification step. After mixing overnight at 4°C, the sample was transferred to a column, the liquid drained, and the matrix washed with 2 mL of 20 mM Tris/150 mM NaCl/5 mM CHAPSO, pH 7.5, followed by 2 mL of 20 mM Tris/5 mM CHAPSO. Divalent cations (0.1 mM) Mn, Mg, and Ca were included in all buffers during binding and washing of the Con A matrix. The receptor-TC-Cbl complex bound to the lectin matrix was eluted by incubating for 4 hours with 1 mL eluting buffer containing 0.4 M each of α-methyl-D-mannoside and maltose in 20 mM Tris, pH 7.5/5 mM CHAPSO. The eluting buffer was collected, and the matrix was washed with another milliliter of the same buffer. This sample was dialyzed, concentrated, and separated in an 8% reducing SDS-PAGE gel, and the protein was stained with Coomassie blue dye. The band corresponding to the size of TCblR was excised, digested with trypsin, and analyzed by LC-MS. Peptide peaks were subjected to further MS-MS analysis to yield amino acid sequences. The raw data files were processed using the MassLynx ProteinLynx software (Waters Corp, Milford, MA), and the data files were submitted to www.matrixscience.com for protein matching using the mascot algorithm.
Results
A definitive measure of receptor protein as determined by binding of TC-Cbl was critical for monitoring the receptor activity during solubilization and purification of TCblR. The binding of TCblR to WGA and Con A was used to develop a functional assay18 to monitor receptor activity during various stages of purification. The assay is not suitable for monitoring receptor activity in crude soluble membrane preparations because of the large amount of lectin agarose required for binding all glycoproteins in the sample. Therefore, detergent-soluble receptor activity in placental membranes was determined by size exclusion column chromatography as previously described.17 Our initial protocol for the preparation of placental membranes, solubilization of TCblR, and affinity purification was based on the original procedure of Seligman and Allen,13 except for the use of photo-labile aminopropyl Cbl27 or the amide side chain (e-isomer of the monocarboxylic acid derivative28 ) coupled to Sephacryl S-200 or Ultralink matrix. rhTC bound to the Cbl on the matrix served as the affinity ligand. The receptor bound to the TC-Cbl matrix was eluted with 10 mM of EDTA. This purification yielded a product that was only 25% pure based on the functional activity and protein concentration, and multiple protein bands were observed by SDS page of this sample (Figure 1A). Trypsin digestion of the protein band corresponding to the size of the receptor followed by LC-MS/MS failed to identify a unique peptide sequence that could be assigned to a potential TCblR protein. Moreover, functional activity in the EDTA eluted fraction could not be restored readily by simply adding a molar excess of Ca++. Extensive dialysis in the presence of Ca++ was necessary to recover at best partial functional activity. Low yield of the functional receptor coupled with loss of activity during purification made it difficult to process large volumes of the solubilized receptor through multiple purification steps to obtain adequate amounts of a homogeneous single protein with functional activity. Therefore, different elution approaches and 3 separate affinity purification steps were applied to obtain the pure protein.
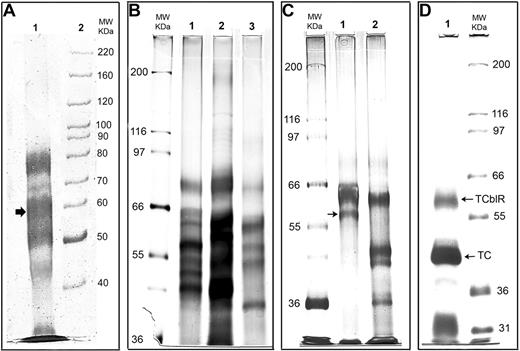
Reducing SDS-PAGE (7.5%) analysis of the TCblR protein during various stages of purification. (A) Protein eluted with EDTA from the TC-Cbl-Sephacryl affinity matrix. The 58-kDa region corresponding to the size of TCblR is marked with an arrow. (B) Protein eluted with 0.5 M of MgCl2 from the TC-Cbl affinity matrix. Lanes 1, 2, and 3 represent 3 batches of purification showing similar profiles by silver staining. (C) Elution from the affinity matrix after the purified protein from the first matrix was reapplied to the same matrix. Lane 1, protein retained on the matrix and was eluted with 0.5 M of MgCl2; lane 2, protein in the effluent that was not retained on the matrix. (D) The protein product after the final purification. The receptor-TC-Cbl complex released by photolysis of the Cbl-matrix was applied to a WGA agarose matrix, and the TCblR-TC complex bound was eluted. The upper band corresponds to the expected size of the receptor and the lower band represents TC that was bound to the receptor.
