Abstract
Mesenchymal stem cells (MSCs), in addition to their multilineage differentiation, exert immunomodulatory effects on immune cells, even dendritic cells (DCs). However, whether they influence the destiny of full mature DCs (maDCs) remains controversial. Here we report that MSCs vigorously promote proliferation of maDCs, significantly reduce their expression of Ia, CD11c, CD80, CD86, and CD40 while increasing CD11b expression. Interestingly, though these phenotypes clearly suggest their skew to immature status, bacterial lipopolysaccharide (LPS) stimulation could not reverse this trend. Moreover, high endocytosic capacity, low immunogenicity, and strong immunoregulatory function of MSC-treated maDCs (MSC-DCs) were also observed. Furthermore we found that MSCs, partly via cell-cell contact, drive maDCs to differentiate into a novel Jagged-2–dependent regulatory DC population and escape their apoptotic fate. These results further support the role of MSCs in preventing rejection in organ transplantation and treatment of autoimmune disease.
Introduction
Bone marrow (BM)–derived mesenchymal stem cells (MSCs) are rare residents of the BM microenvironment.1 Isolated from their BM companions by their characteristic adherence to plastic, MSCs can be expanded from a single BM aspiration to produce millions of cells.2 In addition to their powerful replicative capacity, MSCs are multipotential and capable of differentiating into osteocytes, chondrocytes, adipocytes, myocytes, endotheliocytes, and neurocytes.3,4 Immunologically, MSCs have unique immunologic characteristics, such as low immunogenicity and immunoregulatory property.5 They express negligible levels of major histocompatibility complex (MHC) class I and no MHC class II or Fas ligand, nor do they express CD80, CD86, CD40, or CD40L.1,2 MSCs have also been reported to inhibit T-cell proliferation induced in a mixed lymphocyte reaction (MLR) or by nonspecific mitogens.6
Dendritic cells (DCs), the most potent antigen-presenting cells (APCs), are pivotal and ubiquitously distributed in immune response. They are derived from CD34+ BM stem cells and can be generated from monocytes in vitro by incubation with granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-4 (IL-4).7 DCs plays a major role in the uptake, transport, and presentation of antigens with the unique capacity to stimulate naive T cell.8 The ability of DCs to initiate an immune response depends on its transition from antigen processing to antigen-presenting cell, during which it up-regulates MHC class II and costimulatory molecules (CD80 and CD86) on the cell surface, a process referred to as DC maturation.9 This transition is indispensable for mounting an immune response because immature DCs (imDCs) not only fail to prime T cells effectively10 but also serve to promote tolerance induction.11 In addition to their polarizing capacity on naive T cells, they can interact with B cells12 and natural killer (NK) cells.13
Notch is an evolutionarily conserved transmembrane protein that was first described as the product of a neurogenic gene in Drosophila. Vertebrate notch homologs have now been identified in various tissues, which play a critical role not only in embryogenesis, but also in the regulation of cell growth and differentiation of adult tissues.14 There are 4 identified notch receptors (Notch1-4) and 5 ligands of the Jagged families (Jagged1, 2) and Delta-like families (Delta-like 1, 3, 4), but the precise functions of each ligand for receptor are not well understood.15,16 Many studies have implicated functions of notch signaling in hematopoiesis and thymic development,17,18 that determine all the choices between T- and B-cell development,19 between TCRαβ and TCRγδ decision20 and between CD4+ and CD8+ T-cell production.21 Moreover, they also play an essential role in the peripheral immune regulation. Hoyne et al observed that murine DCs overexpressed Serrate-1 (the homolog of the human Jagged-1) could generate antigen-specific regulatory T cell that transferred tolerance to naive recipient mice.22 Ohishi et al also demonstrated that the Delta-like 1 could inhibit the differentiation of monocytes into macrophages, but allow their differentiation into DCs.23
Although previous reports clearly display the immunomodulatory effects of MSCs on complicated interactions between T cells6 and even DCs,24 whether they also regulate the destiny of full mature DCs (maDCs) remains unknown. Here we reported that mouse BM-derived MSCs interrupted apoptotic fate of maDCs and induced them into a distinct regulatory DC (regDC) population. Compared with maDCs, they had lower expression of Ia, CD80, CD86, CD40, and CD11c, but higher expression of CD11b. Furthermore, they had stronger endocytosic capacity and greatly inhibited lymphocyte proliferation through a Jagged-2–dependent mechanism.
Methods
Mice
Six- to 8-week-old BALB/C and C57BL/6 mice were purchased from the Laboratory Animal Center of Chinese Academy of Medical Sciences (Beijing, China). Enhanced green fluorescent protein (EGFP)–transgenic mice (C57BL/6-EGFP) were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were bred and maintained under specific pathogen-free conditions. The animal handling and experimental procedures were approved by the Animal Care and Use Committee of the Chinese Academy of Medical Sciences.
Culture of mouse BM-derived MSCs
MSCs were prepared from mouse BM cells as we previously described,25 with minor modifications. Briefly, mononuclear cells isolated by Ficoll-Paque (Sigma-Aldrich, St Louis, MO) density-gradient centrifugation from BM cells flushed from the femurs of BALB/C or C57BL/6 mice were depleted of CD45+ and Ter119+ cells by micromagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen, Raritan, NJ) supplemented with 10% fetal calf serum (FCS; Hyclone, Logan, UT), 10 ng/mL leukemia inhibitory factor, 10 ng/mL platelet-derived growth factor BB (R&D Systems, Minneapolis, MN), 10 ng/mL epidermal growth factor, 10−9 M dexamethasone, and 10−4 M ascorbic acid 2-phosphate (all from Sigma-Aldrich) at 3 × 105/cm2 at 37°C in 5% CO2. The nonadherent cells were removed after 24 to 48 hours, and the adherent cells were harvested by trypsinization (0.25% trypsin) when reaching 90% confluence, and then depleted of Sca-1+ cells by micromagnetic beads (Miltenyi Biotec). The immunophenotypes of these cells were persistently positive for Flk-1, CD29, CD44, and CD13, but negative for Sca-1, Ter119, VWF, GlyA, CD34, CD45, and CD31 for more than 3 passages (data not shown).
Preparation of DCs from BM
Mouse BM–derived DCs were generated from BM progenitors according to an established protocol,26 with minor modifications. BM mononuclear cells were prepared from BALB/C mouse femur BM suspensions by depletion of red cells and then cultured at a density of 2 × 106 cells/mL in 6-well plates in PRMI-1640 (Invitrogen, Grand Island, NY) medium supplemented with 10% FCS, 2 mM l-glutamine (Sigma-Aldrich), 10 ng/mL GM-CSF, and 5 ng/mL IL-4 (all from PeproTec, London, United Kingdom). For imDCs, nonadherent cells were gently washed out on day 4, and the remaining loosely adherent cell clusters were collected and purified by anti-CD11c micromagnetic beads (Miltenyi Biotec). Purified imDCs cultured for a further 4 to 5 days under the stimulation of 10 ng/mL bacterial lipopolysaccharide (LPS; Sigma-Aldrich) were used as maDCs. The purity was greater than 90%. GFP+ DCs were prepared from the EGFP-transgenic mice using the same protocol.
