Abstract
Non–Fc-receptor binding anti-CD3 Ab therapy, in the setting of several different autoimmune disorders, can induce antigen-specific and long-lasting immunologic tolerance. Because factor VIII (FVIII) inhibitor formation is the most serious treatment-related complication for hemophilia A patients, we tested the efficacy of anti-CD3 to prevent FVIII inhibitor formation in hemophilia A BALB/c and C57BL/6 mice. A short course of low-dose anti-CD3 significantly increased expression of CD25 and the proportion of CD4+CD25+ regulatory T cells in the spleen and potently prevented the production of inhibitory and non-neutralizing anti-FVIII antibodies in both strains of mouse. Depleting the CD4+CD25+ cells during anti-CD3 therapy completely ablated tolerance to FVIII. Further phenotypic characterization of regulatory cells in tolerant mice showed a consistently higher number of CD4+GITR+ and CD4+FoxP3+ cells in both strains of mice. In addition, in tolerant C57BL/6 mice we observed an increase in CD4+CD25+CTLA-4+ and CD4+CD25+mTGF-β1+ cells. Finally, in vitro cytokine profiling demonstrated that splenocytes from tolerant BALB/c and C57BL/6 were polarized toward a Th1-immune response. Taken together, these findings indicate that anti-CD3 induces tolerance to FVIII and that the mechanism(s) regulating this response almost certainly occurs through the generation of several distinct regulatory T-cell lineages and by influencing cytokine production and profile.
Introduction
Hemophilia A is the most common severe inherited bleeding disorder. Patients with this disease are treated with recombinant or plasma-derived factor VIII (FVIII), which allows them to lead relatively normal lives.1 In approximately 25% of treated patients, however, the development of anti-FVIII antibodies (FVIII inhibitors) severely complicates FVIII replacement therapy and significantly increases morbidity within the hemophilia population.2-5 These antibodies neutralize the procoagulant cofactor activity of FVIII or enhance its clearance from plasma.5
In economically developed countries, there are 2 approaches to the clinical management of FVIII inhibitors: the treatment or prevention of bleeding and long-term immune tolerance induction (ITI). Bleeding is controlled with variably effective and expensive FVIII-bypassing agents, such as recombinant (r) FVIIa and FEIBA (FVIII-inhibitor bypassing agent). In contrast, ITI is usually attempted through the administration of FVIII at a dose and frequency that depends on the ITI protocol.6 This treatment approach is practically challenging, costly, and can take months to years to become effective. In light of the significant limitations of the current treatment options, the development of effective, rapid, and economical ITI strategies is a clinical priority.
Currently, the most consistent model to study FVIII inhibitors is the hemophilia A mouse (FVIII−/−).7-9 Repeated intravenous infusion of human FVIII into hemophilia A mice results in high titer inhibitor formation. This is a CD4+ T cell–dependent process that requires costimulation.9-12 The dependence on CD4+ T cells for inhibitor formation also occurs in humans. Evidence of this first came from hemophilia A patients with FVIII inhibitors who were also HIV+: as patient CD4+ levels declined, there was concomitant disappearance of FVIII inhibitors.13 Therefore, therapies that blocked T-cell activation seemed to be promising candidates to prevent inhibitor formation.
Indeed, Qian et al demonstrated that FVIII−/−B7.2−/− double-knockout mice will not develop anti-FVIII antibodies (Abs) after repeated immunization with FVIII, and that blocking the CD80-CD28 costimulatory interaction with soluble cytotoxic T lymphocyte antigen-4 (CTLA-4)–immunoglobulin (Ig) in FVIII−/− mice also prevented inhibitor formation.10 Additional studies in FVIII−/− mice showed that blockade of the CD40-CD40L interaction with anti-CD40L monoclonal Ab (mAb) also protects against FVIII inhibitor formation.11,12 However, costimulatory blockade must be applied with each FVIII administration to maintain tolerance, and once the blockade is removed, the protective effect is lost. As the potential health risks of long-term costimulatory blockade have not yet been determined and because many hemophilia A patients are treated frequently with FVIII and would most likely need to coadminister blockade with each infusion, this therapy is not a viable option.
To reach the clinic, a therapy that induces tolerance to FVIII should be of short duration, have long-lasting therapeutic benefit, and allow the patient to develop a normal immune response against pathogens. One therapeutic agent that meets these criteria is the non–Fc receptor (FcR)–binding anti-CD3ϵ Ab (anti-CD3), generated as F(ab′)2 fragments for animal studies, or as a humanized Ab with a mutated Fc region for clinical use.14-16 Anti-CD3 modifies the CD3–T-cell receptor (TCR) complex, causing incomplete immune synapse formation and partial T-cell signaling, which together can lead to T-cell anergy or apoptosis, or to the expansion of regulatory CD4+CD25+ T cells.14,17,18
Anti-CD3 has been studied as a tolerance-inducing agent for several autoimmune and inflammatory disorders. To date, the most extensive clinical and preclinical data on anti-CD3 have been generated from studies of type 1 diabetes (T1D).14 In NOD mice, which spontaneously develop overt T1D, low doses of anti-CD3 (eg, 40 μg/day for 5 consecutive days) will suppress the onset of diabetes in 50% to 90% of the treated animals.19-22 The mechanism mediating this tolerance, at least in part, depended on a transient decrease in the pathogenic effector CD4+ and CD8+ T-cell levels concomitant with an increased CD4+CD25+ T-regulatory cell population after anti-CD3 therapy.19,22
Based on these potential benefits, and because the immune response to FVIII, similar to T1D, is highly dependent on CD4+ T cells, we have assessed the efficacy of anti-CD3 as a preventative therapy for FVIII inhibitors in hemophilia A mice. We hypothesized that anti-CD3 would transiently increase the CD4+CD25+ regulatory T-cell (Treg) population19,22 and decrease the frequency of FVIII-specific CD4+ effector cells. Together, these conditions would establish a tolerogenic therapeutic window, within which FVIII administration would result in FVIII tolerance as opposed to immunity.
Here we report that very small doses of anti-CD3 (10 μg/day) can prevent the generation of FVIII inhibitors in hemophilia A mice. We studied the efficacy of this treatment on 2 genetic backgrounds and herein provide evidence of the cellular and molecular immunologic mechanisms that regulate this response.
Methods
Mice
Hemophilia A mice on a C57BL/6 background were generated by targeted disruption of exon 16 in the FVIII gene7,8 (generously provided by Dr H. H. Kazazian, University of Pennsylvania, Baltimore, MD). Congenic hemophilia A mice were generated by crossing the hemophilia A phenotype onto the BALB/c background for 10 or more generations. A mix of age-matched male and female mice, between 8 and 16 weeks of age, were used in all experiments. All animal procedures were reviewed and approved by the Queen's University Animal Care Committee. Blood was taken by orbital plexus bleeding and was mixed with a one-tenth volume of 3.2% sodium citrate, and plasma was separated by centrifugation of citrated blood at 10 000g for 5 minutes at 4°C. Plasma was stored at −86°C for analysis at a later time.