Reducing SDS-PAGE (7.5%) analysis of the TCblR protein during various stages of purification. (A) Protein eluted with EDTA from the TC-Cbl-Sephacryl affinity matrix. The 58-kDa region corresponding to the size of TCblR is marked with an arrow. (B) Protein eluted with 0.5 M of MgCl2 from the TC-Cbl affinity matrix. Lanes 1, 2, and 3 represent 3 batches of purification showing similar profiles by silver staining. (C) Elution from the affinity matrix after the purified protein from the first matrix was reapplied to the same matrix. Lane 1, protein retained on the matrix and was eluted with 0.5 M of MgCl2; lane 2, protein in the effluent that was not retained on the matrix. (D) The protein product after the final purification. The receptor-TC-Cbl complex released by photolysis of the Cbl-matrix was applied to a WGA agarose matrix, and the TCblR-TC complex bound was eluted. The upper band corresponds to the expected size of the receptor and the lower band represents TC that was bound to the receptor.
Figure 1B shows 3 separate batches of TCblR purified through the first affinity matrix. The proteins were separated in a 7.5% reducing SDS-PAGE and visualized by silver staining. All 3 purifications show a similar protein profile with a number of protein bands in these preparations. Additional washing with buffers before elution resulted in further loss of functional receptor protein and did very little to improve the purity. Reapplying the TCblR eluted from the first affinity matrix to a second TC-Cbl-Ultralink matrix or an anti-TC affinity matrix on which mouse monoclonal antibody to TC was covalently immobilized, eluting with 0.5 M of MgCl2 as described for the first affinity purification and analyzing the samples as described for Figure 1B, improved the purity as shown in Figure 1C lane 1 (lane 2 shows proteins that did not adhere to the affinity matrix on the second application). However, there were 4 or 5 bands in the proximity of the putative receptor band (arrow); therefore, we concluded that the purity was not sufficient for unequivocal sequence identification.
From the initial affinity purifications, it appeared that a large number of proteins were sticking to the matrix nonspecifically or by a Ca++-dependent mechanism and were eluting with EDTA used in the initial purification or the MgCl2 used in the later purification. To circumvent this problem, we decided to test a different elution procedure. We had previously shown that the TCblR-TC-Cbl complex could be released from the aminopropyl Cbl-TC affinity matrix by photo-dissociation of the matrix bound Cbl.18 We adapted this procedure for the second affinity purification step. Even though this procedure was deemed not practical for large-scale purification, it was done to circumvent the problems associated with the MgCl2 elution. The obvious disadvantages of this procedure are that all of the TC on the affinity matrix is released contributing to a large amount of nonreceptor protein, and the affinity matrix cannot be reused. To remove most of the TC not bound to the receptor, the protein released from the second affinity purification step was further purified through a Con A-agarose lectin column.
Shown in Figure 1D is the SDS-PAGE separation of the protein eluted from the third affinity matrix (Con A agarose). As can be seen, a single homogeneous band of approximately 58 to 60 kDa was observed by Coomassie blue dye staining (upper band). The lower more intense band represents TC associated with the receptor to form the holo receptor. LC-MS/MS analysis of the trypsin digested protein band corresponding to the size of TCblR provided 4 peptide sequences (identified in Figure 2), which matched a single protein in the database with 100% identity and a probability-based Mowse score of 200, indicating a definite match with the protein.