Coculture experiment
Once MSCs reached 50% confluence, maDCs were seeded onto MSC monolayers at a density of 106 per 5 mL per well in 6-well plates, and MSC culture medium was replaced with RPMI 1640 supplemented with 5% FCS. DCs cultured on MSCs for at least 10 days were washed off with 0.1% trypsin and 5 mM EDTA, purified with anti-CD11b micromagnetic beads (Miltenyi Biotec). GFP+ DCs were visualized by fluorescence microscopy.
Cell-cycle analysis
Briefly, 5 × 105 of maDCs or MSC-treated maDCs (MSC-DCs) were harvested and fixed overnight with 75% cold ethanol, washed twice with cold phosphate-buffered saline (PBS), and then incubated in PBS containing 50 μg/mL propidium iodide (PI) and 20 μg/mL RNase A for 30 minutes at 37°C. PI and forward light scattering were detected using a flow cytometer (FACSCalibur; Becton Dickinson, San Jose, CA) equipped with the ModFit LT software package.
Transmission electron microscopy
For transmission electron microscopy (TEM) observation, imDCs, maDCs, or MSC-DCs were respectively fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 10 hours at 4°C, postfixed in osmium tetride, and dehydrated. Fixed cells were subsequently embedded in Epon812 and sectioned at 800A°, stained with uranyl acetate and lead citrate, and viewed on a JEM-1010 transmission electron microscope operating at 80 kV.
FACS analysis
Cultured DCs were harvested and their phenotypes evaluated after micromagnetic bead purification. The cells were incubated for 15 minutes at 4°C with antibody against CD16/CD32 at a concentration of 1 μg per 106 cells per 100 μL for blockade of Fc receptors, washed twice with ice-cold PBS (pH 7.2) containing 0.1% NaN3 and 0.5% bovine serum albumin (BSA), and then incubated with respective fluorescent antibodies for 30 minutes at 4°C, washed twice, and resuspended in 300 μL PBS. Fluorescent antibodies included fluorescein isothiocyanate (FITC)–conjugated anti–mouse CD11c, CD80, CD86, and CD40, and phycoerythrin (PE)–conjugated anti–mouse CD11b and I-A/E, and same-species, same-isotype IgG was used as isotype control (all from Becton Dickinson). Purified antibody to CD16/CD32 (rat IgG2b; clone 2.4G2), FITC-conjugated rabbit anti–goat IgG, FITC-conjugated goat anti–rabbit IgG were from BD Pharmingen (San Diego, CA). Purified antibodies against mouse Jagged-1, Delta-1 (goat, IgG), and Jagged-2 (rabbit, IgG) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Analysis was performed on a FACS using CellQuest software (Becton Dickinson).
Assay for cytokines
For detection of IL-12, IL-10, and transforming growth factor β (TGF–β) produced by APCs, cells were cultured for 24 hours with or without LPS (0.5 μg/mL) stimulus. Cytokines in supernatants at day 3 of mixed lymphocyte culture (MLC) were detected using ELISA kits (R&D Systems) for IL-2 and interferon γ (IFN–γ).
Endocytosis assay
For determination of the phagocytic ability of DCs, purified maDCs or MSC-DCs were incubated at 37°C or at 4°C as negative control for 4 hours with FITC-conjugated OVA (Sigma-Aldrich) at a final concentration of 100 μg/mL in RPMI 1640 containing 10% FCS, washed twice with ice-cold PBS (pH 7.2) containing 0.1% NaN3 and 0.5% BSA, and resuspended in chilled PBS for immediate flow cytometry.
Mixed lymphocyte culture
After labeled by carboxyfluorescein diacetate succinimidyl diester (CFSE) and suspended in RPMI 1640 supplemented with 10% FCS, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% HEPES buffer, and 10 μM 2-mercaptoethanol, splenocytes (5 × 105 cells/well) from C57BL/6 (H-2Kb) mice together with APCs (maDCs or MSC-DCs; 5 × 104 cells/well) from BALB/C (H-2Kd) mice, or splenocytes (5 × 105 cells/well) from normal or recipient BALB/C (H-2Kd) mice treated by C57BL/6–MSC-DCs together with lethally irradiated (30 Gy) splenocytes (5 × 105 cells/well) from donor C57BL/6 (H-2Kb) mice in allogeneic delayed type hypersensitivity (allo-DTH) assay, in a total volume of 0.2 mL medium were cocultured for 3 days in 96-well U-bottom plates (NUNC, Roskilde, Denmark) at 37°C in 5% CO2 and subsequently counted by FACS.
Mitogen proliferative assay
CFSE-labeling splenocytes (5 × 105 cells/well) were incubated with 5 μg/mL concanavalin A (ConA; Sigma-Aldrich) in the presence or absence of MSC-DCs (5 × 104 cells/well) in a total volume of 0.2 mL medium in 96-well U-bottom plates at 37°C in 5% CO2, harvested 72 hours later, and counted by FACS.
In vivo allo-DTH assay
An in vivo assay for allo-DTH was performed as reported previously,27 but with some changes. Briefly, BALB/C mice were immunized on the dorsal flank by subcutaneous inoculation of C57BL/6 splenocytes (108 cells/mouse) on day 0 and challenged on day 7 and day 14 at the hind footpad by injecting the same antigens (108 cells/mouse). H-2Kd–APCs from BALB/C mice or H-2Kb–MSC-DCs from C57BL/6 (−EGFP) mice were injected intraperitoneally (3 × 106 cells/mouse) on days −6, −4, 0, 3, and 6. Footpad thickness was then measured on days 8 and 15, respectively, with a calipers-type engineers' micrometer by a third experimenter who did not know the sample identity. The extent of swelling was calculated as the thickness of the right footpad (receiving C57BL/6 splenocytes) minus the baseline thickness of the left footpad (receiving BALB/C splenocytes). For detection of the immunologic characteristics of MSC-DCs in vivo, we analyzed all of the splenocytes isolated from recipient BALB/C (H-2Kd) mice treated by C57BL/6-EGFP–MSC-DCs by FACS on days 8 and 15, and an I-A/E and EGFP double-staining analysis of CD11c+cells, sorting-enriched by micromagnetic beads from splenocytes, was subsequently performed by flow cytometry. To determine whether MSC-DCs induced a donor-specific tolerance, MLCs of splenocytes from normal or C57BL/6-MSC-DC–treated recipient BALB/C (H-2Kd) mice (responders) with lethally irradiated splenocytes from donor C57BL/6 (H-2Kb) mice (stimulators) was executed 15 days later, and subsequently mitogen proliferative assay was also elicited with ConA (5 μg/mL).