Effect of anti-CD3 on splenic T-cell populations
Hemophilia A BALB/c mice (n = 15) were treated intravenously with non–FcR-binding anti-CD3ϵ F(ab′)2 (anti-CD3, clone 145-2C11; Bioexpress, Lebanon, NH) at 50 μg/day for 5 consecutive days. At 1, 8, and 15 days after the final anti-CD3 injection, 5 mice per time point were killed and single-cell suspensions were prepared from their spleens. Untreated and age-matched mice (n = 6) were included to determine basal T-cell levels. Cells were incubated with Abs to CD4–phycoerythrin (PE), CD8-PE, and CD25–fluorescein isothiocyanate (FITC; eBioscience, San Diego, CA). The staining protocol is detailed in “Abs and flow cytometry.”
Treatments
Before FVIII immunizations, mice were treated with anti-CD3 diluted in Hank balanced salt solution (HBSS) daily for 5 consecutive days at 10, 25, 50, 100, or 200 μg/day. Control mice received a volumetric equivalent of HBSS, in place of anti-CD3, daily for 5 days. Three days after the final anti-CD3 or HBSS injection, mice received the first of 4 weekly intravenous immunizations with 0.2 μg recombinant human FVIII, approximately 50 U/kg (Kogenate FS; Bayer, West Haven, CT). This dose of FVIII is equivalent to that given to bleeding hemophilia A patients, and, in the absence of immunomodulation, results in FVIII inhibitor formation in all mice that receive this immunization regimen.
Abs and flow cytometry
Abs specific for CD4-PE-Cy5, CD25-FITC (clone 7D4; BD Biosciences, San Jose, CA), CD4-PE, CD8-PE, CD25-FITC (clone PC61), CTLA-4–PE, glucocorticoid-induced tumor necrosis factor receptor (GITR)–PE, CD62L-PE, FoxP3-PE (eBioscience), and transforming growth factor-β1 (TGF-β1)–PE (IQ Products; Rozenburglaan, The Netherlands) were used. Single-cell suspensions were pooled from the spleens of “protected” anti-CD3–treated animals (mice that did not develop FVIII inhibitors after 4 FVIII immunizations), HBSS-treated (HBSS instead of anti-CD3), or untreated mice (n = 2-3/group). Cells were incubated with Fc blocker (CD16/32; eBioscience) for 15 minutes and then stained for expression of surface markers for 30 minutes at 4°C. To detect FoxP3 expression, cells were permeabilized with the Cytofix/Cytoperm Kit (BD Biosciences), incubated with Fc blocker as before, and stained with anti-FoxP3–PE for 30 minutes at 4°C. The cells were fixed with 1% paraformaldehyde; the data were acquired on an EPICS Altra HSS flow cytometer (Beckman Coulter, Fullerton, CA) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Bethesda assay
Inhibitory FVIII Abs were measured using the Bethesda assay and were reported as Bethesda units per mL (BU/mL),23 where 1 BU/mL is defined as the dilution of plasma containing FVIII inhibitory activity that results in 50% inhibition of FVIII activity (FVIII:C) after a 2-hour incubation at 37°C. Mouse plasma was serially diluted in buffer (50 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 100 mM NaCl, 0.1% bovine serum albumin [BSA] wt/vol, pH 7.4), such that the residual FVIII:C for each sample was between 25% and 75%, mixed 1:1 with pooled normal human plasma (Precision Biologics, Dartsmouth, NS), and incubated for 2 hours at 37°C. The remaining FVIII activity was quantified by a one-stage FVIII coagulation assay on a Coag-A-Mate MAX (Biomerieux, Durham, NC) automated coagulometer. For all samples, to reduce error in the assay and inconsistency between samples, the reported inhibitor titer in BU/mL was calculated from the dilution of plasma that yielded a residual FVIII:C of approximately 50%.
ELISA experiments
Anti-FVIII isotype measurement.
Ninety-six–well microtiter plates were coated with FVIII (0.05 μg/well) in 50 mM carbonate buffer (pH 9.6) overnight at 4°C. Plates were washed with 0.1% Tween 20 in HBSS and blocked for 2 hours in blocking buffer (2% BSA wt/vol in HBSS) at room temperature. Plasma from “protected” anti-CD3–treated or HBSS-treated mice was serially diluted in blocking buffer and incubated on the plate for 2 hours at room temperature. Secondary Abs (Southern Biotechnology Associates, Birmingham, AL), specific for the heavy chain region of mouse IgM, IgG1, IgG2a, and IgG2b, and conjugated to horseradish peroxidase, were added and incubated for 1 hour at room temperature. Color was generated by the addition of 3,3′,5,5′ tetramethlybenzidine substrate reagent (BD Biosciences) for 15 minutes, and the reaction was stopped with 1 M H2SO4. Optical density was read at 490 nm on a VERSA max microplate reader (Molecular Devices, Sunnyvale, CA). The Ab titer that is reported was the highest dilution of mouse plasma that showed a positive signal (optical density > 0.1).
Cytokine measurements.
Single-cell suspensions were prepared from the spleens of “protected” anti-CD3–treated, HBSS-treated, or untreated mice (n = 2-3/group) 1 week after the final FVIII immunization. Splenocytes (5 × 106 cell/mL) were cultured in vitro with an anti-CD3/anti-CD28 mixture (1 μL of each; BD Biosciences) to generate nonspecific activation, with 1.0 μg/mL FVIII to generate antigen-specific activation, or were not stimulated, for 72 hours at 37°C (the optimal concentration of FVIII for restimulation was determined by adding 0.01, 0.1, or 1.0 μg/mL FVIII to splenocytes), and enzyme-linked immunosorbent assay (ELISA) for interleukin-2 (IL-2) was performed (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Sandwich ELISA was performed on culture supernatants to determine levels of IL-2, IL-4, IL-5, IL-10, interferon-γ (IFN-γ; eBioscience), and TGF-β1 (BD Biosciences). Data acquisition and analysis were performed using a VERSA max microplate reader (Molecular Devices) and analyzed with Softmax Pro software.
Depletion of CD4+CD25+ cells
Hemophilia A BALB/c mice were injected intraperitoneally with 1 mg anti-CD25 (clone PC61) or an isotype control Ab (rat IgG1; Bioexpress) on days 0 and 5, and on days 3 to 7, mice received 10 μg/day of anti-CD3 (Figure 4A). Three days after the final anti-CD3 injection, mice were killed and splenocytes were analyzed for expression of CD4 and CD25, using anti-CD4–PE and anti-CD25–FITC clone 7D4. An identical treatment regimen was performed as just described, but at 3 days after the anti-CD3 injections (day 10), the mice received the first of 4 weekly FVIII immunizations (the time point at which the first FVIII immunization was administered for the previous inhibitor experiments). The subsequent immune response to FVIII was determined 1 week after the final FVIII immunization by the Bethesda assay.
Anti-CD3 transwell experiment
Sex- and age-matched hemophilia A BALB/c mice were treated with anti-CD3 (10 μg/day) and immunized with FVIII as described in “Treatments.” Spleens were pooled from tolerized anti-CD3–treated animals, and CD4+CD25+ cells were isolated using the CD25+ Treg Isolation Kit (Miltenyi Biotec, Auburn, CA); and CD4+CD25− cells were collected as the cellular fraction that did not bind to the magnetic column. Total splenocytes (5 × 106 cell/mL) from HBSS control animals (FVIII immunized but no anti-CD3 therapy) were cultured with CD4+CD25+ or CD4+CD25− cells (2 × 105) in the same well (coculture ([CC]) or were separated by a 0.4-μm transwell membrane (Transwell, TW; Corning, Corning NY) and were restimulated with 1.0 μg/mL FVIII for 72 hours at 37°C. Sandwich ELISA was performed on culture supernatants to determine IFN-γ and IL-10 levels. Reduction of cytokine secretion was calculated by dividing cytokine levels in the coculture media (ie, total splenocytes + CD4+CD25+ or CD4+CD25− cells from tolerant animals) by HBSS control splenocytes alone (ie, not cultured with CD4+CD25+ or CD4+CD25− cells).