Amino acid sequence of TCblR and homology with TCblR from other mammalian species analyzed using the CLUSTAL 2.0.5 alignment. The structural features of the proteins with conserved domains along with the cysteine repeats (+) and potential N-glycosylation (*) sites are identified. The boxed regions correspond to the 4 peptides identified by LC-MS.
Amino acid sequence of TCblR and homology with TCblR from other mammalian species analyzed using the CLUSTAL 2.0.5 alignment. The structural features of the proteins with conserved domains along with the cysteine repeats (+) and potential N-glycosylation (*) sites are identified. The boxed regions correspond to the 4 peptides identified by LC-MS.
Figure 2 shows the complete amino acid sequence of TCblR from different species. The human receptor protein consists of 282 residues accounting for a core polypeptide of 29 kDa and includes a putative signal peptide with predicted cleavage of the signal peptide occurring between residues 30 and 35 providing glutamic acid as probably the first residue of the receptor protein on the plasma membrane. This 252–amino acid membrane bound receptor is composed of an extracellular domain of 199 residues, a transmembrane sequence of 21 residues, and cytoplasmic domain of 32 residues. Beginning at amino acid 54, there are 18 cysteine residues spanning a sequence of 111 amino acids comprising 2 low-density lipoprotein (LDL)–receptor class A domains. Based on the structural similarity to other LDL receptor family of proteins with cysteine repeats, residues 54-67, 61-80, 74-89, 132-145, 139-158, and 152-167 may be involved in disulfide bonding. Potential N-glycosylation sites are located at residues 126, 195, and 213 along with numerous O glycosylations that could occur at the various serine/threonine residues. The 58-kDa size on SDS-PAGE suggests extensive glycosylation of the 252-amino acid (28 kDa) membrane-anchored polypeptide. The extracellular domain of TCblR expressed as a secreted protein in NSO mouse myeloma cells was purchased from R&D Systems. This protein binds TC-Cbl with the same affinity and specificity (Figure 3B) as the native receptor protein solubilized and purified from human placental membranes (Figure 3A). The full-length native protein binds to Con A as well as to WGA, whereas the extracellular domain only binds to WGA (data not shown). Neither intrinsic factor nor haptocorrin (TC I) binds to TCblR (data not shown). Considering the structural similarity of this receptor to the LDL receptor with type A domains and complement-type repeats, we tested the effect of LDL and receptor-associated protein (RAP) on the binding of TC-Cbl to the receptor. LDL at 20- to 400-fold molar excess (0.3-6 pmol) and RAP at 300- to 3000-fold molar excess (4.5-44 pmol) do not affect TC-Cbl binding to TCblR (data not shown). A polyclonal antiserum generated to the recombinant extracellular domain is also available from RPI. This antiserum reacts specifically with the receptor protein. SDS-PAGE followed by Western blot analysis of the fully glycosylated 200–amino acid recombinant extracellular domain produced in mammalian cells and the 282–amino acid native full-length receptor purified from human placenta showed no difference in the mobility of the 2 proteins (Figure 3C). The broad diffuse staining and the lack of any difference in the mobility of the 2 proteins are characteristic of highly glycosylated proteins. The polyclonal antiserum also binds to the native apo receptor and, as a consequence, blocks the binding of TC-Cbl. A dose-dependent inhibition of binding of radiolabeled TC-Cbl was observed with 97% of TC-Cbl binding blocked by as little as 1 μg of the antibody (Figure 4A). This blocking of TC-Cbl binding and inhibition of uptake can be demonstrated in live cells in culture as well, whereby in cells preincubated with the antiserum to the receptor, binding of TC-Cbl at 4°C was decreased by 90% and uptake at 37°C was decreased by 70% (Figure 4B). The functional specificity of this protein is evident from the decrease in TC-Cbl binding of greater than 50% (Figure 4C) in CD320 gene knockout ES cells where one allele is rendered inactive, and a 3-fold increase in uptake when HEK 293 cells were transfected with plasmid containing the CD320 cDNA (Figure 4D). The specific gene knockout in the ES cells was confirmed by TCblR mRNA determination, which was decreased by 50%, and the insertion site of the gene trap within the gene was confirmed by polymerase chain reaction amplification as well as sequence analysis.