Western blot
The expression levels of Jagged-1, Jagged-2, and Delta-1 in DCs were analyzed by Western blot as previously described.28 DCs (2 × 106) were lysed in 100 μL lysis buffer (10% glycerol, 2 mM EDTA [pH 8.0], 0.5% Nonidet P-40, 137 mM NaCl, and 50 mM Tris-HCl [pH 8.0]), and an amount of 30 μg total protein from whole-cell lysates was separated on 10% SDS–polyacrylamide gels and transferred onto a polyvinylidene difluoride membrane. The following primary antibodies were used for Western blot analysis: anti–Jagged-1, anti–Jagged-2, anti–Delta-1 (1/500; Santa Cruz Biotechnology), and antiactin (1/1000; Santa Cruz Biotechnology). After incubation with the appropriate secondary HRP-labeled antibodies, detection was performed using an ECL Western blotting substrate (Millipore, Billerica, MA).
mRNA interference
siRNAs were designed by an online target finder (http://www.ambion.com/techlib/tb/tb_506.html). Two sequences specific to the Jagged-2 gene were selected: target 1 (CGA CTT CTT TGG CCA CTA TAC) and target 2 (GGA CAT ACT CTA CCA GTG CAA). All siRNA duplexes were obtained from Sangon Biotech (Shanghai, China). To generate knockdown vectors, annealed sets of oligonucleotides were ligated into pMSCVneo/eGFP-U6 (Clontech, Mountain View, CA) according to the manufacturer's instructions. RNAi retroviruses were produced by the 293T packaging line according to an established protocol.29 After centrifuged at 250g for 10 minutes and incubated (1.5 × 106 cells/25 cm2 flask) in 4 mL serum-free Iscove modified Dulbecco medium (IMDM) combined with 1 mL pMSCVneo/eGFP-U6-RNAi retroviral vector solution at a multiplicity of infection (MOI) of 10 for 4 hours in the presence of 8 μg/mL polybrene, purified MSC-DCs were washed and recultured in IMDM supplemented with 10% FCS and 400 μg/mL G418 (Sigma-Aldrich), harvested for subsequent assays at day 5. Transduction efficiency was evaluated by counting GFP+ cells by flow cytometry at 48 hours after transduction, and interference efficiency was analyzed by real-time PCR and Western blot (see a detailed RNAi protocol in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Transwell culture assay
In this assay, a transwell system with 0.3-μm pore size permeable membrane (Corning Costar, Cambridge, MA) was used to separate maDCs physically from MSCs. Briefly, maDCs were directly seeded onto MSCs, or into the upper insert of a transwell system for physical separation from MSCs, at a density of 5 × 105 cells/well in 6-well plates in RPMI 1640 supplemented with 5% FCS at 37°C in 5% CO2. After 7 days of culture, DCs were harvested for FACS analysis and MLCs.
Statistical analysis
All experiments were performed at least 3 times. Mean plus or minus SD of independent experiments was analyzed using the statistical SPSS 11.5 software (Chicago, IL). For intergroup comparison, paired t test was used and P values less than .05 were considered statistically significant.
Results
MSCs induce maDCs into a novel DC population
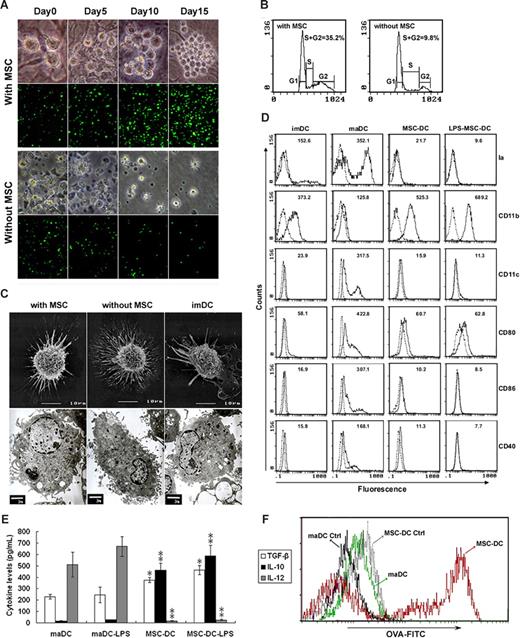
To investigate the influence of MSCs on the proliferation of maDCs, we seeded maDCs prepared from EGFP-transgenic mice on MSC monolayers. The maDCs adhered to the MSCs and proliferated vigorously (Figure 1A). In contrast to the short-term survival (∼ 1 week) of maDCs in the absence of MSCs, maDCs cocultured with MSCs showed long-term survival and continued to proliferate for several months (data not shown). After 15 days of coculture with MSCs, the number of maDCs increased approximately 10-fold (Figure 1A). Cell-cycle analysis demonstrated that 35% of maDCs were in the S and G2 phases after coculture with MSCs for 5 days, whereas fewer than 10% of maDCs were in the S and G2 phases in the absence of MSCs (Figure 1B). Therefore, these results indicate that MSCs can induce maDCs to undergo active cell cycling and promote the proliferation of maDCs.
MSCs induce maDCs into a novel DC population. (A) Expansion of maDCs seeded onto MSCs for 15 days. Increased cell number was obvious in culture with MSCs (top panel, maDCs from normal mice, ×400; bottom panel, maDCs from EGFP-transgenic mice, ×100). (B) Cell-cycle analysis of maDC cultured with or without MSCs. Values are the percentages of S/G2 cells. Data are representative of at least 3 independent experiments. (C) Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of maDC cultured with or without MSCs and imDC. During coculture with MSC, the nonadherent maDC with many long dendrites (without MSC group) gradually became larger with less and shorter dendrites (with MSC group). Similar to imDC, it displayed heterogenous morphology and measured on average 10 to 12 μm in diameter, possessed scattered chromatin and a relatively large nucleus. It had fewer mitochondria, endoplasmatic reticulum and vesicles, but much scattered ribosomes compared with maDC without MSC. (D) Phenotype of MSC-treated DCs. The imDC, maDC, MSC-DC (maDC cocultured with MSC for 7 days), and LPS-MSC-DC (MSC-DC stimulated by LPS for 3 days) were analyzed by flow cytometry. Dotted lines indicate background staining. Numbers in histograms indicate the mean fluorescence of each DC population. (E) Cytokine secretion profiles of maDC, MSC-DC, and MSC-DC stimulated with 500 ng/mL LPS. Data are mean (± SD) of triplicate wells. *P < .05; **P < .001. (F) MSC-DC has an enhanced phagocytic ability than maDC evaluated by OVA-FITC phagocytosis using FACS. One of at least 3 independent experiments with similar results is shown.
MSCs induce maDCs into a novel DC population. (A) Expansion of maDCs seeded onto MSCs for 15 days. Increased cell number was obvious in culture with MSCs (top panel, maDCs from normal mice, ×400; bottom panel, maDCs from EGFP-transgenic mice, ×100). (B) Cell-cycle analysis of maDC cultured with or without MSCs. Values are the percentages of S/G2 cells. Data are representative of at least 3 independent experiments. (C) Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of maDC cultured with or without MSCs and imDC. During coculture with MSC, the nonadherent maDC with many long dendrites (without MSC group) gradually became larger with less and shorter dendrites (with MSC group). Similar to imDC, it displayed heterogenous morphology and measured on average 10 to 12 μm in diameter, possessed scattered chromatin and a relatively large nucleus. It had fewer mitochondria, endoplasmatic reticulum and vesicles, but much scattered ribosomes compared with maDC without MSC. (D) Phenotype of MSC-treated DCs. The imDC, maDC, MSC-DC (maDC cocultured with MSC for 7 days), and LPS-MSC-DC (MSC-DC stimulated by LPS for 3 days) were analyzed by flow cytometry. Dotted lines indicate background staining. Numbers in histograms indicate the mean fluorescence of each DC population. (E) Cytokine secretion profiles of maDC, MSC-DC, and MSC-DC stimulated with 500 ng/mL LPS. Data are mean (± SD) of triplicate wells. *P < .05; **P < .001. (F) MSC-DC has an enhanced phagocytic ability than maDC evaluated by OVA-FITC phagocytosis using FACS. One of at least 3 independent experiments with similar results is shown.