Statistics
The data are expressed as the mean plus or minus SEM or plus or minus SD. In all experiments, statistical comparisons were calculated using a 2-tailed Student t test. The data were considered statistically significant at P less than .05.
Results
Increase in the splenic CD4+CD25+ T-cell compartment after treatment with anti-CD3
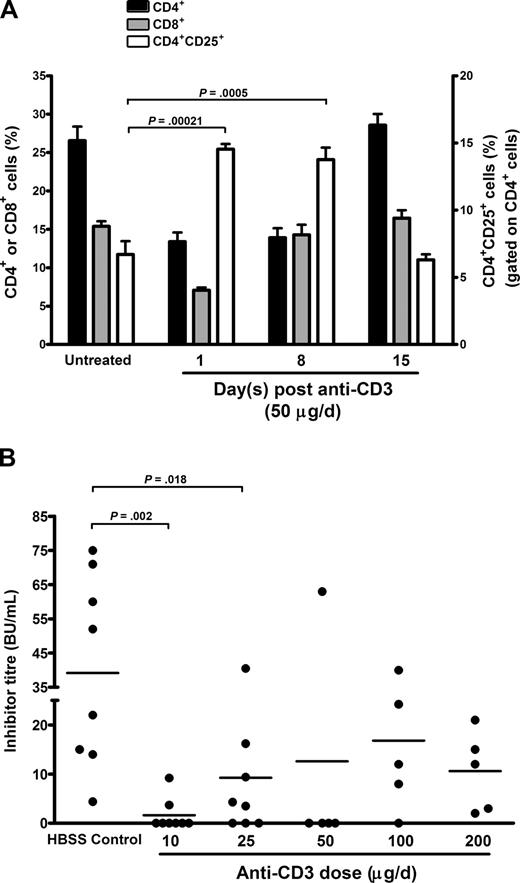
In the NOD mouse, a short course of treatment with anti-CD3 will increase the CD4+CD25+ Treg population in the peripheral blood22 and mesenteric and pancreatic lymph nodes.19 Because the immune response to FVIII in humans and mice probably occurs in the spleen,9,24 we first determined the effect of anti-CD3 (at suboptimal doses of 50 μg/day for 5 consecutive days, the standard dose used in NOD mice) on splenic CD4+, CD8+, and CD4+CD25+ populations 1, 8, and 15 days after anti-CD3 injections in BALB/c hemophilia A mice (Figure 1A). In addition to this, T-cell populations were studied in naive (untreated) hemophilia A BALB/c mice to establish their basal levels. At 1 day after anti-CD3 therapy, there was a greater than 2-fold decrease in the effector CD4+ (P = 9.0 × 10−5) and CD8+ (P = 8.0 × 10−6) populations compared with untreated mice. At the same time point, the CD4+CD25+ T-cell population in anti-CD3–treated mice increased by greater than 2.2-fold (P = 2.1 × 10−4). The absolute CD4+CD25+ T-cell levels (ie, not gated on CD4+ cells) also increased (Figure S3). This increased CD4+CD25+ population was maintained until 8 days after anti-CD3 injections, but by 15 days, the CD4+, CD8+, and CD4+CD25+ populations returned their pretreatment levels. Together, these data suggest that anti-CD3 treatment creates a transient therapeutic window, where effector CD4+ levels are low and relative levels of CD4+CD25+ cells are high. These conditions are sustained for at least 8 days after anti-CD3 treatment and represent a time during which FVIII administration may be more tolerogenic.
Anti-CD3 therapy protects hemophilia A mice from FVIII inhibitor formation
To determine whether there was an effect on inhibitor formation, we administered anti-CD3 at various doses to hemophilia A BALB/c mice (n = 5-8/group), whereas control mice (n = 8) received HBSS rather than anti-CD3, and 3 days later, all mice were immunized with the previously described FVIII infusion schedule (Figure 1B). The FVIII inhibitor titers were determined via the Bethesda assay 1 week after the fourth and final FVIII immunization. All doses of anti-CD3 protected mice from inhibitor formation compared with the HBSS-treated controls; however, low anti-CD3 doses provided the greatest level of protection, with the lowest dose of 10 μg/day (P = .002) being most protective.
Anti-CD3 treatment in vivo increases regulatory CD4+CD25+ T cells and prevents FVIII inhibitor formation. (A) The spleens from hemophilia A BALB/c mice (n = 5/time point) were analyzed 1, 8, and 15 days after treatment with anti-CD3 (50 μg/day for 5 days) for expression of CD4, CD8, and CD25 (gated on CD4+ cells). Untreated and age-matched hemophilia A BALB/c mice (n = 6) were also studied to determine basel T-cell levels. The transient nature of this response may be due to the very short half-life of anti-CD3 F(ab)′2 fragments (< 1 day).22 Data are shown as the mean plus or minus SEM. (B) Dose optimization of anti-CD3 therapy in hemophilia A BALB/c mice. The mice (n= 5-8/group) were treated with 10, 25, 50, 100, or 200 μg/day of anti-CD3 for 5 days. Three days after the final anti-CD3 treatment, mice were immunized with 0.2 μg human FVIII weekly for 4 consecutive weeks. The control mice (n = 8) received HBSS, in place of anti-CD3, followed by 4 FVIII immunizations. One week after the final FVIII immunization, plasma was collected and the immune response to FVIII was assessed by the Bethesda assay. Each point in the figure represents the inhibitor titer in an individual animal and the horizontal bars indicate the mean inhibitor titer in each group.
Anti-CD3 treatment in vivo increases regulatory CD4+CD25+ T cells and prevents FVIII inhibitor formation. (A) The spleens from hemophilia A BALB/c mice (n = 5/time point) were analyzed 1, 8, and 15 days after treatment with anti-CD3 (50 μg/day for 5 days) for expression of CD4, CD8, and CD25 (gated on CD4+ cells). Untreated and age-matched hemophilia A BALB/c mice (n = 6) were also studied to determine basel T-cell levels. The transient nature of this response may be due to the very short half-life of anti-CD3 F(ab)′2 fragments (< 1 day).22 Data are shown as the mean plus or minus SEM. (B) Dose optimization of anti-CD3 therapy in hemophilia A BALB/c mice. The mice (n= 5-8/group) were treated with 10, 25, 50, 100, or 200 μg/day of anti-CD3 for 5 days. Three days after the final anti-CD3 treatment, mice were immunized with 0.2 μg human FVIII weekly for 4 consecutive weeks. The control mice (n = 8) received HBSS, in place of anti-CD3, followed by 4 FVIII immunizations. One week after the final FVIII immunization, plasma was collected and the immune response to FVIII was assessed by the Bethesda assay. Each point in the figure represents the inhibitor titer in an individual animal and the horizontal bars indicate the mean inhibitor titer in each group.