Binding specificity and mobility in SDS-PAGE gel of the full-length native TCblR and the recombinant extracellular domain. (A) Binding of TC-Cbl to detergent soluble full-length TCblR purified from placental membranes and (B) to the recombinant extracellular fragment lacking the transmembrane and cytoplasmic domains produced as a secretory protein in mammalian cells. [57Co]Cbl-TC (0.015-1.2 pmol) was incubated with 0.1 pmol of TCblR for 1 hour at room temperature, and the [57Co]Cbl-TC-receptor complex formed was captured on 50 μL of WGA agarose by mixing for an additional 1 hour at 4°C. (C) Western blot of TCblR separated in a reducing 8% SDS-PAGE gel. The protein was visualized by incubating with a polyclonal antiserum to the extracellular fragment of TCblR followed by peroxide conjugated secondary antibody. Lane 1, native TCblR purified from human placental membranes; lane 2, the recombinant extracellular domain expressed in mammalian cells.
Binding specificity and mobility in SDS-PAGE gel of the full-length native TCblR and the recombinant extracellular domain. (A) Binding of TC-Cbl to detergent soluble full-length TCblR purified from placental membranes and (B) to the recombinant extracellular fragment lacking the transmembrane and cytoplasmic domains produced as a secretory protein in mammalian cells. [57Co]Cbl-TC (0.015-1.2 pmol) was incubated with 0.1 pmol of TCblR for 1 hour at room temperature, and the [57Co]Cbl-TC-receptor complex formed was captured on 50 μL of WGA agarose by mixing for an additional 1 hour at 4°C. (C) Western blot of TCblR separated in a reducing 8% SDS-PAGE gel. The protein was visualized by incubating with a polyclonal antiserum to the extracellular fragment of TCblR followed by peroxide conjugated secondary antibody. Lane 1, native TCblR purified from human placental membranes; lane 2, the recombinant extracellular domain expressed in mammalian cells.
Effect of TCblR antiserum gene knockdown, and cDNA transfection on TC-Cbl binding. (A) Blocking of TC-Cbl binding to purified native TCblR by a polyclonal antiserum to the recombinant extracellular domain of the receptor. Apo receptor (0.1 pmol) was incubated overnight with antibody followed by measuring the binding of [57Co]Cbl-TC (0.015 pmol) as described for Figure 3. The vertical bars represent the TC-Cbl-TCblR complex formed as determined by the Con A binding assay. The numbers in parentheses show percentage blocking in the presence of antibody. (B) Inhibition of TC-Cbl uptake in K-562 cells by anti-TCblR antibody. After incubation of 106 cells with antibody for 1 hour at 4°C, binding of [57Co]Cbl-TC (0.015 pmol) was measured for 1 hour at 4°C or 37°C. TC-Cbl binding to the receptors on the cell surface was blocked by 92% (■) when incubated with 1 μg of the antibody. Increased binding in control cells as well as cells preincubated with antiserum at 37°C represents new receptors expressed at 37°C (▧). (C) TC-Cbl uptake in CD320 knockout and wild-type ES cells. (D) TC-Cbl uptake in wild-type and TCblR cDNA-transfected HEK293 cells. For these studies, 106 cells were seeded in 6-well plates for 24 hours, [57Co]Cbl-TC (0.015 pmol) was added to 1 mL medium in the wells and incubated at 37°C for 1 hour.