During the coculture, the nonadherent maDCs with many long dendrites gradually became larger DCs with less and shorter dendrites (Figure 1A,C). Interestingly, in contrast to maDCs, phenotype analysis (Figure 1D) showed that this MSC-treated maDCs expressed more myeloid lineage marker CD11b but less CD11c and the functional markers such as Ia, CD80, CD86, and CD40, similar to imDCs. However, in contrast to imDCs, addition of LPS to these cells could not restore the expression of the above markers (Figure 1D), indicating MSCs induced maDCs into a novel DC population (MSC-DC) with a stable phenotype similar to imDCs.
To further characterize MSC-DCs, we determined their cytokine expression patterns by ELISA (Figure 1E). In contrast to maDCs, MSC-DCs spontaneously secreted more TGF-β and IL-10 but less IL-12. This profile was significantly enhanced after LPS stimulation, suggesting that MSC-DCs maybe be involved in immune regulation. We also found that MSC-DCs had greater phagocytic capacity compared with maDCs (Figure 1F). These results demonstrate that mouse BM-derived MSCs induce maDCs to differentiate into a unique population of DCs.
The low immunogenicity and immunoregulatory property of MSC-DCs in vitro
To investigate whether the ability of MSC-DCs to stimulate lymphocyte proliferation was different from that of maDCs, MLC was performed. CFSE-labeled splenic lymphocytes were used as responders and cocultured with allogenic maDCs or MSC-DCs. Our results demonstrated that MSC-DCs could only slightly stimulate lymphocyte proliferation (Figure 2A), even in the presence of 10 ng/mL LPS for 5 days (data not shown).
The low immunogenicity and immunoregulatory property of MSC-DCs. (A) The influence of MSC-DCs on lymphocyte proliferation. The top panel shows that lymphocytes (H-2Kb) were stained with CFSE and cocultured for 3 days with maDCs (H-2Kd), ConA (5 μg/mL), MSC-DCs (H-2Kd), MSC-DCs (H-2Kd) + Trans, MSC-DCs (H-2Kd) + maDCs (H-2Kd), MSC-DCs (H-2Kd) + ConA (5 μg/mL), MSC-DCs (H-2Kd) + Trans + maDCs (H-2Kd), and MSC-DCs (H-2Kd) + Trans + ConA (5 μg/mL) respectively, then assessed by FACS. Data represent mean (± SD) of triplicate samples. *P < .05. In the bottom panel, 1 FACS analysis of at least 3 independent experiments with similar results is shown. Dotted line, unstimulated lymphocytes. Trans indicates a transwell system used in the MSC-DCs/maDCs/lymphocytes or MSC-DCs/ConA/lymphocytes cocultural system to determine whether intercellular contact or soluble factors were involved in the inhibitory function of MSC-DCs. (B) The influence of MSC-DCs on IFN-γ and IL-2 secretion by lymphocytes. Lymphocytes (H-2Kb) were cocultured for 5 days with maDCs (H-2Kd) or ConA (5 μg/mL) in the absence or presence of MSC-DCs (H-2Kd) or MSC-DCs (H-2Kd) + Trans. IFN-γ and IL-2 in each well were assayed by ELISA. Data are mean (± SD) of triplicate wells. *P < .05.
The low immunogenicity and immunoregulatory property of MSC-DCs. (A) The influence of MSC-DCs on lymphocyte proliferation. The top panel shows that lymphocytes (H-2Kb) were stained with CFSE and cocultured for 3 days with maDCs (H-2Kd), ConA (5 μg/mL), MSC-DCs (H-2Kd), MSC-DCs (H-2Kd) + Trans, MSC-DCs (H-2Kd) + maDCs (H-2Kd), MSC-DCs (H-2Kd) + ConA (5 μg/mL), MSC-DCs (H-2Kd) + Trans + maDCs (H-2Kd), and MSC-DCs (H-2Kd) + Trans + ConA (5 μg/mL) respectively, then assessed by FACS. Data represent mean (± SD) of triplicate samples. *P < .05. In the bottom panel, 1 FACS analysis of at least 3 independent experiments with similar results is shown. Dotted line, unstimulated lymphocytes. Trans indicates a transwell system used in the MSC-DCs/maDCs/lymphocytes or MSC-DCs/ConA/lymphocytes cocultural system to determine whether intercellular contact or soluble factors were involved in the inhibitory function of MSC-DCs. (B) The influence of MSC-DCs on IFN-γ and IL-2 secretion by lymphocytes. Lymphocytes (H-2Kb) were cocultured for 5 days with maDCs (H-2Kd) or ConA (5 μg/mL) in the absence or presence of MSC-DCs (H-2Kd) or MSC-DCs (H-2Kd) + Trans. IFN-γ and IL-2 in each well were assayed by ELISA. Data are mean (± SD) of triplicate wells. *P < .05.
As shown in Figure 1, MSC-DCs had a stable immature-like phenotype and secrete TGF-β, an important inhibitory cytokine.30,31 We postulated that MSC-DCs may have the immune regulatory function. To confirm this, we added MSC-DCs into maDCs/lymphocytes or ConA/lymphocytes coculture system to see whether MSC-DCs could inhibit lymphocyte proliferation. The results confirmed that MSC-DCs significantly suppressed the lymphocyte proliferation stimulated by both maDCs and ConA (Figure 2A). Meanwhile, we also observed that the concentrations of IFN-γ and IL-2 in culture supernatant were greatly reduced in the presence of MSC-DCs (Figure 2B).
To explore whether immunoregulatory property of MSC-DCs depends on a direct contact with lymphocytes or is mediated by soluble factors, we added MSC-DCs into the upper insert of a transwell system physically separated from maDCs/lymphocytes or ConA/lymphocytes by permeable membrane. The results showed that MSC-DCs significantly suppressed lymphocyte proliferation and production of IFN-γ and IL-2, but this inhibitory effect almost disappeared in the transwell system (Figure 2), indicating that MSC-DCs might exert their inhibitory effects through direct contact between cells rather than by soluble factors present in culture supernatant.
Accordingly, our results show that MSC-DCs alone fail to promote proliferation of lymphocytes and have a powerful ability to inhibit maDC- or ConA-induced lymphocyte proliferation and IFN-γ and IL-2 generation through a cell-cell contact mechanism. Thus MSC-DCs are a novel DC population with low immunogenicity and high immunoregulatory potential.
In vivo infusion of MSC-DCs suppresses allo-DTH reaction
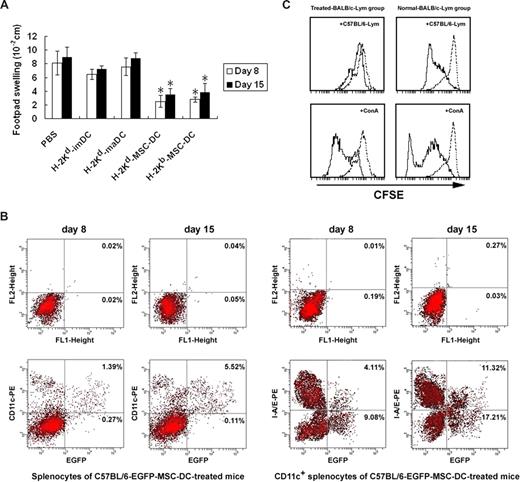
Because MSC-DCs were potent in inhibiting the lymphocyte proliferation in vitro, we wondered whether MSC-DCs could suppress allospecific immune reaction in vivo. An allo-DTH experiment was performed for its simplicity and reliability to study allospecific immune reaction.32 As shown in Figure 3A, the footpad swelling of BALB/C mice receiving the alloantigen (C57BL/6 splenocytes) was suppressed more significantly by infusion of BALB/C or C57BL/6 ([mice]EGFP) mice-derived MSC-DCs.