In 4 separate experiments, this optimal dose (10 μg/day) was administered to BALB/c hemophilia A mice, and 3 days later these mice were immunized with FVIII as before (Figure 2A,B). By 1 week after the final FVIII immunization, 98% of the HBSS-treated control (n = 30) and 22% of anti-CD3–treated mice (n = 33) were inhibitor positive. Twenty-seven of 33 anti-CD3–treated mice were inhibitor negative (P = 6.5 × 10−5; Figure 2A). Importantly, at the same time, the mean inhibitor titer in the anti-CD3–treated mice was 1.6 plus or minus 1.1 BU/mL compared with the HBSS-treated mice at 50.1 plus or minus 14.9 BU/mL (P = .001; Figure 2B). Tolerance was maintained for as long as the animals were studied (12 weeks, data not shown), and the tolerant animals did not show a memory response when rechallenged with FVIII 6 weeks after the final FVIII immunization (Figure S4). Collectively, these data demonstrate that the protective effect of anti-CD3 is highly reproducible and extremely robust.
Anti-CD3 prevents anti-FVIII Ab formation in BALB/c hemophilia A mice. (A) In 4 separate experiments using the optimized dose of anti-CD3 (10 μg/day for 5 days), the incidence of FVIII inhibitor formation was determined in HBSS-treated mice (n = 30) and anti-CD3–treated mice (n = 33). The incidence of FVIII inhibitor formation shown was determined 1 week after the final FVIII immunization. Data are mean plus or minus SD. (B) The FVIII inhibitor titer in Bethesda units per milliliter in the mice from panel A is shown at 1 week after the final FVIII immunization. (C) The anti-FVIII Ab isotypes were analyzed in the plasma of “protected” anti-CD3–treated (n = 5) (anti-CD3–treated mice that did not form inhibitors after 4 FVIII immunizations) and HBSS-treated mice (n = 8) 3 weeks after the final FVIII immunization. Data are mean plus or minus SEM. (D) The effect of anti-CD3 at 10 μg/day for 5 days (the optimized dose) on T-cell populations in hemophilia A BALB/c mice (n = 5) is shown at 3 days after the final anti-CD3 injection and is compared with untreated and age-matched animals (n = 5).
Anti-CD3 prevents anti-FVIII Ab formation in BALB/c hemophilia A mice. (A) In 4 separate experiments using the optimized dose of anti-CD3 (10 μg/day for 5 days), the incidence of FVIII inhibitor formation was determined in HBSS-treated mice (n = 30) and anti-CD3–treated mice (n = 33). The incidence of FVIII inhibitor formation shown was determined 1 week after the final FVIII immunization. Data are mean plus or minus SD. (B) The FVIII inhibitor titer in Bethesda units per milliliter in the mice from panel A is shown at 1 week after the final FVIII immunization. (C) The anti-FVIII Ab isotypes were analyzed in the plasma of “protected” anti-CD3–treated (n = 5) (anti-CD3–treated mice that did not form inhibitors after 4 FVIII immunizations) and HBSS-treated mice (n = 8) 3 weeks after the final FVIII immunization. Data are mean plus or minus SEM. (D) The effect of anti-CD3 at 10 μg/day for 5 days (the optimized dose) on T-cell populations in hemophilia A BALB/c mice (n = 5) is shown at 3 days after the final anti-CD3 injection and is compared with untreated and age-matched animals (n = 5).
The Bethesda assay is a functional assay that strictly measures the titer of the anti-FVIII Abs that inhibit the procoagulant cofactor activity of FVIII yet provides no information regarding the total Ab titer, which includes both inhibitory and noninhibitory Abs. Thus, we studied the spectrum of anti-FVIII Ab isotypes in “protected” anti-CD3 mice (anti-CD3–treated BALB/c mice that remained inhibitor negative after FVIII immunizations) and the HBSS-treated mice by ELISA (Figure 2C). The HBSS-treated control mice predominately produced anti-FVIII Abs of the type IgG1 and IgG2a, whereas in contrast, the anti-CD3–treated mice did not produce significant levels of any of the isotypes (IgM, IgG1, IgG2a, and IgG2b). These results, taken together with the Bethesda data, suggest anti-CD3 protects mice from total anti-FVIII Ab formation.
We initially studied the effect of anti-CD3 on T-cell populations at a dose of anti-CD3 (50 μg/day) previously used in T1D studies (Figure S2). In addition, FVIII was administered 3 days after anti-CD3 injections, and we did not study T-cell levels at this time point. Thus, we studied the effect of the optimal dose (10 μg/day) of anti-CD3 on T-cell populations (CD4+, CD8+, and CD25+) in BALB/c hemophilia A mice 3 days after the final anti-CD3 injection, and this was compared with untreated mice (Figure 2D). At 3 days after the 10 μg/day anti-CD3 treatment, the CD4+ and CD8+ levels decreased by 1.6-fold compared with the untreated mice, whereas the CD4+CD25+ population increased by 1.8-fold (P = .002). These data are consistent with those using 50 μg/day anti-CD3 (Figure S2) and indicate that the increased CD4+CD25+ and decreased effector CD4+ populations, at least in part, may be responsible for the reduced immune response to the FVIII immunizations.
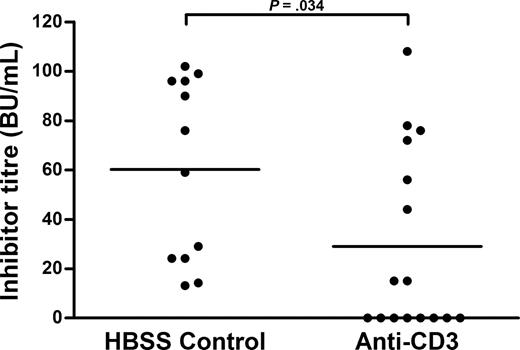
In 2 independent experiments, hemophilia A mice on the C57BL/6 background were treated to determine whether this therapy could be generalized to another genetic population. These mice received the dose of anti-CD3 optimized in the BALB/c model (10 μg/day for 5 days), and 3 days later, were immunized with FVIII as before (Figure 3). One week after the final FVIII immunization, all HBSS-treated C57BL/6 hemophilic mice (n = 11) were inhibitor positive, whereas only 50% of the anti-CD3–treated mice (n = 18) had inhibitors (Figure 3). Furthermore, the mean inhibitor titer in the HBSS-treated mice was 60.2 (± 36.7) BU/mL compared with the anti-CD3 group at 29.0 (± 37.2) BU/mL (P = .034). Taken together, these data indicate that anti-CD3 therapy is effective at preventing inhibitor formation on 2 distinct genetic backgrounds, albeit to lesser extent, at a nonoptimized dose, in the C57BL/6 mice than in the BALB/c model.
Anti-CD3 prevents FVIII inhibitor formation in C57BL/6 hemophilia A mice. In 2 independent experiments, anti-CD3, at the dose optimized in the BALB/c model (10 μg/day for 5 days), was administered to C57BL/6 hemophilia A mice (n = 16). The C57BL/6 control mice (n = 11) received HBSS rather than anti-CD3 daily for 5 days. Three days later, they were immunized with FVIII as outlined for BALB/c hemophilia A mice.
Anti-CD3 prevents FVIII inhibitor formation in C57BL/6 hemophilia A mice. In 2 independent experiments, anti-CD3, at the dose optimized in the BALB/c model (10 μg/day for 5 days), was administered to C57BL/6 hemophilia A mice (n = 16). The C57BL/6 control mice (n = 11) received HBSS rather than anti-CD3 daily for 5 days. Three days later, they were immunized with FVIII as outlined for BALB/c hemophilia A mice.