Effect of TCblR antiserum gene knockdown, and cDNA transfection on TC-Cbl binding. (A) Blocking of TC-Cbl binding to purified native TCblR by a polyclonal antiserum to the recombinant extracellular domain of the receptor. Apo receptor (0.1 pmol) was incubated overnight with antibody followed by measuring the binding of [57Co]Cbl-TC (0.015 pmol) as described for Figure 3. The vertical bars represent the TC-Cbl-TCblR complex formed as determined by the Con A binding assay. The numbers in parentheses show percentage blocking in the presence of antibody. (B) Inhibition of TC-Cbl uptake in K-562 cells by anti-TCblR antibody. After incubation of 106 cells with antibody for 1 hour at 4°C, binding of [57Co]Cbl-TC (0.015 pmol) was measured for 1 hour at 4°C or 37°C. TC-Cbl binding to the receptors on the cell surface was blocked by 92% (■) when incubated with 1 μg of the antibody. Increased binding in control cells as well as cells preincubated with antiserum at 37°C represents new receptors expressed at 37°C (▧). (C) TC-Cbl uptake in CD320 knockout and wild-type ES cells. (D) TC-Cbl uptake in wild-type and TCblR cDNA-transfected HEK293 cells. For these studies, 106 cells were seeded in 6-well plates for 24 hours, [57Co]Cbl-TC (0.015 pmol) was added to 1 mL medium in the wells and incubated at 37°C for 1 hour.
The gene encoding the human TCblR is located on chromosome 19 at p13.2, spans a region of 6.2 kb, and contains 5 exons and 4 introns with a predicted promoter region of approximately 650 nt. The closely related chimp and the monkey also have this gene on chromosome 19. In the rat, bull, and horse, this gene is located on chromosome 7 and on chromosome 20 in the dog. The mouse ortholog of TCblR is located on chromosome 17 B1 and spans a region of 6.792 kb (Figure 5). A comparison of the gene structure of TCblR from available sequence data shows highly conserved exons and introns with the exception of exons 1 and 2 in the horse and intron 2 among species. In most species with chromosomal localization determined, the gene is flanked by NAD dehydrogenase and LAG 1 homolog 4. This gene along with the flanking DNA has been carried on different chromosomes to its current location on chromosome 19 in primates and humans, indicating conservation of shared synteny during evolutionary translocation of this locus.
Comparison of human TCblR gene structure with the receptor from other mammalian species. The intron-exon boundaries and genomic organization of the TCblR gene was compiled from sequence information available from gene data banks.
Comparison of human TCblR gene structure with the receptor from other mammalian species. The intron-exon boundaries and genomic organization of the TCblR gene was compiled from sequence information available from gene data banks.
Discussion
Since the first demonstration of a B12 binding protein in serum needed for the uptake of vitamin B12 in tissues,1 the search for a membrane receptor that specifically binds the plasma protein saturated with B12 has continued for the past several decades. During this period, it was unequivocally established that the plasma protein necessary for cellular uptake of Cbl is transcobalamin and that a receptor protein expressed on the plasma membrane of all cell types specifically binds TC saturated with Cbl.29 The binding requires the divalent cation Ca++ and could be blocked by a molar excess of a chelator, such as EDTA or ethyleneglycoltetraacetic acid. This binding was saturable and required metabolic energy for expression of the receptor protein and for cellular uptake of the Cbl.15 Purification of this receptor proved to be problematic because of the low level of expression and complex multistep procedures, such as functional affinity purification and assay to accomplish this. Placenta is one human tissue that can be readily obtained and proved to be the source of membranes for further characterization and purification of the receptor protein. The first attempted purification of the receptor reported by Seligman and Allen13 described a Triton-soluble 50 kDa of protein with comparable affinity for both apo and holo TC. Their procedures proved difficult to reproduce in our hands and subsequently led to a detailed characterization of the receptor protein,17 with results that contradicted many of their earlier conclusions. In 1995, Bose et al14 published the characterization of a receptor protein using the procedure