MSC-DCs inhibit allo-DTH. (A) Treatment of allo-DTH by MSC-DCs. BALB/C mice were immunized on the dorsal flank by subcutaneous inoculation of C57BL/6 spleen cells (108 cells/mouse) on day 0, and challenged on day 7 and day 14 at the hind footpad by injecting the same antigens (108 cells/mouse). H-2Kd–APCs from BALB/C mice or H-2Kb–MSC-DCs from C57BL/6 (−EGFP) mice were injected intraperitoneally (3 × 106 cells/mouse) on days −6, −4, 0, 3, and 6. Footpad thickness was then measured on day 8 and day 15. Data are representative of 3 independent experiments, showing the mean (± SD) of footpad swelling on indicated day. *P < .05. (B) MSC-DCs proliferated and up-regulated their CD11c and Ia expression in vivo. EGFP+MSC-DC–treated BALB/C mice were killed on days 8 and 15, and all of the splenocytes isolated from these mice were analyzed for the levels of EGFP and CD11c expression by flow cytometry. Subsequently, CD11c+ cells were enriched from splenocytes by magnetic-activated cell sorting. MSC-DC proliferation and Ia expression in vivo were measured by I-A/E and EGFP double-staining FACS analysis and showed with a percentage change. A representative of at least 3 independent experiments is shown. (C) MSC-DCs induced a donor-specific tolerance. Normal or C57BL/6-MSC-DC–treated BALB/C mice were killed 15 days later, and splenocytes were used as responder cells in MLC and mitogen proliferative assay. Lethally irradiated splenocytes from C57BL/6 mice or ConA (5 μg/mL) were used as stimulators. Splenocytes from normal mice served as control. The proliferative responses were assessed by CFSE label and FACS. Dotted line, unstimulated splenocytes.
MSC-DCs inhibit allo-DTH. (A) Treatment of allo-DTH by MSC-DCs. BALB/C mice were immunized on the dorsal flank by subcutaneous inoculation of C57BL/6 spleen cells (108 cells/mouse) on day 0, and challenged on day 7 and day 14 at the hind footpad by injecting the same antigens (108 cells/mouse). H-2Kd–APCs from BALB/C mice or H-2Kb–MSC-DCs from C57BL/6 (−EGFP) mice were injected intraperitoneally (3 × 106 cells/mouse) on days −6, −4, 0, 3, and 6. Footpad thickness was then measured on day 8 and day 15. Data are representative of 3 independent experiments, showing the mean (± SD) of footpad swelling on indicated day. *P < .05. (B) MSC-DCs proliferated and up-regulated their CD11c and Ia expression in vivo. EGFP+MSC-DC–treated BALB/C mice were killed on days 8 and 15, and all of the splenocytes isolated from these mice were analyzed for the levels of EGFP and CD11c expression by flow cytometry. Subsequently, CD11c+ cells were enriched from splenocytes by magnetic-activated cell sorting. MSC-DC proliferation and Ia expression in vivo were measured by I-A/E and EGFP double-staining FACS analysis and showed with a percentage change. A representative of at least 3 independent experiments is shown. (C) MSC-DCs induced a donor-specific tolerance. Normal or C57BL/6-MSC-DC–treated BALB/C mice were killed 15 days later, and splenocytes were used as responder cells in MLC and mitogen proliferative assay. Lethally irradiated splenocytes from C57BL/6 mice or ConA (5 μg/mL) were used as stimulators. Splenocytes from normal mice served as control. The proliferative responses were assessed by CFSE label and FACS. Dotted line, unstimulated splenocytes.
To evaluate the immunologic characteristics of MSC-DCs in vivo, we analyzed all of the splenocytes of C57BL/6-EGFP-MSC-DC–treated recipient mice by FACS on days 8 and 15. The results showed that C57BL/6-EGFP mice–derived MSC-DCs were present in spleen, consisting of 1.66% (on day 8) and 5.63% (on day 15) of splenocytes of treated mice (Figure 3B), implicating the allogeneic C57BL/6-EGFP mice–derived MSC-DCs underwent an obvious proliferation in spleen of recipient mice after infusion. Furthermore, we also found that splenocytes consisted of EGFP+CD11c+, EGFP+CD11c−, EGFP−CD11c+, and EGFP−CD11c− cells, and most of EGFP+ cells expressed CD11c (Figure 3B). As MSC-DCs generated in vitro lacked CD11c expression, MSC-DCs up-regulated CD11c levels during proliferation in vivo. We consequently collected magnetic-activated cell sorting–enriched CD11c+ cells by micromagnetic beads from these splenocytes for further FACS analysis of Ia expression of the CD11c+EGFP+ cell population. Interestingly, a proportion of EGFP+Ia+ cells were subsequently detected (Figure 3B), indicating that expression of Ia on some MSC-DCs also increased in vivo.
Importantly, what concerned us most was whether the allo-DTH suppression described above was donor specific. For this purpose, MLC assay and mitogen proliferative assay were respectively performed 15 days later. In MLC assay, the CFSE-labeled splenocytes from C57BL/6-MSC-DC–treated recipient BALB/C mice showed an obviously low response against irradiated splenocytes from donor C57BL/6 mice. In contrast, a strong alloreactivity was observed in normal mice (Figure 3C). In subsequent mitogen proliferative assays, both responder splenocytes from treated mice and normal mice proliferated intensively, and no significant difference was observed (Figure 3C). These data clearly demonstrated that splenocytes from treated mice had normal proliferative responses to nonspecific mitogen, but low proliferative response against donor-specific antigen. Thus, we suggested that MSC-DCs, even from allogeneic donors, might be used as a negative regulator for immune response and have the potential to prevent or treat transplant rejection or autoimmune diseases.
Immunologic characteristics of MSC-DCs are attributed to up-regulation of Jagged-2
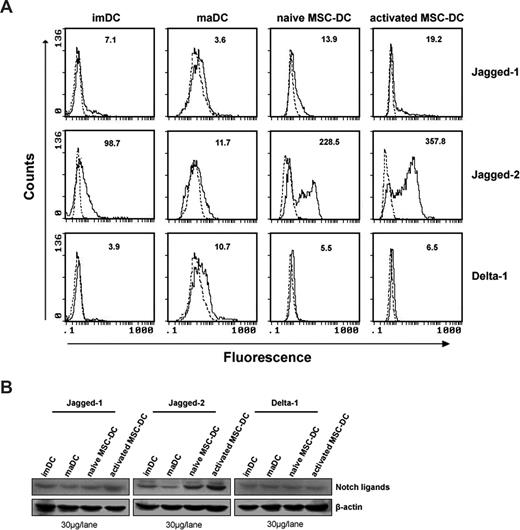
Our research has shown that the in vitro immunoregulatory property of MSC-DCs depends on a cell-cell contact mechanism. Yvon et al reported that overexpression of Jagged-1 in the context of alloantigen presentation represented the critical signal for induction of a specific regulatory T cell able to regulate alloantigen responses.33 To further investigate the exact molecular mechanisms involved in the inhibitory function of MSC-DCs, we evaluated the expression levels of Jagged-1, Jagged-2, and Delta-1 of their cellular surface as previously described.34 Surprisingly, we observed that MSC-DCs expressed more Jagged-2 molecules than imDCs and maDCs. Furthermore, the expression of Jagged-2 was obviously up-regulated after 48 hours in MLC (Figure 4A). The same results were confirmed by Western blot (Figure 4B).