CD4+CD25+ Tregs from tolerant anti-CD3–treated mice are suppressive in vivo and in vitro and require cellular contact
Because anti-CD3 treatment increased CD4+CD25+ T cells (Figures 1A, S3, and 2D), we depleted this population to test its importance in the FVIII tolerogenic process. Anti-CD25 (clone PC61) is highly effective at depleting murine CD4+CD25+ cells in vivo.25-28 To determine the extent of depletion of the CD4+CD25+ compartment with anti-CD25 in anti-CD3–treated mice, we injected hemophilia A BALB/c mice intraperitoneally with anti-CD25 (PC61, n = 3) or isotype control (rat IgG1, n = 3) on days 0 and 5 (Figure 4A). Mice were then treated with 10 μg/day anti-CD3 on days 3 to 7, and on day 10 splenocytes were analyzed for expression of CD4 and CD25 by flow cytometry using anti-CD25 clone 7D4, which binds to a different epitope on the CD25 complex than the depleting anti-CD25 mAb (clone PC61) (Figure 4B,C). Importantly, there was nearly complete ablation of the CD4+CD25+ compartment in anti-CD25/anti-CD3–treated mice (1.4% ± 0.6%) compared with the isotype control/anti-CD3–treated mice (15.2% ± 0.6%, P = 9.0 × 10−4; Figure 4C). Furthermore, as expected, because of anti-CD3 treatments, the CD4+ T cell levels in both the anti-CD25/anti-CD3 and isotype control/anti-CD3 mice were reduced by approximately 2-fold compared with the untreated mice (Figure 4C, compare with Figures S2, 2D). In addition, the isotype control/anti-CD3 mice showed an increase in the CD4+CD25+ population consistent with that of all mice previously treated with anti-CD3 (Figure 4C).
CD4+CD25+ cells are required in vivo and in vitro for anti-CD3–derived tolerance and mediate suppression through a contact-dependent mechanism. (A) Experimental outline for the in vivo depletion of CD4+CD25+ cells. Anti-CD25 was administered intraperitoneally on days 0 and 5, whereas anti-CD3 was given intravenously on days 3 to 7. On day 10, mice were killed or were immunized with FVIII weekly for 4 weeks. (B) Raw data demonstrating that the spleen was completely depleted of CD4+CD25+ cells after injection of anti-CD25 (clone PC61, n = 3) or isotype control (IgG1, n = 3) during anti-CD3 treatment (10 μg/day for 5 days). Splenocytes from untreated aged-matched mice are shown for reference (n = 3). (C) Quantification of the effect of anti-CD3/anti-CD25 on CD4+ and CD4+CD25+ populations as shown in panel B. Anti-CD3, as shown in previous experiments, can deplete CD4+ cells, and this is clearly evident in the isotype control and anti-CD25 group compared with untreated mice. An increase in CD4+CD25+ cell levels is also evident in the anti-CD3–treated isotype control mice. However, despite anti-CD3 treatment, which increases CD4+CD25+ cell levels, anti-CD25 almost completely depleted this population. (D) Evaluation of FVIII inhibitor formation after depletion of CD4+CD25+ cells. During anti-CD3 treatment (10 μg/day for 5 days), mice were treated with anti-CD25 (n = 6) or isotype control antibody (n = 6), which was followed with 4 FVIII immunizations as previously described. The subsequent immune response to FVIII was analyzed by the Bethesda assay 1 week after the final FVIII immunization. Tolerance to FVIII was completely abrogated in the anti-CD25 mice, but not in the isotype controls. (E) CD4+CD25+ or (F) CD4+CD25− cells isolated from tolerant anti-CD3–treated mice were cocultured, in the presence of FVIII, with total splenocytes from FVIII-immunized, HBSS control animals (no anti-CD3 therapy) in the same well (coculture, CC), or were separated by a transwell membrane (transwell, TW). Data are mean plus or minus SEM.
CD4+CD25+ cells are required in vivo and in vitro for anti-CD3–derived tolerance and mediate suppression through a contact-dependent mechanism. (A) Experimental outline for the in vivo depletion of CD4+CD25+ cells. Anti-CD25 was administered intraperitoneally on days 0 and 5, whereas anti-CD3 was given intravenously on days 3 to 7. On day 10, mice were killed or were immunized with FVIII weekly for 4 weeks. (B) Raw data demonstrating that the spleen was completely depleted of CD4+CD25+ cells after injection of anti-CD25 (clone PC61, n = 3) or isotype control (IgG1, n = 3) during anti-CD3 treatment (10 μg/day for 5 days). Splenocytes from untreated aged-matched mice are shown for reference (n = 3). (C) Quantification of the effect of anti-CD3/anti-CD25 on CD4+ and CD4+CD25+ populations as shown in panel B. Anti-CD3, as shown in previous experiments, can deplete CD4+ cells, and this is clearly evident in the isotype control and anti-CD25 group compared with untreated mice. An increase in CD4+CD25+ cell levels is also evident in the anti-CD3–treated isotype control mice. However, despite anti-CD3 treatment, which increases CD4+CD25+ cell levels, anti-CD25 almost completely depleted this population. (D) Evaluation of FVIII inhibitor formation after depletion of CD4+CD25+ cells. During anti-CD3 treatment (10 μg/day for 5 days), mice were treated with anti-CD25 (n = 6) or isotype control antibody (n = 6), which was followed with 4 FVIII immunizations as previously described. The subsequent immune response to FVIII was analyzed by the Bethesda assay 1 week after the final FVIII immunization. Tolerance to FVIII was completely abrogated in the anti-CD25 mice, but not in the isotype controls. (E) CD4+CD25+ or (F) CD4+CD25− cells isolated from tolerant anti-CD3–treated mice were cocultured, in the presence of FVIII, with total splenocytes from FVIII-immunized, HBSS control animals (no anti-CD3 therapy) in the same well (coculture, CC), or were separated by a transwell membrane (transwell, TW). Data are mean plus or minus SEM.
We repeated the experiment (Figure 4A), but on day 10, mice received the first of 4 weekly FVIII immunizations. One week after the fourth and final FVIII immunization, plasma was assessed for FVIII inhibitor formation by the Bethesda assay (Figure 4D). Tolerance to FVIII was completely ablated in the anti-CD25/anti-CD3–treated mice (mean titer 110 ± 36 BU/mL, P = 3.0 × 10−5), whereas the isotype control/anti-CD3–treated mice remained tolerant (1.2 ± 1.9 BU/mL), at a level consistent with that of mice previously treated with anti-CD3 (Figures 1, 2A,B).
To further study the function of CD4+CD25+ T cells, we performed a transwell experiment (Figure 4E,F). CD4+CD25+ and CD4+CD25− cells were isolated from tolerant anti-CD3–treated mice and cocultured with total splenocytes from HBSS control mice (FVIII immunized but no anti-CD3) in the same well (CC), or in the same well separated by a transwell (TW) membrane. When CD4+CD25+ cells were cultured in the same well as total splenocytes, there was a 31% and 20% suppression of IFN-γ and IL-10 production, respectively. In contrast, when these cell populations were separated by a TW membrane, there was only a 13% and 10% suppression of IFN-γ and IL-10 secretion, respectively. In addition, when CD4+CD25− cells were cocultured in the same well as total control splenocytes, the IFN-γ and IL-10 production increased by 6.5- and 1.9-fold, respectively. Together, these data indicate that the CD4+CD25+ population is immunosuppressive both in vivo and in vitro and requires cellular contact to maximize suppression.