of Seligman and Allen.13 They identified a 72 kDa/144 kDa monomer/dimer as the receptor protein14 and followed with a number of publications describing the complex physiochemical properties of this receptor.19-25 The lack of progress in identifying the gene encoding the receptor for nearly 10 years after their first report claiming purification of substantial quantities of the receptor protein motivated us to reevaluate our original data and attempt to purify the protein. Extensive modifications to the solubilization, affinity chromatography, and elution procedures finally yielded a pure functional protein for amino acid sequence analysis and the gene encoding this sequence was identified. The cDNA sequence encoding this receptor protein was reported in 2000 by a group studying signaling molecules expressed on dendritic cells and involved in B-cell development and maturation.30 They generated mAbs to membrane proteins derived from dendritic cells and used these to screen a cDNA library. One such mAb 8D6 reacted with an antigen that was reported as 8D6 antigen and suggested that the protein may be involved in signaling by dendritic cells in the maturation of B cells31 and in lymphoid proliferation.32 The aberrant mobility of the protein by SDS-PAGE is probable given the extensive complex glycosylation resulting from the 200 amino acid recombinant extracellular fragment produced fully glycosylated in mammalian cells has the same mobility as the 282–amino acid full-length native receptor. Highly glycosylated proteins are known to deviate from their true size because of conformations of the attached sugars and decreased binding of SDS. The binding of the full-length native protein to both Con A as well as WGA and binding of the extracellular fragment only to WGA suggests that the cytoplasmic domain contains gamma-linked mannose and the extracellular domain contains terminal N-acetyl glucosamine. The 200–amino acid extracellular fragment is fully functional in that it binds the TC-Cbl complex with the specificity and affinity of the native receptor expressed on the cell surface and purified from placental membranes. The polyclonal antiserum to the extracellular fragment reacts with the native receptor with the same specificity and with the characteristic blocking of TC-Cbl binding as well as binding to the receptor-TC-Cbl complex. We have now expressed the extracellular fragment in HEK 293 cells, have generated monoclonal antibodies to this purified fragment, and have isolated binding and blocking antibodies. One blocking antibody tested in cell culture showed continuous blocking of B12 uptake in K562 cells over 4 days, and a similar effect could be elicited by adding the extracellular fragment of the receptor to the culture medium whereby binding of this fragment to TC-Cbl prevents subsequent binding of TC-Cbl to the receptor on the cell surface. The specificity of TC-Cbl binding and the abrogation of this function by specific antibodies decreased TC-Cbl uptake in CD320 knockout cells and the increased uptake in cells transfected with the cDNA, all pointing to this protein as the probable protein ubiquitously present on all cell types as the receptor for cellular uptake of TC-Cbl. The affinity of this receptor for holo TC and the total lack of any interference by other ligands, such as LDL and RAP for which the protein has putative structural domains, suggest evolution of this receptor from the LDL receptor family with structural modifications for the highly specialized function of TC-Cbl transport into cells.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work has been supported by a grant from the National Institutes of Health (DK064732).
National Institutes of Health
Authorship
Contribution: E.V.Q. designed the study, analyzed data, and wrote the manuscript; Y.N. performed the experiments and analyzed data; and J.M.S. performed studies, analyzed data, and participated in writing the manuscript.
Conflict-of-interest disclosure: E.V.Q. and J.M.S. are coinventors on a patent pertaining to the content of this manuscript filed by the Research Foundation of the State University of New York and licensed to KYTO Biopharma (Toronto, ON). Y.N. declares no competing financial interests.
Correspondence: Edward V. Quadros, Department of Medicine, State University of New York Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203; e-mail: Edward.Quadros@downstate.edu.