MSC-DCs up-regulate Jagged-2 expression. (A) FACS analysis for expression of notch ligands on MSC-DCs. Expression of Jagged-1, Jagged-2, and Delta-1 on the surface of imDC, maDC, MSC-DC, and activated MSC-DC (48 hours after MLC) were analyzed by flow cytometry. Dotted lines indicate background staining. Numbers in histograms indicate the geometric mean fluorescence of each DC population. (B) Western blot detection for notch ligands of MSC-DCs. The expression of Jagged-1, Jagged-2, and Delta-1 protein were further detected by Western blot, using 30 μg protein per lane of lysates from imDC, maDC, MSC-DC, and activated MSC-DC. Results are representative of at least 5 independent experiments.
MSC-DCs up-regulate Jagged-2 expression. (A) FACS analysis for expression of notch ligands on MSC-DCs. Expression of Jagged-1, Jagged-2, and Delta-1 on the surface of imDC, maDC, MSC-DC, and activated MSC-DC (48 hours after MLC) were analyzed by flow cytometry. Dotted lines indicate background staining. Numbers in histograms indicate the geometric mean fluorescence of each DC population. (B) Western blot detection for notch ligands of MSC-DCs. The expression of Jagged-1, Jagged-2, and Delta-1 protein were further detected by Western blot, using 30 μg protein per lane of lysates from imDC, maDC, MSC-DC, and activated MSC-DC. Results are representative of at least 5 independent experiments.
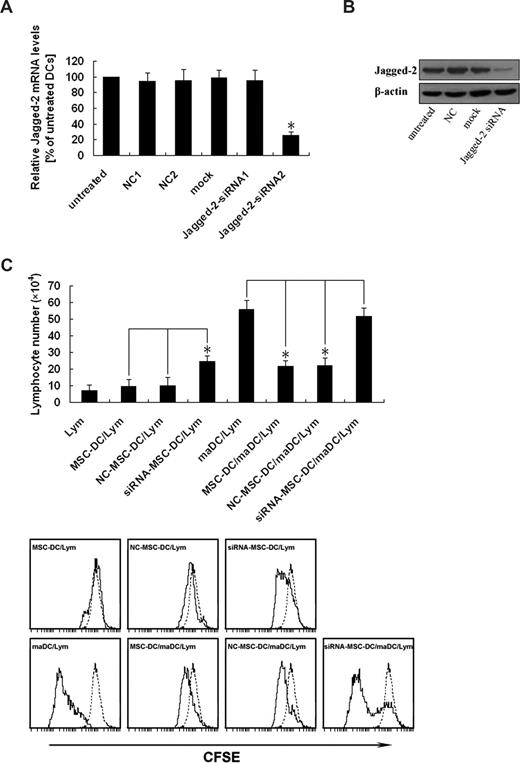
To further determine whether the inhibitory effect of MSC-DCs was really mediated by Jagged-2, Jagged-2 expression of MSC-DCs was silenced by RNAi. To identify the specific siRNA targeted against Jagged-2, we analyzed the mRNA level of Jagged-2 by real-time PCR. Figure 5A demonstrated the knockdown efficiency of different Jagged-2–targeted sequences. The Jagged-2 mRNA level of untreated group was regarded as the calibrator that was set 100% (Figure 5A column 1). For the group of NC1, NC2, Mock, and Jagged-2–siRNA1 (Figure 5A columns 2-5), no significant effect on Jagged-2 mRNA could be observed. A significant reduction of the total amount of Jagged-2 mRNA to approximately 25% to 30% was detected in case of Jagged-2–siRNA2 (P < .01, marked by asterisks; Figure 5A column 6). The Jagged-2–siRNA2 and the relevant NC groups were chosen for Jagged-2 protein analysis.
Up-regulation of Jagged-2 determines the immunologic characteristics of MSC-DCs. (A) Identification of an efficient and specific siRNA targeted against Jagged-2. The MSC-DC was infected with different Jagged-2–siRNA retroviral vectors. After 5 days of transfection, total RNA was isolated and reverse transcribed, and real-time PCR was performed with Jagged-2 mRNA as the target and β-actin as an internal control. The normalized ratio of Jagged-2 to β-actin in untreated cells was set to 100% (column 1). The transfection with scrambled negative control (NC) 1 (column 2), NC2 (column 3), mock (column 4) siRNA, and Jagged-2–siRNA1 demonstrated no significant down-regulation of the Jagged-2 mRNA. A significant effect (*P < .01) on Jagged-2 mRNA was detected for Jagged-2–siRNA2 (column 6). The specific mRNA level was reduced up to 70%. The data are presented as mean (± SD) of 3 independent experiments. Changes were considered as significant if P < .01. (B) Jagged-2 knockdown is confirmed at the protein level. After 5 days of transfection, the total cellular protein of extract of DCs was separated for analysis of Jagged-2 expression. Jagged-2-siRNA–MSC-DCs showed dramatically reduced Jagged-2 protein levels compared with untreated, NC, and mock vector treated cells. The data are representative of 3 independent experiments with cells. (C) Knockdown of Jagged-2 enhances the immunogenicity while decreases the immunoregulatory function of MSC-DCs. The top panel shows that CFSE-labeled lymphocytes (H-2Kb) were cultured with maDCs (H-2Kd), MSC-DCs (H-2Kd), NC-MSC-DCs, (H-2Kd) and siRNA–MSC-DCs (H-2Kd), respectively, in the absence or presence of maDCs (H-2Kd) stimulation for 3 days, and the total number of live lymphocytes in each well was measured by flow cytometry. Data represent mean (± SD) of triplicate samples. *P < .05. In the bottom panel, FACS analysis showed that the effects of Jagged-2 RNAi on the lymphocyte proliferation. Data are representative of at least 3 independent experiments. Dotted line, unstimulated lymphocytes.