Analysis of Treg phenotypes in anti-CD3–treated C57BL/6 and BALB/c hemophilia A mice
To further investigate the mechanisms regulating the suppressed immune response to FVIII, we studied the T-cell phenotypes to search for Treg markers 1 week after the fourth and final FVIII immunization (Figures 5, 6). The spleens were pooled from “protected” anti-CD3–treated, HBSS-treated, or untreated BALB/c and C57BL/6 mice, and we studied total CD4+ T-cell levels and expression of 6 Treg markers: the alpha chain of the IL-2 receptor (CD25), the GITR, the homing receptor CD62L, the transcriptional repressor FoxP3, CTLA-4, and membrane-bound TGF-β1 (mTGF-β1). We also studied the expression of these markers in C57BL/6 mice that were treated with anti-CD3 but were not tolerant after 4 FVIII immunizations (anti-CD3–nt).
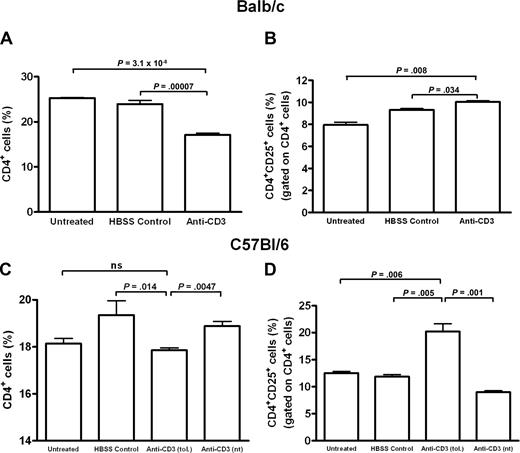
Evaluation of CD4+ and CD4+CD25+ populations in BALB/c and C57BL/6 anti-CD3–treated mice after FVIII immunizations. Splenocytes were pooled and analyzed from anti-CD3–treated, HBSS-treated, or untreated animals (n = 2-3/treatment group) 1 week after the final of 4 FVIII immunizations (1 of 3 and 1 of 2 representative experiments for the BALB/c and C57BL/6 mice, respectively, is shown). The number of CD4+ cells is significantly reduced in the anti-CD3–treated BALB/c (A) and C57BL/6 (C) mice after FVIII immunizations, whereas the CD4+CD25+ populations are increased in both the BALB/c (B) and C57BL/6 (D) mice, although to a much greater extent in the latter. Anti-CD3 (tol.) and anti-CD3 (nt) refer to anti-CD3–treated C57BL/6 mice inhibitor negative or positive, respectively, 1 week after FVIII immunizations. ns indicates not significant.
Evaluation of CD4+ and CD4+CD25+ populations in BALB/c and C57BL/6 anti-CD3–treated mice after FVIII immunizations. Splenocytes were pooled and analyzed from anti-CD3–treated, HBSS-treated, or untreated animals (n = 2-3/treatment group) 1 week after the final of 4 FVIII immunizations (1 of 3 and 1 of 2 representative experiments for the BALB/c and C57BL/6 mice, respectively, is shown). The number of CD4+ cells is significantly reduced in the anti-CD3–treated BALB/c (A) and C57BL/6 (C) mice after FVIII immunizations, whereas the CD4+CD25+ populations are increased in both the BALB/c (B) and C57BL/6 (D) mice, although to a much greater extent in the latter. Anti-CD3 (tol.) and anti-CD3 (nt) refer to anti-CD3–treated C57BL/6 mice inhibitor negative or positive, respectively, 1 week after FVIII immunizations. ns indicates not significant.
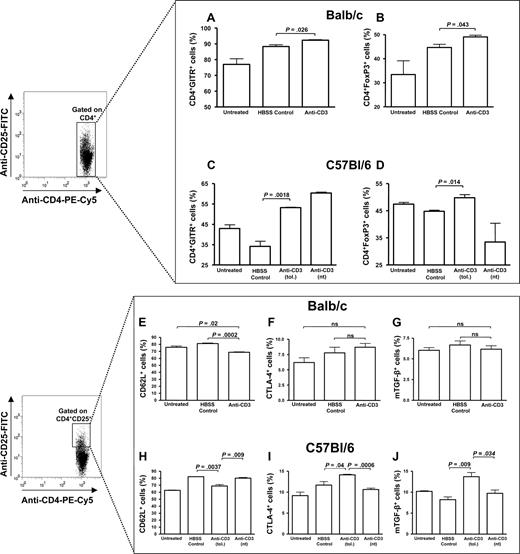
Treatment with anti-CD3 increases the number of CD4+ cells that express FoxP3 and GITR. Splenocytes were pooled and analyzed from anti-CD3–treated, HBSS-treated, or untreated animals (n = 2-3/treatment group) 1 week after the final of 4 FVIII immunizations (1 of 3 and 1 of 2 representative experiments for the BALB/c and C57BL/6 mice, respectively, is shown). There is a significant increase in the number of CD4+ cells expressing GITR and FoxP3 from anti-CD3–treated BALB/c (A,B) and C57BL/6 (C,D) mice. In addition, the C57BL/6 anti-CD3–treated mice expressed significantly more CTLA-4 (I) and mTGF-β (J) on CD4+CD25+ cells than the HBSS-treated and untreated mice, but this did not occur in tolerant BALB/c mice (F,G). Furthermore, there was a significant reduction in CD62L on CD4+CD25+ cells from both the BALB/c (E) and C57BL/6 (H) mice. Anti-CD3 (tol.) and anti-CD3 (nt) refer to anti-CD3–treated C57BL/6 mice inhibitor negative or positive, respectively, 1 week after FVIII immunizations. ns indicates not significant.
Treatment with anti-CD3 increases the number of CD4+ cells that express FoxP3 and GITR. Splenocytes were pooled and analyzed from anti-CD3–treated, HBSS-treated, or untreated animals (n = 2-3/treatment group) 1 week after the final of 4 FVIII immunizations (1 of 3 and 1 of 2 representative experiments for the BALB/c and C57BL/6 mice, respectively, is shown). There is a significant increase in the number of CD4+ cells expressing GITR and FoxP3 from anti-CD3–treated BALB/c (A,B) and C57BL/6 (C,D) mice. In addition, the C57BL/6 anti-CD3–treated mice expressed significantly more CTLA-4 (I) and mTGF-β (J) on CD4+CD25+ cells than the HBSS-treated and untreated mice, but this did not occur in tolerant BALB/c mice (F,G). Furthermore, there was a significant reduction in CD62L on CD4+CD25+ cells from both the BALB/c (E) and C57BL/6 (H) mice. Anti-CD3 (tol.) and anti-CD3 (nt) refer to anti-CD3–treated C57BL/6 mice inhibitor negative or positive, respectively, 1 week after FVIII immunizations. ns indicates not significant.
In the BALB/c animals, 1 week after FVIII immunizations, the total CD4+ levels in the spleen were significantly less in anti-CD3 mice (17.1% ± 0.7%) compared with the untreated (25.3% ± 0.3%) and HBSS-treated control mice (23.9% ± 1.7%) (Figure 5A). In addition, the level of CD4+CD25+ cells in anti-CD3 mice was significantly increased compared with the other groups, but this increase was modest (Figure 5B).
