Although normal B-cell development depends on the expression of a functional B-cell receptor (BCR) on the cell surface, the degree to which established tumors depend on its continued signaling is unclear. While early (pre-B) and late (plasma cell) tumors would not appear to depend on this signal, there are a number of tumors in between that clearly do. The first clinical attempt to target BCR signaling targeted the BCR directly, using patient-specific anti-idiotype monoclonal antibody for the treatment of follicular lymphoma. A revealing observation from these early studies was that the treatment appeared to exert a selective pressure for tumors that continued to express a functional BCR, albeit with a mutated idiotype.1 If BCR signaling was dispensable, one would have expected to also see loss-of-function mutants. Clinical evidence for the importance of antigen-driven BCR signaling derives from the observation of gastric lymphoma regression after the successful treatment of Helicobacter pylori (presumably it is, or is responsible for eliciting, the offending antigen).2
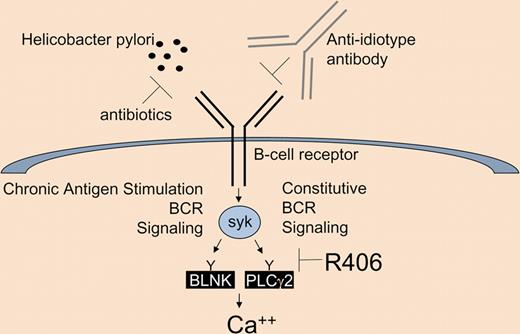
There are 2 qualitatively different signals from the B-cell receptor (BCR; a constitutive one, and one associated with antigen stimulation), both transduced by the tyrosine kinase syk, eventually resulting in a robust calcium response. BCR signaling can be inhibited by removing the antigen (eg, Helicobacter pylori), directly targeting the BCR (eg, anti-idiotype antibodies), or by inhibition of the critical mediator, syk (eg, with the tyrosine kinase inhibitor R406).
There are 2 qualitatively different signals from the B-cell receptor (BCR; a constitutive one, and one associated with antigen stimulation), both transduced by the tyrosine kinase syk, eventually resulting in a robust calcium response. BCR signaling can be inhibited by removing the antigen (eg, Helicobacter pylori), directly targeting the BCR (eg, anti-idiotype antibodies), or by inhibition of the critical mediator, syk (eg, with the tyrosine kinase inhibitor R406).
There are at least 2 types of qualitatively different signals that originate from the BCR. There is a basal constitutive signal associated with the presence on the cell surface of a functional BCR, and there is one associated with cognate antigen binding. Both of these signals are transduced by the nonreceptor tyrosine kinase syk. Its substrates include BLNK, PLCγ2, and the signal results in a calcium response. Modeling this signaling complexity is impossible using human tumors or cell lines because of the difficulty of replicating the complex in vivo environment and because of the unidentified nature of the offending antigen. Young et al overcome these limitations by using a transgenic mouse that spontaneously develops lymphoma expressing a defined anti-HEL BCR (Eμ-MYC/BCRHEL).3 The nature of the resultant tumor depends on the presence or absence of the cognate antigen (sHEL), introduced on a transgene. Using this model, Young et al show that syk expression is required by these lymphomas, and that pharmacologic inhibition of its kinase activity results in tumor regression in vivo. Syk is an attractive target because it is expressed primarily in hematopoietic cells, and orally available syk inhibitors (R406/FosD/TAMF from Rigel Pharmaceuticals) are being developed for the treatment of rheumatoid arthritis. These studies will help to inform the clinical development of syk inhibitors for the treatment of lymphoma where promising activity is emerging in early clinical trials.4
Conflict-of-interest disclosure: The author declares no competing financial interests. ■