Up-regulation of Jagged-2 determines the immunologic characteristics of MSC-DCs. (A) Identification of an efficient and specific siRNA targeted against Jagged-2. The MSC-DC was infected with different Jagged-2–siRNA retroviral vectors. After 5 days of transfection, total RNA was isolated and reverse transcribed, and real-time PCR was performed with Jagged-2 mRNA as the target and β-actin as an internal control. The normalized ratio of Jagged-2 to β-actin in untreated cells was set to 100% (column 1). The transfection with scrambled negative control (NC) 1 (column 2), NC2 (column 3), mock (column 4) siRNA, and Jagged-2–siRNA1 demonstrated no significant down-regulation of the Jagged-2 mRNA. A significant effect (*P < .01) on Jagged-2 mRNA was detected for Jagged-2–siRNA2 (column 6). The specific mRNA level was reduced up to 70%. The data are presented as mean (± SD) of 3 independent experiments. Changes were considered as significant if P < .01. (B) Jagged-2 knockdown is confirmed at the protein level. After 5 days of transfection, the total cellular protein of extract of DCs was separated for analysis of Jagged-2 expression. Jagged-2-siRNA–MSC-DCs showed dramatically reduced Jagged-2 protein levels compared with untreated, NC, and mock vector treated cells. The data are representative of 3 independent experiments with cells. (C) Knockdown of Jagged-2 enhances the immunogenicity while decreases the immunoregulatory function of MSC-DCs. The top panel shows that CFSE-labeled lymphocytes (H-2Kb) were cultured with maDCs (H-2Kd), MSC-DCs (H-2Kd), NC-MSC-DCs, (H-2Kd) and siRNA–MSC-DCs (H-2Kd), respectively, in the absence or presence of maDCs (H-2Kd) stimulation for 3 days, and the total number of live lymphocytes in each well was measured by flow cytometry. Data represent mean (± SD) of triplicate samples. *P < .05. In the bottom panel, FACS analysis showed that the effects of Jagged-2 RNAi on the lymphocyte proliferation. Data are representative of at least 3 independent experiments. Dotted line, unstimulated lymphocytes.
The effect of RNA interference on protein level of Jagged-2 was identified by Western blot. Figure 5B demonstrated the different protein level of Jagged-2. There was significant reduction of Jagged-2 protein level in Jagged-2–siRNA, but not in untreated, NC, and Mock groups (Figure 5B lanes 1-4). All of the data suggested that the siRNA of targeted sequence could knock down the gene expression of Jagged-2 at the level of mRNA and protein.
To explore whether low-immunogenicity and high-immunoregulatory properties of MSC-DCs were influenced by knockdown of Jagged-2, we used the MLC assay again. As shown in Figure 5C, significant difference was only observed in the group of Jagged-2 RNAi–MSC-DC, compared with the NC–MSC-DC and MSC-DC groups. Thus the ability of stimulating CFSE-labeled lymphocyte proliferation of MSC-DCs was strikingly elevated, and their inhibitory function of maDC-stimulating lymphocyte proliferation was markedly reduced after suppression of Jagged-2 expression by RNAi. Therefore these results suggest the immunologic characteristics of MSC-DCs are dependent on Jagged-2.
Generation of MSC-DC partly depends on a cell-cell contact mechanism
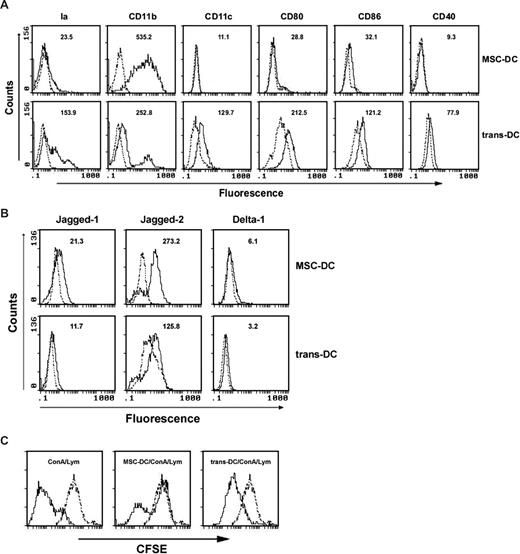
To investigate whether the induction of maDCs into MSC-DCs in coculture with MSCs depends on direct contact with MSCs or soluble factors, maDCs were cultured with MSC monolayers or in the upper insert of a transwell system, physically separated from MSCs by permeable membrane. By the transwell assay, we detected that DCs cultured in the transwell system (trans-DCs) had a phenotypic down-regulation of CD11b and up-regulation of CD11c, Ia, CD80, CD86, and CD40 in comparison with DCs in direct contact culture with MSC monolayers (Figure 6A). Furthermore, the down-regulation of Jagged-2 of trans-DCs was obviously observed, compared with MSC-DCs, by FACS (Figure 6B). Surprisingly, the capacity of trans-DCs to inhibit the lymphocyte proliferation was significantly reduced (Figure 6C). These results suggested that induction of MSC-DC production was partly dependent on a cell-cell contact mechanism.
Cell-cell contact is required for MSC-DC generation. (A) FACS for the influence of transwell system on phenotype of MSC-DC. The MSC-DCs and trans-DCs (maDCs cultured in the transwell plates to avoid cell-cell contact with MSC monolayers for 7 days) were stained with antibodies to Ia, CD11b, CD11c, CD80, CD86, and CD40, analyzed by flow cytometry. Dotted lines, background staining. Numbers in histograms indicate the geometric mean fluorescence of each DC population. (B) FACS for the influence of transwell system on expression of notch ligands on the surface of MSC-DC. The MSC-DCs and trans-DCs were stained with antibodies to Jagged-1, Jagged-2, and Delta-1, analyzed by flow cytometry. Dotted lines, background staining. Numbers in histograms indicate the geometric mean fluorescence of each DC population. (C) Effects of transwell system on the immune inhibitory function of MSC-DC. CFSE-labeled lymphocytes (H-2Kb) were cultured with MSC-DCs (H-2Kd) or trans–MSC-DCs (H-2Kd) in the absence or presence of ConA (5 μg/mL) stimulation, and 3 days later, cells in each well were collected and measured by FACS. Dotted line, unstimulated lymphocytes.
Cell-cell contact is required for MSC-DC generation. (A) FACS for the influence of transwell system on phenotype of MSC-DC. The MSC-DCs and trans-DCs (maDCs cultured in the transwell plates to avoid cell-cell contact with MSC monolayers for 7 days) were stained with antibodies to Ia, CD11b, CD11c, CD80, CD86, and CD40, analyzed by flow cytometry. Dotted lines, background staining. Numbers in histograms indicate the geometric mean fluorescence of each DC population. (B) FACS for the influence of transwell system on expression of notch ligands on the surface of MSC-DC. The MSC-DCs and trans-DCs were stained with antibodies to Jagged-1, Jagged-2, and Delta-1, analyzed by flow cytometry. Dotted lines, background staining. Numbers in histograms indicate the geometric mean fluorescence of each DC population. (C) Effects of transwell system on the immune inhibitory function of MSC-DC. CFSE-labeled lymphocytes (H-2Kb) were cultured with MSC-DCs (H-2Kd) or trans–MSC-DCs (H-2Kd) in the absence or presence of ConA (5 μg/mL) stimulation, and 3 days later, cells in each well were collected and measured by FACS. Dotted line, unstimulated lymphocytes.
Discussion
MSCs can inhibit T-cell proliferation and mediate a systemic immunosuppression. Bartholomew et al reported that MSCs suppressed lymphocyte proliferation in vitro and prolonged skin graft survival in vivo.35 However, the mechanisms underlying these inhibitory effects are still poorly understood. It has been demonstrated that MSCs produce hepatocyte growth factor (HGF) and TGF-β to mediate T-cell suppression.6 Some researches have also demonstrated that the ability of MSC to inhibit T-cell proliferation is independent on the MHC, as the third-party or autologous MSCs can exert immunomodulatory effect.35,36 In this report, we found that MSCs could unexpectedly induce maDCs into a distinct Jagged-2–dependent regDC population, and thus revealed a new mechanism of their immunoregulation. Our data showed that MSCs significantly down-regulated the expression of myeloid lineage molecule CD11c, presentation molecule Ia, and costimulatory molecules CD80, CD86, and CD40 of maDCs, but greatly up-regulated the CD11b expression, and LPS stimulation could not reverse this trend, demonstrating that a different DC population was induced by MSCs. Further research displayed that MSC-DCs secreted less IL-12, but more TGF-β and IL-10, a characteristic of regDC population.37 Indeed, our results prove that MSC-DCs have the capacity to inhibit lymphocyte proliferation in vitro and suppress allo-DTH in vivo, suggesting that MSC-DCs are a regDC population. Moreover, we also found a significant higher expression of Jagged-2, which is critical for the immunologic characteristics of MSC-DC as indicated by a Jagged-2 RNAi assay.