Furthermore, in the C57BL/6 mice, the total number of CD4+ cells was slightly decreased in the tolerant anti-CD3 mice (17.9% ± 0.2%) compared with HBSS-treated (19.4% ± 1.2%, P = .01) and nontolerant anti-CD3 mice (18.9% ± 0.4%, P = .005), but not untreated mice (18.1% ± 0.5%; Figure 5C). In contrast, the CD4+CD25+ population in tolerant anti-CD3 C57BL/6 mice was significantly increased compared with all other groups (Figure 5D). Based on these data, the ratio of CD4+CD25+ to total CD4+ cells in both strains of tolerant mice was greater than all other groups (data not shown).
Anti-CD3 therapy in the BALB/c mice correlated with a significant increase in the CD4+GITR+ and CD4+FoxP3+ cells compared with the untreated and HBSS-treated control mice (Figure 6A,B). However, all groups of mice produced high levels of GITR and FoxP3 in the CD4+CD25+ population (Figure S5). Furthermore, tolerant BALB/c mice had reduced numbers of CD4+CD25+CD62L+ cells compared with untreated and HBSS-treated controls (Figure 6E). Similar to the BALB/c mice, anti-CD3 therapy in tolerant C57BL/6 mice was correlated with a higher number of CD4+GITR+ and CD4+FoxP3+ cells (Figure 6C,D), and there was a significant decrease in CD4+CD25+CD62L+ cells (Figure 6H). However, CD4+CD25+ cells from tolerant anti-CD3–treated C57BL/6 mice expressed significantly higher levels of CTLA-4 and mTGF-β1 compared with all other groups (Figure 6I,J).
Shift to an in vitro Th1 cytokine profile in tolerant anti-CD3–treated BALB/c and C57BL/6 mice
We evaluated the production of T-helper 1 (Th1; IL-2 and IFN-γ) and Th2 (IL-4, IL-5, and IL-10) cytokines by splenocytes from both strains of hemophilic mice (Figure 7). Splenocytes from untreated, HBSS-treated, tolerant, or nontolerant anti-CD3–treated mice were restimulated in vitro with a mixture of anti-CD3/CD28 Abs (to mediate nonspecific activation), with FVIII (to mediate antigen-specific activation) or were not stimulated. Interestingly, splenocytes from tolerant anti-CD3–treated BALB/c and C57BL/6 mice were polarized toward a Th1 cytokine profile (Figure 7A). Splenocytes from these animals produced detectable levels of IL-2 and IFN-γ yet failed to produce significant amounts of IL-4, IL-5, and IL-10 compared with untreated or HBSS-treated mice (Figure 7B). In contrast, splenocytes from both the BALB/c and C57BL/6 HBSS-treated mice secreted both Th1 and Th2 cytokines, both of which to a higher level than the anti-CD3–treated mice. In addition, of note, the anti-CD3–treated C57BL/6 mice that were not tolerant after FVIII immunizations were not Th1-shifted (their cytokine profile was nearly identical to the HBSS-treated control mice). TGF-β secretion was also studied, but there was no difference in production by anti-CD3–treated mice compared with the controls (data not shown).
Tolerant anti-CD3–treated BALB/c and C57BL/6 mice are polarized toward a Th1 cytokine profile. Splenocytes were pooled from anti-CD3–treated, HBSS-treated, or untreated animals (n = 2-3/treatment group) 1 week after the final of 4 FVIII immunizations (1 of 3 and 1 of 2 representative experiments for BALB/c and C57BL/6 mice, respectively). Cells were restimulated in vitro with a combination of anti-CD3 and anti-CD28 mAbs, FVIII, or were not stimulated (unstimulated). Both the BALB/c and C57BL/6 mice treated with anti-CD3 (in vivo) were able to produce Th1 cytokines in vitro (IL-2 and IFN-γ; A) but were limited in their ability to secrete Th2 cytokines (IL-4, IL-5, and IL-10) compared with HBSS-treated and untreated animals after antigen-specific restimulation (B). “Anti-CD3 (tol.)” and “anti-CD3 (nt)” refer to anti-CD3–treated C57BL/6 mice inhibitor negative or positive, respectively, 1 week after FVIII immunizations. *P < .01, **P < 1.0 × 10−5 for anti-CD3 compared with FVIII control; +P < .02, ++P < .002, +++P < 1.0 × 10−4 for anti-CD3 (tol.) compared with anti-CD3 (nt).
Tolerant anti-CD3–treated BALB/c and C57BL/6 mice are polarized toward a Th1 cytokine profile. Splenocytes were pooled from anti-CD3–treated, HBSS-treated, or untreated animals (n = 2-3/treatment group) 1 week after the final of 4 FVIII immunizations (1 of 3 and 1 of 2 representative experiments for BALB/c and C57BL/6 mice, respectively). Cells were restimulated in vitro with a combination of anti-CD3 and anti-CD28 mAbs, FVIII, or were not stimulated (unstimulated). Both the BALB/c and C57BL/6 mice treated with anti-CD3 (in vivo) were able to produce Th1 cytokines in vitro (IL-2 and IFN-γ; A) but were limited in their ability to secrete Th2 cytokines (IL-4, IL-5, and IL-10) compared with HBSS-treated and untreated animals after antigen-specific restimulation (B). “Anti-CD3 (tol.)” and “anti-CD3 (nt)” refer to anti-CD3–treated C57BL/6 mice inhibitor negative or positive, respectively, 1 week after FVIII immunizations. *P < .01, **P < 1.0 × 10−5 for anti-CD3 compared with FVIII control; +P < .02, ++P < .002, +++P < 1.0 × 10−4 for anti-CD3 (tol.) compared with anti-CD3 (nt).
Discussion
For some hemophilia A patients, with ready access to FVIII products free of viral contamination, the formation of FVIII inhibitors is now the most serious complication of their FVIII infusion therapy. In this study, we have demonstrated that modulating the TCR/CD3 complex with a non–FcR-binding anti-CD3 Ab can prevent anti-FVIII Ab formation in 2 different murine models of hemophilia A. Importantly, we found the efficacy of this treatment was greatest with very small doses of anti-CD3 and that tolerance was dependent on splenic CD4+CD25+ cells. In addition, we observed several different Treg phenotypes and a polarization to a Th1 cytokine profile in both strains of tolerant animals.
We optimized the dose of anti-CD3 in the hemophilia A BALB/c model because these mice consistently produce a robust humoral immune response after repeated intravenous immunization with human FVIII. The optimal dose of anti-CD3 in these mice was very low: 10 μg/day for 5 days, administered before FVIII immunizations. This is consistent with a recent suggestion that a low dose of anti-CD3 (< 50 μg/day) favors the survival and expansion of Tregs while destroying effector T cells.14 This clearly has significant implications for clinical translation.
This optimal dose of anti-CD3 protected both the BALB/c and C57BL/6 hemophilic mice from FVIII inhibitor formation. However, inhibitors developed less frequently and at a lower level in anti-CD3–treated BALB/c mice compared with C57BL/6 mice. It is most probable that the difference in efficacy in the C57BL/6 mice was the result of the lack of dose optimization in this strain. Interestingly, the magnitude of the immune response to FVIII was greater in C57BL/6 than in BALB/c control animals (this study; and unpublished observations). Thus, the C57BL/6 mice might be less responsive to the very low-dose anti-CD3 therapy used in this study.