![Figure 3. Binding specificity and mobility in SDS-PAGE gel of the full-length native TCblR and the recombinant extracellular domain. (A) Binding of TC-Cbl to detergent soluble full-length TCblR purified from placental membranes and (B) to the recombinant extracellular fragment lacking the transmembrane and cytoplasmic domains produced as a secretory protein in mammalian cells. [57Co]Cbl-TC (0.015-1.2 pmol) was incubated with 0.1 pmol of TCblR for 1 hour at room temperature, and the [57Co]Cbl-TC-receptor complex formed was captured on 50 μL of WGA agarose by mixing for an additional 1 hour at 4°C. (C) Western blot of TCblR separated in a reducing 8% SDS-PAGE gel. The protein was visualized by incubating with a polyclonal antiserum to the extracellular fragment of TCblR followed by peroxide conjugated secondary antibody. Lane 1, native TCblR purified from human placental membranes; lane 2, the recombinant extracellular domain expressed in mammalian cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/1/10.1182_blood-2008-05-158949/5/m_zh80220826930003.jpeg?Expires=1765900609&Signature=drXWynDgIWNZdOtqVUFN6EPxoumXpELT0IZaliCFI7cwQez80hT4~2JvaDvLSfv7Tj4tl5JWQtnDBlH55rY-3JAGqU4w36NeDvS57Q2GQLgkwXHeh4ZU47ti6vUUu5HaVL7KnXMC~zm1MqhIJPr6IfmrULgcvfBk38UMHCoZQh6ZgkLIBBpAYT2DVGqyR8BMg9MXHOC3qmvSAPfxEEVuM4bWYFcPtqRNQHiL~cIvneOvK5M--zikB5NA3bfWiWKPYd~di~HnLCtqCftfkTi-vwOyLhHX1~USwB61lhwE1OCjhZOvElS-4Wc9CMLQLqsISty1DQj~PL1VNyldJuRviQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Effect of TCblR antiserum gene knockdown, and cDNA transfection on TC-Cbl binding. (A) Blocking of TC-Cbl binding to purified native TCblR by a polyclonal antiserum to the recombinant extracellular domain of the receptor. Apo receptor (0.1 pmol) was incubated overnight with antibody followed by measuring the binding of [57Co]Cbl-TC (0.015 pmol) as described for Figure 3. The vertical bars represent the TC-Cbl-TCblR complex formed as determined by the Con A binding assay. The numbers in parentheses show percentage blocking in the presence of antibody. (B) Inhibition of TC-Cbl uptake in K-562 cells by anti-TCblR antibody. After incubation of 106 cells with antibody for 1 hour at 4°C, binding of [57Co]Cbl-TC (0.015 pmol) was measured for 1 hour at 4°C or 37°C. TC-Cbl binding to the receptors on the cell surface was blocked by 92% (■) when incubated with 1 μg of the antibody. Increased binding in control cells as well as cells preincubated with antiserum at 37°C represents new receptors expressed at 37°C (▧). (C) TC-Cbl uptake in CD320 knockout and wild-type ES cells. (D) TC-Cbl uptake in wild-type and TCblR cDNA-transfected HEK293 cells. For these studies, 106 cells were seeded in 6-well plates for 24 hours, [57Co]Cbl-TC (0.015 pmol) was added to 1 mL medium in the wells and incubated at 37°C for 1 hour.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/1/10.1182_blood-2008-05-158949/5/m_zh80220826930004.jpeg?Expires=1765900609&Signature=aeEVGuyGW5oj-r818BdXTzYiHYYain3AV0yaPLpP3Czt50H2sMoSfTiMYmYdNB-hiitmosgA8x4MXEccre1ujEseqgv~ABkpSFFul4GTSSK6X78u~OuR~Tg3trR-WxySdjxXc5Bk6rledF34mlc61lk17m8Yk4Fc54FdZKfW0b1jZ~C~zEkUjGppyM0VM20POXxUpXAU8Dgq18Wwmzzx84NZY9VwgOSGHQz8G-7XmYrRee3WO31WeZPRohZMIdNWaNo6-ffYqNtGAJizA5ZtrqbVWEHaWGOHmVfn~4~ModGG9R8WdJuBJfFOLIH2FK6-ovcUlRuxhAntaw5xX25uvA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)