Several kinds of regDCs have been reported, which have similar functions but different phenotypes. MSC-DCs, expressing low levels of CD11c and Ia, are different from CD11chiIahi regDCs, induced by combination of mGM-CSF, mIL-10, and hTGF-β.38 Because CD80 and CD86 are expressed at low levels by MSC-DCs, they are also different from the CD80hiCD86hiCD40+Iaint DCs reported by Akbari et al.39 These results show that, phenotypically, MSC-DCs are a novel regDC population. Furthermore, inhibiting lymphocyte proliferation is the hallmark of regDCs, but its molecular mechanisms are not well understood. Here we detected an increased Jagged-2 expression of MSC-DCs, and knockdown of Jagged-2 effectively reduced the immunoregulatory function of MSC-DCs. These results confirm that, functionally, MSC-DCs are a Jagged-2–dependent regDC population. Strikingly, a regDC subset, CD11bhiIalo DC induced by splenic stroma, has recently been reported.40,41 Because their phenotypes and functions are similar to those of MSC-DCs, whether there is any relationship between them deserves further study.
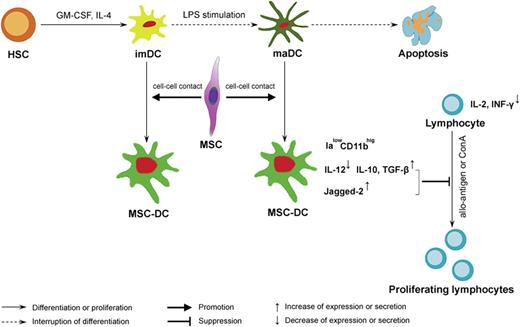
DCs plays a crucial role in the initiation and regulation of immune response,42 and its ability to initiate immune reaction or induce tolerance is strictly dependent on its maturation state or subset. It has been reported that imDC or regDC, which is deficient of costimulatory molecules, can induce T-cell anergy, generate regulatory T cell, and promote alloantigen-specific tolerance.43 Zhang et al showed that DCs were the primary target of the immunosuppressive activity of MSCs, which affected all major stages of the DC life cycle.24 However, this report is the first to define the effects of MSCs on the fate of maDCs, as shown by a proposed model in Figure 7. In this model MSCs not only promote maDC proliferation, but also drive them to differentiate into a novel regDC population capable of suppressing lymphocyte proliferation in vitro and allo-DTH in vivo. In addition, we also explored the influences of MSCs on the fate of imDCs and showed that, besides their phenotypes and functions similar to MSC-DCs, MSC-treated imDCs had also a high level expression of Jagged-2, and LPS stimulation failed to induce them to a more mature status (data not shown). Thus we conclude that MSCs might also induce imDCs directly into MSC-DCs but not maDCs. Although several types of regDCs have been prepared in vitro using such soluble factors as TGF-β, M-CSF, and IL-10,44,45 we have a good reason to postulate that direct cell-cell contact may play a more important role in MSC-DC production. Although it has been reported that bone marrow stroma regulates DC differentiation via different notch ligands,46 the detail surface molecules participating in intercellular contact remain unknown and require further investigation.
A proposed model of regulation of DC destiny by MSCs. We propose that MSCs mediate their immunomodulatory effects by influencing DC fate. MSCs, via a critical cell-cell contact mechanism, not only drive imDCs or maDCs to escape their traditional destiny of apoptosis, but also induce them into a novel IalowCD11bhig regDC population, MSC-DCs, capable of suppressing lymphocyte activities through up-regulation of Jagged-2 and increasing secretion of IL-10 and TGF-β. Ialow indicates a low-level expression of Ia molecules; CD11bhig, a high level expression of CD11b.
A proposed model of regulation of DC destiny by MSCs. We propose that MSCs mediate their immunomodulatory effects by influencing DC fate. MSCs, via a critical cell-cell contact mechanism, not only drive imDCs or maDCs to escape their traditional destiny of apoptosis, but also induce them into a novel IalowCD11bhig regDC population, MSC-DCs, capable of suppressing lymphocyte activities through up-regulation of Jagged-2 and increasing secretion of IL-10 and TGF-β. Ialow indicates a low-level expression of Ia molecules; CD11bhig, a high level expression of CD11b.
To avoid contamination of hematopoietic stem cell (HSC) in MSC preparation, BM cells were depleted of CD45+ and Ter119+ cells by micromagnetic beads, which were subsequently used to further deplete Sca-1+ cells before coculture with maDCs, and the cell purity was also confirmed by FACS, which showed that the expression of Sca-1, Ter119, CD34, and CD45 were negative (data not shown). By these procedures, we gained a rather pure cell population, consisting of more than 90% Flk-1+Sca-1− cells. Due to their multipotentials, we first tested whether MSCs could differentiate into DC-like cells in the presence of maDCs and even LPS by coculture of EGFP+ MSCs with C57BL/6 mice–derived maDCs. Results showed that no GFP+ DC-like cells were observed (data not shown), thus excluding the possibility of DC-like cell differentiation from MSCs.
Our study shows that MSCs can induce maDCs into a novel Jagged-2–dependent regDC population. Whether this function of MSCs could be used to prevent rejection in organ transplantation or treatment of autoimmune disease deserves further research.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Chunjing Bian and Yongqiang Guo for excellent technical assistance; Zengxuan Song for expert assistance on bioinformatics discussions; Kai Cheng and Shali Wang for reagents; Yehua Han for TEM; and Yumei Li for help with FACS analysis.
This work was supported by grants from the 863 Projects of the Ministry of Science and Technology of China (no. 2006AA02A109), the National Natural Science Foundation of China (no. 30570771), the Beijing Ministry of Science and Technology (no. D07050701350701), and the Cheung Kong Scholars program.
Authorship
Contribution: B.Z., R.L., and D.S. designed research, performed experiments, analyzed data, and wrote the paper; X.L. and Y.C. collected data and wrote the paper; X.D., X.Z., C.L., and W.L. collected and analyzed data; L.L. evaluated the data and corrected the paper; and M.Z. and R.C.H.Z. reviewed the manuscript. All authors read and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert C. H. Zhao, Center of Excellence in Tissue Engineering, Department of Cell Biology, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences and School of Basic Medicine, Peking Union Medical College, 5 Dong Dan San Tiao, Beijing 100005, China; e-mail: chunhuaz@public.tpt.tj.cn; or Martin Zenke, Institute for Biomedical Engineering, Department of Cell Biology, RWTH Aachen University Medical School, Pauwelsstrasse 30, Aachen 52074, Germany; e-mail: martin.zenke@rwth-aachen.de.
References
Author notes
*B.Z. and R.L. contributed equally to this work.