In this and other studies,19,22,29 anti-CD3 treatment promoted the generation of several different Treg phenotypes. For example, an increase in CD4+CD25+ cells after anti-CD3 therapy was shown in the NOD model.19,22 In addition, in mice with experimental autoimmune encephalomyelitis, anti-CD3 protected mice from developing multiple sclerosis–like symptoms, and depleting CD4+CD25+ cells with anti-CD25 reversed anti-CD3–mediated tolerance.26 Similarly, we demonstrated expansion of CD4+CD25+ cells through anti-CD3 therapy; and when these cells were depleted during anti-CD3 treatment, using an anti-CD25 mAb, tolerance to FVIII was completely abrogated. Thus, in 3 different animal models, including now the hemophilia A mouse, anti-CD3–mediated tolerance is strongly dependent on a population of CD4+CD25+ cells. In addition, CD4+CD25+ cells from tolerant animals inhibited cytokine secretion by total splenocytes from FVIII-immunized control mice while removing the CD25+ population (ie, CD4+CD25− cells) completely abrogated this suppression and significantly enhanced cytokine production. Furthermore, suppression was most effective when the Tregs were in cellular contact with the effector cells from control animals because there was less suppression when the cells were physically separated with a transwell membrane.
Interestingly, when hemophilia A mice are depleted of CD4+CD25+ cells and repeatedly immunized with FVIII, they develop much higher FVIII inhibitor levels compared with mice immunized with FVIII but with an intact CD4+CD25+ population (compare Figure 4D with Figure 2B). This finding would perhaps indicate that the CD4+CD25+ compartment might have an important role in limiting the immune response to FVIII in the absence of anti-CD3 therapy.
Previous studies have shown that tolerant anti-CD3–treated NOD mice are capable of rejecting skin or heart allografts and clearing viral infection, suggesting that tolerance mediated through anti-CD3 was antigen-specific.20,21,30 The question of whether the regulatory CD4+CD25+ cells induced in our tolerant hemophilia A mice were antigen-specific remains unclear. However, it has recently become clear that anti-CD3 increases CD25 levels by inducing its expression on peripheral effector CD4+CD25− cells, rather than by expanding thymically derived CD4+CD25+ Tregs.19,29 This is an important observation because effector CD4+ T cells specific for FVIII in the periphery may have been converted to regulatory cells in this study. In addition to enhancing the suppressive capacity of the splenic lymphocyte population, this would concomitantly decrease the number of effector T cells available to respond to the subsequent FVIII immunizations that followed anti-CD3 therapy.
In addition to this, total CD4+ cell levels were reduced at the time of FVIII immunization in anti-CD3–treated mice. Furthermore, after 4 FVIII immunizations, tolerant animals still had decreased total CD4+ levels and increased numbers of CD4+CD25+ cells compared with the HBSS-treated controls. Therefore, the ratio of regulatory to effector cells was higher in anti-CD3–treated mice both before and after FVIII immunizations, and this ratio is probably an important component of tolerance in this setting.
Recent studies have also shown that anti-CD3 can increase expression of GITR and FoxP3 by CD4+ cells.22,29 We observed an expansion in CD4+GITR+ and CD4+FoxP3+ cells in both the tolerant anti-CD3–treated BALB/c and C57BL/6 strains of hemophilia A mice after repeated immunizations with FVIII. Recent studies have clearly demonstrated that FoxP3, and not CD25 expression, correlates with Treg function in mice31 and humans,32 and that CD4+FoxP3+ cells are considered a Treg population.33,34 We would thus propose that the increase in FoxP3, and perhaps GITR expression, is also in part responsible for tolerance to FVIII in both mouse models.
In the tolerant C57BL/6 mice, anti-CD3 treatment also correlated with an increase in the number of CD4+CD25+ cells expressing the Treg markers CTLA-4 and mTGF-β1, but this did not occur in the tolerant BALB/c mice. Indeed, Bresson et al22 demonstrated a similar increase in CTLA-4 expression in the CD4+CD25+ population in diabetic mice treated with anti-CD3 and intranasal insulin peptides, whereas Belghith et al19 showed that a blocking Ab to CTLA-4 prevented anti-CD3–mediated tolerance in the same model. To our knowledge, however, the current study is the first to show that anti-CD3 can correlate with an increase in mTGF-β1 in tolerant animals. From these data, we can speculate that populations of CD4+CD25+CTLA-4+ and CD4+CD25+mTGF-β+ Tregs may contribute to tolerance induction for FVIII in the C57BL/6, but not the BALB/c mice.
In this and previous studies, splenocytes from FVIII immunized hemophilia A mice cultured in vitro with FVIII produce both Th1 and Th2 cytokines.12,19,35,36 However, in the current study, only tolerant C57BL/6 and BALB/c mice treated with anti-CD3 were polarized toward a Th1 cytokine profile (IL-2 and IFN-γ), and this response was reduced by approximately 2-fold compared with the HBSS controls.
In addition, C57BL/6 mice that were anti-CD3–treated but did not remain tolerant after 4 FVIII vaccinations were not Th1-shifted. These results indicate that cytokine shifting, to favor a Th1 response, may play a role in anti-CD3–mediated tolerance.
Polarization of cytokine production in anti-CD3–treated mice has been extensively documented in the diabetic mouse.17,19,20,22,37 In these instances, a robust Th1-mediated immune response that is associated with disease progression develops, but when treated with anti-CD3, the response is polarized to an IL-4–dominant (ie, Th2-shifted) immune response that is associated with tolerance.17,19,20,22,37 Our transwell experiment demonstrated that CD4+CD25+ cells from tolerant animals can reduce both Th1 (IFN-γ) and Th2 (IL-10) cytokines, and it may be that this population is suppressing cytokine secretion in the tolerant animals, thereby reducing the overall immune response to FVIII.
This shift in Th responses to FVIII also shares some similarity with a study by Reding et al.38 These authors analyzed the isotype of anti-FVIII Abs in hemophilia A patients undergoing ITI and found that, after successful ITI, patients mainly produced Th1-driven IgG1 and IgG2 isotypes, whereas patients in whom ITI was unsuccessful primarily had Th2-driven IgG4 Abs.
Currently, there are 2 non-FcR–binding anti-CD3 mAbs that bind to the human CD3 complex.15,16 Both of these mAbs have been successfully tested in phase 1 clinical trials to prevent the onset of T1D. Based on the present study, there may be significant benefits to using anti-CD3 as a preventative therapy for FVIII inhibitor formation. Young boys at high risk for inhibitor development can be identified through the consideration of several factors, including a family history of inhibitors, ethnicity, and high-risk immunomodulatory and FVIII genotypes.39-43 In these patients, a short course of low-dose anti-CD3 treatment with subsequent exposure to FVIII under prophylactic “noninflammatory” conditions may significantly reduce the likelihood of inhibitor development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
B.W. thanks Dr Todd Hatchette (Dalhousie University) for his continuous support.
This work was supported by an operating grant from the Canadian Institutes of Health Research (MOP 10912). B.W. is the recipient of a Canadian Blood Services Graduate Fellowship, and D.L. is the recipient of a Canada Research Chair in Molecular Hemostasis.
Authorship
Contribution: B.W. designed and performed research, analyzed data, and wrote the paper; M.Q. and R.C. designed research; E.B., A.L., P.T., and C.H. performed research; and D.L. directed the study, designed research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Lillicrap, Department of Pathology and Molecular Medicine, Richardson Laboratory, Queen's University, Kingston, ON K7L 3N6; e-mail: lillicrap@cliff.path.queensu.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal