Abstract
Exosomes are nanovesicles harboring proteins important for antigen presentation. We compared the potency of differently loaded exosomes, directly loaded with OVA323-339 peptide (Pep-Exo) or exosomes from OVA-pulsed DCs (OVA-Exo), for their ability to induce specific T-cell proliferation in vitro and in vivo. Both Pep-Exo and OVA-Exo elicited specific transgenic T-cell proliferation in vitro, with the Pep-Exo being more efficient. In contrast, only OVA-Exo induced specific T-cell responses in vivo highlighting the importance of indirect loading strategies in clinical applications. Coadministration of whole OVA overcame the unresponsiveness with Pep-Exo but still elicited a lower response compared with OVA-Exo. In parallel, we found that OVA-Exo not only augmented the specific T-cell response but also gave a Th1-type shift and an antibody response even in the absence of whole OVA. We detected IgG2a and interferon-γ production from splenocytes showing the capability of exosomes to provide antigen for B-cell activation. Furthermore, we found that B cells are needed for exosomal T-cell stimulation because Bruton tyrosine kinase–deficient mice showed abrogated B- and T-cell responses after OVA-Exo immunization. These findings reveal that exosomes are potent immune regulators and are relevant for the design of vaccine adjuvants and therapeutic intervention strategies to modulate immune responses.
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells that regulate the induction and outcome of the immune response. DCs process exogenous antigens in the endosomal compartment and multivesicular bodies are formed, which contain vesicles with peptide/major histocompatibility complex class II (MHC II) complexes on their surface.1,2 These small vesicles are known as exosomes when they are secreted from the cells. Exosomes are actively secreted by a diverse range of cells, and especially DC-derived exosomes have acquired much attention because they harbor all the necessary molecules required for the activation of potent immune responses, for example, MHC I and MHC II, CD54, CD80, and CD86, on their surface.2 The initial investigations have shown the biologic significance of exosomes in different areas of research, such as tumor and transplantation immunology,3-7 vaccine therapy against infection,8-10 as well as a biomarker for diagnostic purposes.11 Exosome-based tumor vaccines have recently been tested in phase 1 clinical trials in melanoma, nonsmall cell lung cancer, and colorectal cancer patients.12-14 To fully understand the potential of exosome-based immunotherapy, there is a need to further explore the fundamental mechanisms of exosome-mediated immune stimulation and regulation. Several studies have demonstrated the potency of antigen-pulsed DC-derived exosomes (indirectly loaded exosomes) to elicit in vitro and in vivo antigen-specific activation of T cells.15-17 Hsu et al20 developed a direct peptide-loading method on exosomes and have shown a superiority of the direct peptide loading over indirect loading regarding exosome immunogenicity in vitro; however, they were not compared in vivo.
Based on the studies that antigen-loaded exosomes can serve to amplify DC function and stimulate T cells, we aimed to evaluate and compare the potency of both directly and indirectly antigen-loaded exosomes for their ability to stimulate antigen-specific T cells in vitro and in vivo. As model antigens, we used native ovalbumin (OVA) and the OVA323-339 peptide to load indirectly or directly on exosomes, respectively, and T cells from the OVA T-cell receptor (TCR) transgenic (Tg) mouse DO11.10 were used. We demonstrate that both directly and indirectly loaded exosomes can induce the proliferation of transgenic T cells in vitro. However, on the contrary, only indirectly loaded, but not directly loaded, exosomes elicited T-cell proliferative responses in vivo. Coadministration of the whole OVA overcame the unresponsiveness with the directly loaded exosomes in wild-type mice, indicating that B- and T-cell collaboration is crucial for exosome-mediated specific immune activation in vivo. Interestingly, indirectly loaded exosomes exerted their effect in the absence of whole OVA and modulated the specific response toward Th1 type, which may imply the presence of recycled antigen on indirectly loaded exosomes. The need for B-cell help for T-cell stimulation was verified in Bruton tyrosine kinase (Btk) knockout (KO) mice, which show decreased antibody production and splenocyte proliferation in response to OVA-Exo. Our results suggest an additional role for indirectly loaded exosomes in the presentation of not only MHC II/pep but also native antigen to B cells, thus assisting activation of B cells, which in turn stimulates the efficient priming of antigen-specific T cells.
Methods
Mice
BALB/c and DO11.10 OVA TCR transgenic mice (The Jackson Laboratory, Bar Harbor, ME)18 were kept and bred at the animal facility at the Department of Microbiology, Tumor and Cell Biology (MTC; Karolinska Institutet, Stockholm, Sweden). Age- and sex-matched mice of 6 to 8 weeks were used for experiments. Btk KO mice were kindly provided by Edvard Smith (Clinical Research Center, Karolinska Institutet). Btk KO mice were on a C57BL/6 background and hence were used as wild-type controls in these experiments. All animal experiments were approved by the Ethics Committee at Karolinska University Hospital Solna.
Generation of BMDCs
Bone marrow–derived dendritic cells (BMDCs) were generated as described previously with some modifications.19 Bone marrow cells were cultured in complete RPMI 1640 medium (Invitrogen, Carlsbad, CA; 10% exosome-depleted fetal calf serum, 1 mM sodium pyruvate, 100 IU/mL penicillin streptomycin, 200 mM L-glutamine, 50 μM β-mercaptoethanol) in the presence of 10 ng/mL interleukin-4 (IL-4; Invitrogen) and 10% granulocyte macrophage colony-stimulating factor conditioned medium (Ag8653/X63 clone, a kind gift from Mattias Svensson, Center for Infectious Medicine, Karolinska Institutet). At day 6, 50% of the culture supernatant was replaced with fresh medium. For maturation, lipopolysaccharide (LPS; Sigma-Aldrich, St Louis, MO) or interferon-γ (IFN-γ; Invitrogen) at 30 ng/mL was added on day 6 of culture followed by 48 hours of incubation, and the supernatant was collected and kept at −80°C.
Preparation of exosomes from DC culture supernatants
Exosomes were isolated by differential ultracentrifugation (Beckman Coulter, Fullerton, CA) as described previously1 with modifications. The supernatants were subjected to centrifugation at 3000g, followed by 10 000g for 30 minutes. Exosomes were pelleted at 100 000g for 2 hours and washed at 100 000g. Pelleted exosomes were dissolved in phosphate-buffered saline (PBS). The protein contents were measured by a DC protein assay (Bio-Rad, Hercules, CA).
Sucrose gradient
Exosomes were layered on a linear sucrose gradient (0.25-2 mM sucrose and 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/NaOH, pH 7.4; Sigma-Aldrich).1 The gradients were centrifuged for 21 hours at 79 000g at 4°C. Eighteen fractions were collected, and the density was determined by refraction index measurements.
Phenotypic analysis of exosomes by FACS
A total of 30 μg exosomes was incubated with 10 μL aldehyde/sulfate latex beads (Invitrogen) and rotated overnight at room temperature. The reaction was stopped by 1 mL of 100 mM glycine (Sigma-Aldrich). Beads with exosomes were labeled with a panel of fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated antibodies specific for H-2Kd, CD9, CD54, CD80, CD81, and CD86 (BD Biosciences, San Jose, CA) and the corresponding isotype-matched antibodies.
Western blot analysis
Thirty micrograms of OVA-Exo, Exo, or 1 μg OVA was loaded on 8% to 16% Tris-HCl gel (Bio-Rad) and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA) using semidry blotting. OVA was detected using immune sera from OVA-alum sensitized mice (1:500 diluted). Bands were detected using alkaline phosphatase (ALP)–conjugated anti–mouse IgG (Southern Biotechnology, Birmingham, AL; 1:2000 diluted) and ALP substrate kit (Bio-Rad).
Direct and indirect loading of OVA on exosomes
Direct loading of exosomes with OVA peptide (ISQAVHAAHAEINEAGR; OVA323-339; Innovagen, Lund, Sweden) was done using the acid elution method as described.20 Exosomes were mixed with 0.2 M sodium acetate at pH 5.2 and with OVA323-339 peptide at the concentration 10 μg/mL. The mixture was neutralized to pH 7.0 with 2 M Tris-HCl (2.6% of total volume; Bio-Rad) of pH 11 and incubated at room temperature to allow reassembly of MHC II/peptide on exosomes. Unbound peptides were removed by filtering through 100-kDa Ultrafree Biomax filters (Millipore). Directly loaded exosomes were termed Pep-Exo. The same amount of OVA323-339 peptide as loaded on exosomes was filtrated in parallel and the fraction above the filter was used as control for the removal of free peptides and was termed pep-cont.
For indirect loading, OVA (Serotec, Oxford, United Kingdom) or bovine serum albumin (BSA; Sigma-Aldrich; 300 μg/mL) proteins were added to DC cultures at day 6, for overnight followed by washing once and then LPS was added to the culture. After 48 hours, exosomes were purified. Indirectly loaded exosomes were termed OVA-Exo or BSA-Exo.
DO11.10 CD4+ T-cell isolation and in vitro T-cell proliferation assay
DO11.10 CD4+ T cells from spleen were isolated by positive selection on anti-CD4 beads by magnetic-activated cell sorter (Miltenyi Biotec, Auburn CA) according to the manufacturer's instructions. Purity was checked by fluorescence-activated cell sorter (FACS). To assess T-cell proliferation in vitro, magnetic-activated cell sorter–purified CD4+ DO11.10 T cells were incubated with 5 μM carboxy fluoroscein succinimidyl ester (CFSE; Invitrogen) for 10 minutes at room temperature. Labeling was stopped by adding cold PBS/10% fetal calf serum. Cells were then washed 3 times in PBS and cocultured at a concentration of 106 cells/mL with different concentrations of Pep-Exo, OVA-Exo, and the respective controls followed by incubation at 37°C in a humid incubator with 5% CO2 for 5 days.
In vivo T-cell proliferation assay
Purified DO11.10 CD4+ T cells were adoptively transferred to BALB/c mice intravenously with 5.5 × 106 cells/ mouse at day 0. On day 1, mice were immunized intravenously with Pep-Exo, OVA-Exo, or with respective controls. On day 4, mice were killed and splenocytes were stained with anti–CD3-allophycocyanin together with anti–KJ1-26+-FITC antibodies specific for OVA TCR and the number of KJ1-26+ cells assessed by FACS. In some experiments, DO11.10 splenocytes were labeled with CFSE and adoptively transferred to BALB/c mice before exosome injection. Lymphocyte early activation was checked using biotinylated anti-CD69 and detected using streptavidin-PE by FACS.
Immunohistochemistry
Spleens from the adoptively transferred mice were frozen in OCT medium (Sakura Finetek, Zoeterwoude, The Netherlands) and 8-μm-thin sections were cut in a cryostat microtome. After overnight drying, the slides were fixed in acetone, blocked with 5% goat serum (Dako North America, Carpinteria, CA) together with avidin and followed by biotin (Vector Laboratories, Burlingame, CA). The following antibodies were used: biotinylated KJ1-26, FITC-conjugated anti-B220 and CD11c, allophycocyanin-conjugated anti-B220 (BD PharMingen, San Diego, CA), streptavidin-Qdot605 (Invitrogen/Molecular Probes, Eugene, OR), and biotinylated anti–FDC-M2 (ImmunoKontact, Abingdon, United Kingdom). Images were collected using a Leica DM IRBE confocal laser scanning microscope (Leica Microsystems, Heidelberg, Germany) equipped with 1 argon and 2 HeNe lasers, using an HC PL APO lens at 20×/0.70 IMM CORR and 100×/1.40-0.7 oil and 90% glycerol (MP Biomedicals, Solon, OH). Images were processed with Adobe Photoshop CS3 Extended 10.0.1 (Adobe Systems, San Jose, CA).
Immunization of mice
Female BALB/c mice, or where indicated, C57BL/6 or Btk mice, were primed intravenously with 50 μg OVA/mouse formulated with 50 μg/mouse of different exosome preparations in 100 μL PBS. Priming was done once with loaded exosomes, followed by boosting with 50 μg OVA/mouse 4 weeks later. Animals were bled from the tail vein 7 days after primary immunization and 7 days after boosting.
Determination of serum antibody levels by ELISA
To determine specific antibody responses, microtiter plates (Costar, Corning, Corning, NY) were coated with 10 μg/mL OVA protein. Plates were then incubated overnight at room temperature followed by incubation overnight with serial dilutions of sera. Isotypes of the reactive antibodies were determined by incubating for 2 hours at room temperature with alkaline phosphatase–conjugated goat immunoglobulin specific for mouse μ and γ isotypes and subclasses (Southern Biotechnology). Development was done at room temperature with p-nitrophenyl phosphate disodium (Sigma-Aldrich), and the absorbance was measured at 405 nm at different time points by an enzyme-linked immunosorbent assay (ELISA) reader.
Proliferation and Th1/Th2 cytokine assay
Supernatants, obtained from the 48-hour cocultures of immunized splenocytes with in vitro stimuli, were analyzed for proliferation by thymidine incorporation and for T-helper cytokine production using the Th1/Th2 CBA assay kit measuring IL-2, IL-4, IL-5, IFN-γ, and TNF-α in a single sample. The results were analyzed using the BD Biosciences CBA analysis software.
Statistical analyses
Results were expressed either as individual data and mean or as mean plus or minus SEM from individual mice from each group. Nonparametric Mann-Whitney U test was performed to identify significant differences between experimental groups.
Results
Characterization of exosomes derived from immature and matured DCs
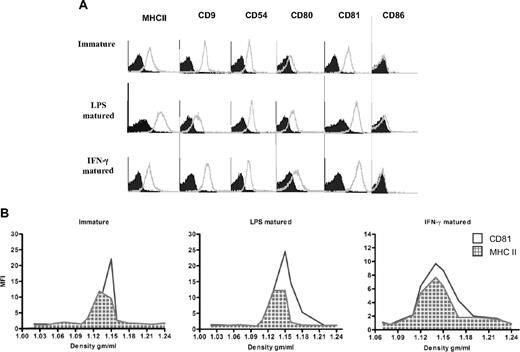
We compared differently matured (LPS- or IFN-γ–matured) or immatured BMDC exosomes by FACS. We obtained approximately 2 μg exosomes/million cells from immature DCs and 0.5 to 1 μg/million cells for LPS- or IFN-γ–matured DCs. Exosomes from both immature and LPS- or IFN-γ–matured DCs displayed the tetraspanins CD9 and CD81, the costimulatory molecule CD80, as well as MHC II and CD54 (Figure 1A). However, LPS and IFN-γ–matured DC exosomes exhibited higher expression of CD80 and CD81 on their surface, whereas MHC II and CD86 were more highly expressed on LPS-matured DC exosomes compared with the other types (Figure 1A).
Exosomes from LPS and IFN-γ–matured BMDCs display higher expression of MHC II and CD80 molecules on their surface than exosomes from immature BMDCs. (A) Exosomes derived from immature, LPS, or IFN-γ–matured BMDCs were coated on aldehyde/sulfate latex beads, stained with a panel of PE- and FITC-conjugated specific (open histogram) and isotype-matched (solid histogram) antibodies, and then analyzed by flow cytometry. One representative experiment of 3 is displayed. (B) BMDC-derived exosomes have similar densities as previously reported for exosomes. Pelleted (100 000g) exosomes from immature, LPS, or IFN-γ–matured BMDCs were loaded on continuous sucrose density gradients and ultracentrifuged. Fractions were collected and directly analyzed by flow cytometry after coating on latex beads and staining with fluorochrome-conjugated antibodies against MHC II (grid histogram) and CD81 (open histogram).
Exosomes from LPS and IFN-γ–matured BMDCs display higher expression of MHC II and CD80 molecules on their surface than exosomes from immature BMDCs. (A) Exosomes derived from immature, LPS, or IFN-γ–matured BMDCs were coated on aldehyde/sulfate latex beads, stained with a panel of PE- and FITC-conjugated specific (open histogram) and isotype-matched (solid histogram) antibodies, and then analyzed by flow cytometry. One representative experiment of 3 is displayed. (B) BMDC-derived exosomes have similar densities as previously reported for exosomes. Pelleted (100 000g) exosomes from immature, LPS, or IFN-γ–matured BMDCs were loaded on continuous sucrose density gradients and ultracentrifuged. Fractions were collected and directly analyzed by flow cytometry after coating on latex beads and staining with fluorochrome-conjugated antibodies against MHC II (grid histogram) and CD81 (open histogram).
To further verify the exosomal nature of BMDC exosomes, exosome preparations were layered on a continuous sucrose gradient, and the distribution of the MHC II and CD81 over the gradient fractions was analyzed by direct coating of each fraction on the latex beads and visualized by flow cytometry.21 As shown in Figure 1B, MHC II and CD81 molecules from each exosome preparation were distributed in fractions with densities between 1.12 and 1.15 g/mL, indicating their exosomal nature.
OVA-loaded exosomes stimulate DO11.10 T-cell proliferation in vitro
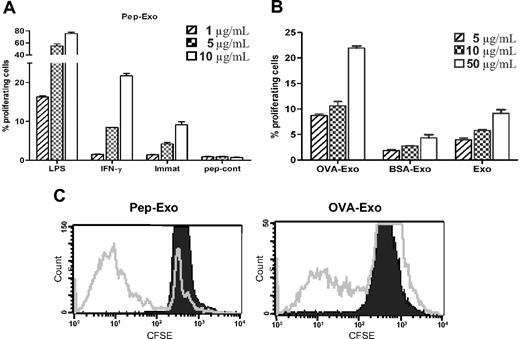
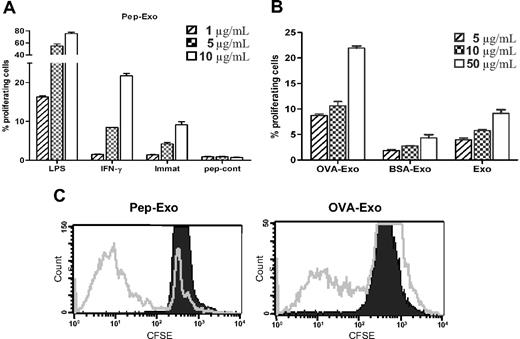
MHC on exosomes can be directly loaded with peptides in a mildly acidic condition, which generates higher numbers of MHC/peptide complexes.20 We compared the efficiency of directly and indirectly OVA-loaded exosomes for their ability to elicit OVA-specific T-cell responses in vitro, in a TCR Tg mouse model (DO11.10). We evaluated the proliferative responses of CFSE-labeled DO11.10T cells induced by exosomes, indirectly loaded (OVA-Exo) or directly loaded with the OVA peptide (Pep-Exo) by detecting the DO11.10 TCR with the mAb KJ1-26. KJ1-26 is a monoclonal antibody that specifically recognizes DO11.10 Tg TCR. We assessed the potency of directly loaded, differently matured (LPS or IFN-γ) exosomes to stimulate T-cell proliferation by CFSE staining. Pep-Exo could stimulate the expansion of DO11.10 CD4+ T cells in a dose-dependent manner (Figure 2A). Similarly, as seen by us and others,22-24 LPS-matured Pep-Exo was 8 times more potent in stimulating OVA-specific T cells compared with immature Pep-Exo. In addition, IFN-γ–stimulated exosomes were more potent than exosomes from immature DCs but only approximately 2 times more potent than immature DC exosomes (Figure 2A). Peptide control (pep-cont) failed to trigger a measurable T-cell response (Figure 2A). OVA-Exo also induced proliferation of DO11.10 T cells in a dose-dependent manner, which was significantly higher than the response achieved with unloaded LPS-matured exosomes (Exo) or exosomes indirectly loaded with a control protein BSA (BSA-Exo; Figure 2B). However, it is noteworthy that 75% of DO11.10 T cells proliferated in response to the highest concentration (10 μg/mL) of LPS-matured Pep-Exo added to the culture (Figure 2A), whereas only 11% of OVA323-339- specific cell populations were expanded in response to the same concentration of OVA-Exo (Figure 2B). Polymyxin treatment did not affect the proliferative response (not shown). LPS content of each exosome preparation was determined, the corresponding amount of LPS was added to the culture, and this gave undetectable response (not shown). Because LPS-matured BMDC exosomes elicited stronger T-cell activation, we used LPS-matured exosomes in the following experiments.
Directly loaded LPS-matured exosomes are more potent in stimulating T-cell proliferation in vitro than indirectly loaded exosomes. (A) Exosomes derived from LPS, IFN-γ–matured, or immature BMDCs were directly loaded with OVA323-339 peptide by acid elution or (B) indirectly loaded by pulsing BMDCs with OVA or BSA protein and cocultured in different concentrations with CFSE-labeled CD4+ T cells, sorted from the splenocytes of DO11.10 mice. Proliferation was detected 5 days later by flow cytometry, and results are expressed as the mean percentage plus or minus SEM of proliferating cells cultured in triplicate according to the dimming intensity of the CFSE-positive cells. One representative experiment of 3 is displayed. (C) Representative FACS plots from experiments shown in panels A and B (shown for 10 μg/mL).
Directly loaded LPS-matured exosomes are more potent in stimulating T-cell proliferation in vitro than indirectly loaded exosomes. (A) Exosomes derived from LPS, IFN-γ–matured, or immature BMDCs were directly loaded with OVA323-339 peptide by acid elution or (B) indirectly loaded by pulsing BMDCs with OVA or BSA protein and cocultured in different concentrations with CFSE-labeled CD4+ T cells, sorted from the splenocytes of DO11.10 mice. Proliferation was detected 5 days later by flow cytometry, and results are expressed as the mean percentage plus or minus SEM of proliferating cells cultured in triplicate according to the dimming intensity of the CFSE-positive cells. One representative experiment of 3 is displayed. (C) Representative FACS plots from experiments shown in panels A and B (shown for 10 μg/mL).
OVA-loaded exosomes enhance the proliferation of DO11.10 T cells in vivo
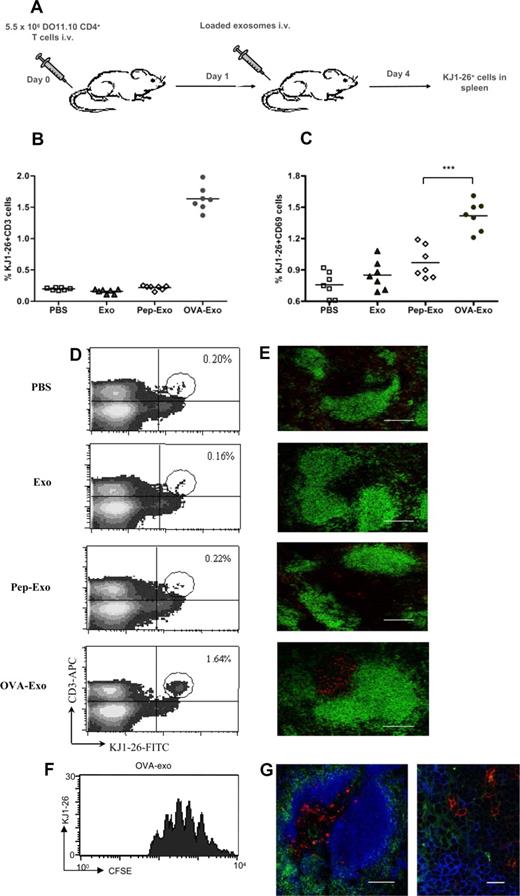
To compare the different loading methods in vivo, BALB/c mice were adoptively transferred with DO11.10 Tg CD4+ T cells and immunized intravenously with differently loaded or unloaded exosomes (Figure 3A). The percentage of OVA-specific T cells in the spleens was determined as CD3+/KJ1-26+ cells by FACS 3 days later. As shown in Figure 3B and D, OVA-Exo potently stimulated the proliferation of OVA-specific DO11.10 T cells as evidenced by an 8- to 10-fold increase in the percentage of KJ1-26+ cells (1.64% ± 0.14%) in the spleen compared with the PBS (0.20% ± 0.018%) or unloaded exosome (0.16% ± 0.028%) treated mice, respectively. Surprisingly, only a trace population (0.22% ± 0.027%) of KJ1-26+ cells was observed for the Pep-Exo–immunized group, which corresponded to the values obtained with PBS or unloaded exosome control (Figure 3B,D). This suggests that an additional factor is needed for the T-cell proliferative response in vivo.
Only indirectly loaded exosomes are able to stimulate OVA-specific T-cell proliferation in vivo. (A) BALB/c mice were adoptively transferred with a total of 5.5 × 106 purified CD4+ T cells from DO11.10 mice and immunized either with PBS or 50 μg unloaded (Exo), directly (Pep-Exo) or indirectly (OVA-Exo) loaded exosomes. After 3 days of immunization, the percentage of (B,D) KJ1-26+/CD3+ or (C) KJ1-26+/CD69+ DO11.10 cells per spleen was determined by flow cytometry or (E) the spleens were sectioned and stained with KJ1-26 (DO11.10 T cells; red) and anti-B220 (B cells; green). The pictures shown are representative of 2 independent experiments. Bar represents 150 μm. For panel C, ***P < .001. (F) Splenocytes from DO11.10 mice were stained with CFSE and adoptively transferred to the BALB/c mice followed by OVA-Exo injection the day after. Spleens were taken 3 days later, and CFSE division was assessed in the flow cytometry. A representative experiment with 1 mouse of 4 is shown. (G) Spleen sections from mice adoptively transferred with DO11.10 CD4+ T cells and immunized with OVA-Exo were also stained with KJ1-26 (DO11.10 T cells; red), anti-B220 (B cells; pseudo-colored blue) and anti-CD11c (DCs; green). Original magnification: left, ×20; bar represents 150 μm; right, ×100; bar represents 15 μm.
Only indirectly loaded exosomes are able to stimulate OVA-specific T-cell proliferation in vivo. (A) BALB/c mice were adoptively transferred with a total of 5.5 × 106 purified CD4+ T cells from DO11.10 mice and immunized either with PBS or 50 μg unloaded (Exo), directly (Pep-Exo) or indirectly (OVA-Exo) loaded exosomes. After 3 days of immunization, the percentage of (B,D) KJ1-26+/CD3+ or (C) KJ1-26+/CD69+ DO11.10 cells per spleen was determined by flow cytometry or (E) the spleens were sectioned and stained with KJ1-26 (DO11.10 T cells; red) and anti-B220 (B cells; green). The pictures shown are representative of 2 independent experiments. Bar represents 150 μm. For panel C, ***P < .001. (F) Splenocytes from DO11.10 mice were stained with CFSE and adoptively transferred to the BALB/c mice followed by OVA-Exo injection the day after. Spleens were taken 3 days later, and CFSE division was assessed in the flow cytometry. A representative experiment with 1 mouse of 4 is shown. (G) Spleen sections from mice adoptively transferred with DO11.10 CD4+ T cells and immunized with OVA-Exo were also stained with KJ1-26 (DO11.10 T cells; red), anti-B220 (B cells; pseudo-colored blue) and anti-CD11c (DCs; green). Original magnification: left, ×20; bar represents 150 μm; right, ×100; bar represents 15 μm.
Although the early activation marker CD69 expression was observed on DO11.10 spleen cells from both Pep-Exo– and OVA-Exo–treated groups, the expression of CD69 was significantly higher in the spleens of the OVA-Exo group (Figure 3C). No significant differences in the CD69 expression could be detected in the splenocytes between mice injected with unloaded exosomes or PBS (Figure 3C).
To visualize T-cell expansion, spleens from the adoptively transferred, exosome-treated mice were sectioned and stained for OVA-specific T cells (KJ1-26 antibody) and for B cells (anti-B220). The number of OVA-specific T cells in the T-cell zone remained low in mice given PBS or unloaded exosomes (Figure 3D), whereas the number was markedly increased in the mouse group treated with OVA-Exo. No change in the number of the specific T cells was observed for the Pep-Exo–treated group (Figure 3E). To further verify that the KJ1-26+ cells had proliferated in response to OVA-Exo, we also adoptively transferred CFSE-labeled splenocytes from DO11.10 mice to wild-type mice followed by OVA-Exo injection. Cells stained for KJ1-26 had proliferated 4 to 8 times at day 4 after OVA-Exo injection (Figure 3F). We also stained the spleen sections of OVA-Exo–treated mice with antibody against the DC marker CD11c together with the B- (B220) and T- (KJ1-26+) cell markers to be able to observe the interaction between these cell types on exosome treatment. We observed that both B and T cells were in close proximity with the DCs (Figure 3G). This observation indicates the involvement of B cells and DCs in the early events in exosome-mediated stimulation of T cells.
OVA-loaded exosomes have the potency to prime the immune system to induce OVA-specific antibody responses in naive mice
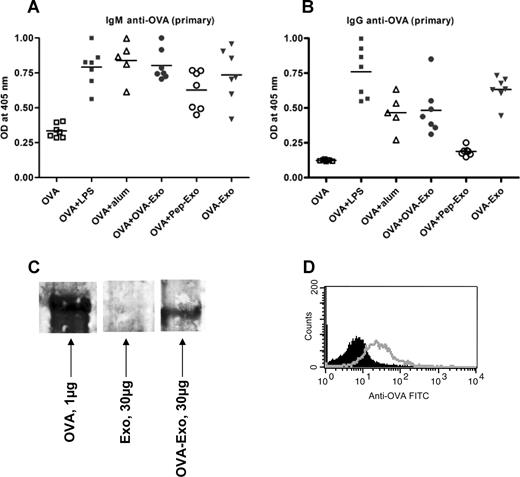
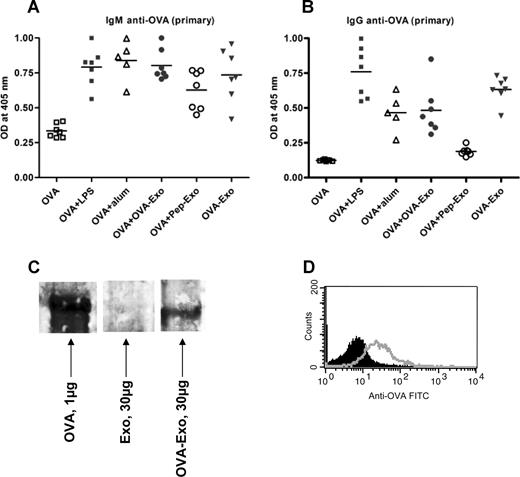
To test whether B-cell help is needed to overcome the nonresponsiveness of Pep-Exo, we delivered the loaded exosomes, thought to contain T-cell epitopes, together with the whole OVA protein, containing B-cell epitopes, to wild-type mice. BALB/c mice were primed intravenously with 50 μg OVA administered either alone or together with OVA-Exo or Pep-Exo. As an exosome control, a group of mice was immunized only with OVA-Exo. We compared the antibody responses induced by loaded exosomes with the response induced by OVA with aluminium hydroxide (Alum) or LPS. The anti-OVA antibody titers were determined 7 days after priming. Figure 4A shows that both Pep-Exo and OVA-Exo given together with OVA promoted the generation of primary IgM antibodies against OVA, which was comparable with the response induced in the presence of Alum and LPS. Nearly undetectable amounts of IgM antibodies were produced in mice immunized with OVA alone (Figure 4A). Interestingly, the mouse group immunized only with OVA-Exo also elicited OVA-specific IgM responses (Figure 4A). IgG antibodies were also detected in the serum of mice primed with OVA plus OVA-Exo or only with OVA-Exo (Figure 4B). Negligible amounts of IgG were induced in the mouse group primed with OVA plus Pep-Exo or only with OVA. These results show the efficiency of OVA-Exo to induce a humoral response in the absence of whole OVA and suggest that indirectly loaded exosomes may carry intact OVA for stimulation of B cells. It also suggests that the B-cell stimulatory function of exosomes is needed for them to be able to evoke a specific T-cell response in vivo.
Indirectly loaded exosomes alone can induce potent OVA-specific primary antibody responses. BMDCs were stimulated with LPS on day 6, and exosomes were harvested on day 8. BALB/c mice were primed intravenously with 50 μg OVA per mouse alone or together with 10 μg LPS, Alum (1:1 ratio with OVA), 50 μg OVA-Exo, or Pep-Exo or only with 50 μg OVA-Exo. Sera were collected 7 days after priming, and the presence of OVA-specific (A) IgM and (B) IgG antibodies was detected by ELISA using serial dilution of the sera. Results are expressed as optical density at 1:100 serum dilutions. Individual data and mean are presented (n = 7 per group, for Alum group n = 5). (C) A total of 30 μg OVA-Exo and Exo or 1 μg native OVA protein was separated on 8% to 16% SDS-acrylamide gel, transferred to polyvinylidene difluoride membrane, and incubated with OVA-specific immune sera (1:500 dilutions), followed by incubation with horseradish peroxidase–conjugated secondary antibodies, and detected by chemiluminescence kit. (D) A total of 30 μg OVA-Exo was coated on anti-CD9–coated latex beads, treated with OVA immune sera (open histogram) or preimmune sera (solid histogram), followed by addition of PE-conjugated anti–mouse Ig, and then analyzed by flow cytometry.
Indirectly loaded exosomes alone can induce potent OVA-specific primary antibody responses. BMDCs were stimulated with LPS on day 6, and exosomes were harvested on day 8. BALB/c mice were primed intravenously with 50 μg OVA per mouse alone or together with 10 μg LPS, Alum (1:1 ratio with OVA), 50 μg OVA-Exo, or Pep-Exo or only with 50 μg OVA-Exo. Sera were collected 7 days after priming, and the presence of OVA-specific (A) IgM and (B) IgG antibodies was detected by ELISA using serial dilution of the sera. Results are expressed as optical density at 1:100 serum dilutions. Individual data and mean are presented (n = 7 per group, for Alum group n = 5). (C) A total of 30 μg OVA-Exo and Exo or 1 μg native OVA protein was separated on 8% to 16% SDS-acrylamide gel, transferred to polyvinylidene difluoride membrane, and incubated with OVA-specific immune sera (1:500 dilutions), followed by incubation with horseradish peroxidase–conjugated secondary antibodies, and detected by chemiluminescence kit. (D) A total of 30 μg OVA-Exo was coated on anti-CD9–coated latex beads, treated with OVA immune sera (open histogram) or preimmune sera (solid histogram), followed by addition of PE-conjugated anti–mouse Ig, and then analyzed by flow cytometry.
To confirm the presence of B-cell epitopes on OVA-Exo, Western blot analysis was performed with OVA-Exo, Exo, or with purified OVA using anti-OVA immune sera. As shown in Figure 4C, the band appeared on the OVA-Exo lane corresponding to that of the band with OVA protein-loaded lane, which is approximately 45 kDa. In addition, we could detect the presence of OVA on the surface of exosomes by FACS analysis (Figure 4D). These results indicate the presence of conformationally intact OVA on the OVA-Exo.
OVA-loaded exosomes can prime for the induction of secondary responses to OVA
Given the ability of indirectly loaded OVA-Exo to prime a primary humoral response, we next addressed whether the primed immune system could induce a memory response after a second injection with OVA. We therefore boosted the aforementioned primed mouse groups with OVA alone 4 weeks after the priming. As shown in Figure 5A, when mice were primed either with OVA plus OVA-Exo or only with OVA-Exo, followed by boosting with OVA alone, anti-OVA IgG antibodies were produced. The antibody titer for those groups attained similar levels as obtained from the group of mice primed with OVA plus Alum or OVA plus LPS and boosted with OVA. The IgG level in the serum of the OVA plus Pep-Exo group was comparatively lower than in the other groups. The control group, primed and boosted only with OVA, produced negligible amounts of IgG anti-OVA antibodies (Figure 5A). To determine whether the priming with loaded exosomes and boosting with OVA conditioned the nature of OVA-specific humoral response, the IgG1 and IgG2a profiles were analyzed. As illustrated in Figure 5B and C, whereas IgG1 was induced in all immunized groups except for the group that received OVA alone, only OVA-Exo–immunized mice elicited strong IgG2a response, delivered either alone or in conjunction with OVA. This indicates the potency of OVA-Exo as an immune-modulating vaccine adjuvant.
Indirectly loaded exosomes induce a potent memory response to OVA that is distinct from the response obtained with other adjuvants. All primed mouse groups were boosted with 50 μg OVA alone per mouse 4 weeks later and bled 7 days after boosting. The presence of OVA-specific (A) IgG, (B) IgG1, and (C) IgG2a antibodies was detected by ELISA using serial dilution of the sera. The results from the 1:2700 dilutions are presented, showing OD (A 405 nm) values for each subject and the mean. Significant differences between groups after nonparametric Mann-Whitney U test are depicted with asterisks: *P < .01, **P < .002.
Indirectly loaded exosomes induce a potent memory response to OVA that is distinct from the response obtained with other adjuvants. All primed mouse groups were boosted with 50 μg OVA alone per mouse 4 weeks later and bled 7 days after boosting. The presence of OVA-specific (A) IgG, (B) IgG1, and (C) IgG2a antibodies was detected by ELISA using serial dilution of the sera. The results from the 1:2700 dilutions are presented, showing OD (A 405 nm) values for each subject and the mean. Significant differences between groups after nonparametric Mann-Whitney U test are depicted with asterisks: *P < .01, **P < .002.
OVA-loaded exosomes can trigger the polarization of T cells to the Th1 type
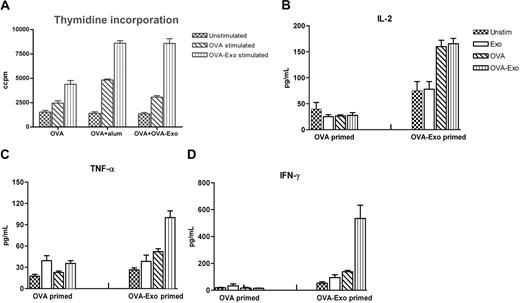
The efficacy of adjuvants in the induction of optimal immune responses has been judged by the isotype and levels of antibodies elicited as well as the cytokine milieu. We sought to determine the immunomodulatory effect of OVA-Exo on splenocyte proliferation and cytokine production in vitro to observe the polarization of T cells. Comparisons were made with the OVA-primed group or OVA plus OVA-Exo– or OVA plus Alum-primed groups. Splenocytes isolated 2 weeks after the OVA boost were restimulated in vitro either with whole OVA or with OVA-Exo or left unstimulated. Proliferation was observed for all groups of mice with different intensities when restimulated with OVA-Exo or whole OVA protein, which was significantly higher than the proliferation achieved with unstimulated cells for each group of mice (Figure 6A). In vitro restimulation with OVA-Exo gave a 1.8 to 3 times higher proliferative response compared with OVA alone (Figure 6A).
In vitro stimulation with OVA-Exo induces a potent Th1 response in mice sensitized with OVA-Exo and challenged with OVA in vivo. OVA + OVA-Exo, OVA + Alum, or OVA alone primed mice groups were boosted with OVA, and 2 weeks later splenocytes were restimulated for 48 hours. (A) Proliferation was detected by thymidine incorporation assay, and the production of (B) IL-2, (C) TNF-α, and (D) IFN-γ was measured by cytometric bead array from the culture supernatant. Results are expressed as picograms per milliliter. Recombinant cytokines were used for standard curves for the quantification of sample cytokines by software provided by BD Biosciences. Data are presented as mean of 7 mice per group plus or minus SEM.
In vitro stimulation with OVA-Exo induces a potent Th1 response in mice sensitized with OVA-Exo and challenged with OVA in vivo. OVA + OVA-Exo, OVA + Alum, or OVA alone primed mice groups were boosted with OVA, and 2 weeks later splenocytes were restimulated for 48 hours. (A) Proliferation was detected by thymidine incorporation assay, and the production of (B) IL-2, (C) TNF-α, and (D) IFN-γ was measured by cytometric bead array from the culture supernatant. Results are expressed as picograms per milliliter. Recombinant cytokines were used for standard curves for the quantification of sample cytokines by software provided by BD Biosciences. Data are presented as mean of 7 mice per group plus or minus SEM.
Cytokine responses, analyzed by CBA assay, were used to determine the T-cell regulatory effect of exosomes. Neither OVA plus OVA Exo- nor OVA-immunized groups mounted a visible IL-4 and IL-5 response (data not shown). As illustrated in Figure 6B through D, splenocytes from the mouse group primed with OVA alone generated low levels of IL-2, TNF-α, and IFN-γ in response to OVA or OVA-Exo. However, all these cytokines were significantly up-regulated for OVA plus OVA-Exo–primed splenocytes in response to OVA-Exo. In particular, higher levels of IFN-γ were released by stimulation with OVA-Exo, whereas OVA stimulation gave low amounts. Taken together, these data suggest that OVA-Exo can influence the polarization of T-cell responses toward Th1.
Exosome-induced T-cell responses are B-cell dependent
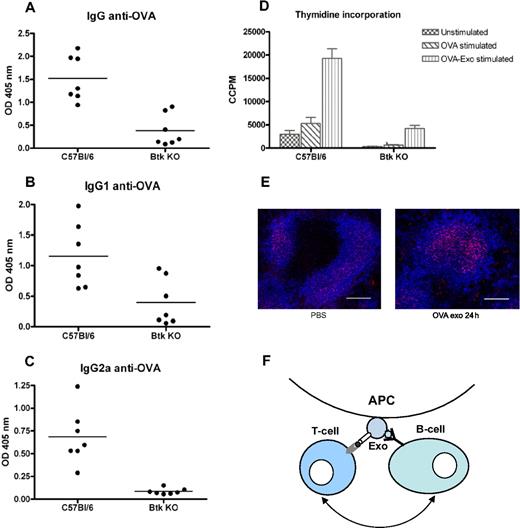
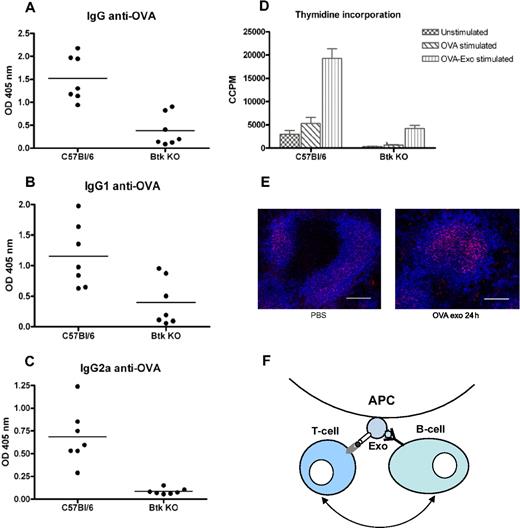
Because T-cell proliferation was only detected where a B-cell response was seen, we speculated that activation of B cells is needed for exosomal T-cell activation. By injecting B-cell signaling-deficient Btk mice with OVA-Exo, we verified this hypothesis. Immunization of Btk KO mice with OVA-Exo resulted, as expected, in a significant reduction in primary antibody production to OVA compared with the wild-type controls (IgM and IgG, P = .001; data not shown). After the second immunization with OVA protein, wild-type C57BL/6 mice generated significantly higher titers of IgG (P = .001) as well IgG1 (P = .017) and IgG2a (P = .001) antibodies, whereas the Btk KO mice responded with very low or negligible amounts of antibodies (Figure 7A-C). Splenocytes from Btk KO mice showed diminished ability to proliferate when restimulated in vitro with OVA (P = .001) or OVA-Exo (P = .001) compared with the wild-type controls (Figure 7D). This shows the requirements of B-cell help for effective activation of T cells by OVA-loaded exosomes.
Btk-deficient mice show abrogated production of antibodies and splenocytes proliferation compared with the wild-type control. C57BL/6 and Btk-deficient mice were primed intravenously with 50 μg per mouse with OVA-Exo and boosted with the OVA protein 4 weeks later. Sera were collected 7 days after boosting, and the presence of OVA-specific (A) IgG and (B) IgG1, and (C) IgG2a antibodies was detected by ELISA using serial dilution of the sera. Results are expressed as optical density at 1:900 serum dilutions. Individual data and mean are presented (n = 7 per group). (D) Splenocytes from the aforementioned boosted mice were restimulated in vitro with OVA or OVA-Exo for 48 hours, and proliferation was detected by thymidine incorporation assay. (E) Spleen sections from mice immunized with OVA-Exo stained with anti–FDC-M2 (follicular dendritic cells; red) and anti-B220 (B cells; pseudo-colored blue). Bar represents 150 μm. (F) A proposed model describing the mechanism of how indirectly loaded exosomes facilitate interaction between B and T cells for efficient activation of the immune response. APC indicates antigen-presenting cell; Exo, exosome.
Btk-deficient mice show abrogated production of antibodies and splenocytes proliferation compared with the wild-type control. C57BL/6 and Btk-deficient mice were primed intravenously with 50 μg per mouse with OVA-Exo and boosted with the OVA protein 4 weeks later. Sera were collected 7 days after boosting, and the presence of OVA-specific (A) IgG and (B) IgG1, and (C) IgG2a antibodies was detected by ELISA using serial dilution of the sera. Results are expressed as optical density at 1:900 serum dilutions. Individual data and mean are presented (n = 7 per group). (D) Splenocytes from the aforementioned boosted mice were restimulated in vitro with OVA or OVA-Exo for 48 hours, and proliferation was detected by thymidine incorporation assay. (E) Spleen sections from mice immunized with OVA-Exo stained with anti–FDC-M2 (follicular dendritic cells; red) and anti-B220 (B cells; pseudo-colored blue). Bar represents 150 μm. (F) A proposed model describing the mechanism of how indirectly loaded exosomes facilitate interaction between B and T cells for efficient activation of the immune response. APC indicates antigen-presenting cell; Exo, exosome.
FDCs accumulate C4 molecules on OVA-Exo injection
According to Denzer et al,25 follicular DCs (FDCs) can bind exosomes on their surface, which might support antigen-specific T and B cells for activation. Because FDCs play an important role during the germinal center reaction and antibody class switch, we wanted to investigate whether OVA-Exo targets the FDCs in the spleen to modulate the immune response. BALB/c mice were injected either with OVA-Exo or with PBS, and spleens were collected after 24 hours. Immunostaining showed that FDCs accumulated C4 components of complement on OVA-Exo immunization, which was comparatively lower in the PBS control group (Figure 7E). This observation suggests that exosomes, besides binding to FDCs, also focus complement components to these cells that might be involved in the OVA-Exo–mediated immune activation and adjuvant effect.
Discussion
The interaction between T cells and DCs is necessary to generate effective T-cell help for the production of high-affinity B cells and long-lived plasma cells. In the present study, we show that exosome-mediated immune activation needs the assistance of B cells in vivo for generating antigen-specific T-cell responses. We evaluated the influence of both directly (Pep-Exo) and indirectly (OVA-Exo) OVA-loaded exosomes on the immune response. The increased efficiency in vitro of Pep-Exo, as seen by others,20 might lead to the conclusion that Pep-Exo should be more optimal also in clinical settings. However, to our surprise, in contrast to the in vitro results, the OVA-Exo induced a potent antigen-specific T-cell response in adoptively transferred mice, whereas no such proliferation was seen after injection of Pep-Exo. This nonresponsiveness with Pep-Exo suggests that additional factors are involved for in vivo activation of T cells with Pep-Exo. Based on the understanding that activation of the immune response is achieved through the coordination of 3 classes of cells, B and T cells and DCs, we therefore designed a new experiment to explore whether B-cell help is crucial to overcome the nonresponsiveness with Pep-Exo in vivo. We combined Pep-Exo with the native OVA as a source of B-cell epitopes, injected to BALB/c mice, and measured the humoral response. For making comparison, we also treated mice with OVA-Exo codelivered with the whole OVA. Together with the native OVA, Pep-Exo could prime the immune system to produce antibodies, although the magnitude of the response was lower than the one achieved with OVA-Exo or with Alum or LPS.
Surprisingly, we observed that OVA-Exo alone also generated a potent primary antibody response in the absence of whole antigen, and this response was boosted after OVA challenge. This finding corroborates what we have noticed in the experiment with in vivo Tg T-cell proliferation with OVA-Exo. Because B cells recognize the conformationally intact antigen, it is thus evident that OVA-Exo carries B-cell epitopes either inside or on the surface of them. It has been shown previously that DCs have an antigen retention compartment enabling DCs to internalize, store, and recycle antigen for the direct presentation of native antigen to B cells.26 Indeed, our Western blot analysis confirmed the presence of conformational OVA on OVA-Exo, and the FACS analyses confirmed its presence on the surface of the exosomes. Colino and Snapper have failed to identify the whole antigen in exosome preparations by ELISA.15 However, Skokos et al could detect the presence of OVA on the mast cell–derived exosomes by Western blot.27 Hence, it is probable that a minimal amount of intact antigen might stimulate the B cells, suggesting an efficient mechanism for exosome-mediated enhancement of the immune response. In addition, one possibility could be that sequential B-cell epitopes might be exposed on the MHC28 of the OVA-Exo. Regardless of this question, we show that activation of antigen-specific B cells is needed for the induction of efficient T-cell proliferation in this system. According to previous studies, mice given repeated injections of anti-μ antisera (μsm mice) showed total lack of B cells and those mice displayed functional T-cell defects.29 Later, Ron and Sprent showed that the impaired T-cell proliferation in μsm mice can be restored by injecting purified B cells before antigen administration.30,31 In support of these findings, we show that Btk KO mice, lacking mature conventional B cells,32 failed to generate antibody and T-cell proliferative responses in the spleen. The B-cell dependence of T-cell activation reveals that not only DCs are needed to help T-cell activation in vivo; however, we think that DCs probably also contribute to full T-cell activation. Denzer et al25 have demonstrated that B cell–derived exosomes specifically bind to FDCs. In line with this, we show an increased C4 staining on FDCs after OVA-Exo injection. FDCs are accessory cells of the immune system essential for affinity maturation and immunoglobulin isotype switching of B-cell clones during the germinal center reaction,33-35 in which they present antigen to B and T lymphocytes.36 FDCs are also pivotal for selection for high-affinity B-lymphocytes. In this line, our data propose a model in which OVA-Exo that display peptide/MHC II molecules as well as conformationally intact antigen dock on FDCs. Thus, in coordination with FDCs, exosomes might facilitate interaction between specific B and T cells, ultimately leading to isotype switching and differentiation into plasma and memory B cells (Figure 7F). Thereby, OVA-Exo served to efficiently prime the immune system to amplify and modulate the humoral response to IgG2a and the T-cell response to the Th1 type.
Our observation that OVA-Exo but not OVA plus Pep-Exo elicited a detectable IgG antibody response on primary immunization is striking. One can speculate that the B-cell epitopes on exosomes are more efficiently presented compared with the native protein, which makes them more accessible to the B cells to recognize, thus lowering the threshold for their activation. In addition to effective presentation of antigen to B cells, it is possible that the OVA-Exo carry B-cell costimulatory molecules, eg, C337 or possibly C4, as shown on the FDCs by us, engaging the CD21 complement receptor and CD54,38 which bind LFA-1 on B cells, which leads to stronger primary IgG response to OVA.
Our findings that immunization with OVA plus Pep-Exo resulted in a comparatively low magnitude secondary antibody response of the IgG1 type whereas OVA-Exo induced strong secondary IgG2a antibodies could be the result of a higher TCR binding affinity of the MHC/OVA-Exo. It has been demonstrated previously that OVA323-339 can bind to the MHC in a different configuration, leading to altered TCR-exposed residues.39 In our case, processing of OVA by DCs may favor a specific peptide orientation on the MHC on exosomes with relatively higher affinity for the TCR than the synthetic OVA323-339 peptide directly loaded on exosomes.40 Thus, the higher binding affinity of the OVA-Exo to TCR might be associated with a prolonged interaction with the TCR, which leads to the induction of a Th1 phenotype and IgG2a production.41,42 An alternative explanation can be that OVA loading might promote distinct sorting of cargo proteins involved in Th1-biased responses. Our results demonstrate that OVA-Exo not only can function as adjuvant to enhance the humoral response but also contribute to the deviation of the response to Th1 type in naive mice, which is different from that obtained with the commonly used adjuvants Alum and LPS. These data support the findings by Colino and Snapper where they have shown that DT-pulsed DC-derived exosomes induce a Th1 type of response; however, they induced a systemic proinflammatory response by injecting complete Freund adjuvant before exosome treatment.15 We show that exosomes are so potent that an additional adjuvant is not needed. In another approach, Toxoplasma gondii antigen-pulsed DC2.4 cell-derived exosomes were reported to trigger Th1 type humoral immunity and IFN-γ production, which they found to be associated with the protection against T gondii infection.8 Recently, Beauvillain et al have reported that Toxoplasma antigen-pulsed DC-derived exosomes induce protective immunity against parasite infection both in syngeneic and allogeneic mice.10 In these systems, whole antigen is added and exosomes probably contain both B- and T-cell epitopes.
Our results are in contrast to the findings by Théry et al,16 who showed that Pep-Exo could induce T-cell responses in vivo. The discrepancy between their and our results might be the result of the nature of the antigen they used, which is a male-specific natural self-antigen. It is probable that that there already exists self-reactive B cells to this antigen, inherited in the B-cell repertoire.
The production of different antibody isotypes shows that the adjuvant effect of exosomes is not the result of LPS contamination of the exosome preparations. Previously, we have shown that LPS stimulated the production of IgG1 antibody in C57BL/6 mice when used as adjuvant.43 We used LPS-matured DC-derived exosomes in this study because exosomes from LPS stimulated DC display higher expression of MHC II and costimulatory molecules than exosomes from immature DCs, and are more potent stimulators than immature exosomes in vitro 23 (our results). Exosomes from LPS-matured DC were also more potent than the IFN-γ–matured exosomes, however, whether the cytokine and immunoglobulin profile after administration of IFN-γ exosomes might be the focus of another study, especially given the discrepancy between in vitro and in vivo results.
The rationale of using DC-derived exosomes in tumor vaccine design is now relatively well established. Because antigen-loaded exosomes are now being considered to have strong implications for the use in clinical settings, especially in the cancer therapy, the loading method should be considered and optimized accordingly. It has been observed that the combination of indirect loading through DC incubation for class II peptides and direct loading for MHC I peptides could be performed for stimulation of both CD8+ and CD4+ T cells.44 Our data show that the indirect loading is essential for inducing CD4+ T-cell proliferation to exosomes in vivo. In addition, in other therapeutic approaches, such as vaccine development for infectious diseases, indirectly loaded exosomes should be considered when Th1-like responses are desired. Vaccine adjuvants are an attractive option to overcome poor immunogenicity of candidate antigens and offer the opportunity to improve vaccine efficacy; thus, antigen-loaded exosomes may mimic the natural adjuvant to enhance and modulate the immune response. Furthermore, using exosomes as adjuvant instead of Alum might reduce the risk of Th2-biasing of the immune response. Our findings might lead to the design of more efficient exosome-based vaccines in the near future.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Swedish Research Council (grant 57X-15 242-05-02), the Karolinska Institutet, the Hesselman, Åke Wiberg, and Magnus Bergvall foundations, the Swedish Heart-Lung Foundation, and the Center for Allergy Research at the Karolinska Institutet.
Authorship
Contribution: K.R.Q., M.C.I.K., and S.G. contributed ideas and hypotheses; K.R.Q. performed most of the experiments and wrote the major part of the manuscript; U.G. provided help in some experiments and gave suggestions to the manuscript; E.D.J. performed the immunohistochemistry and wrote part of the manuscript; M.C.I.K. guided in setting up in vivo experiments and helped edit the manuscript; and S.G. provided guidance for the experimental design and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Khaleda Rahman Qazi, Department of Medicine, Clinical Allergy Research Unit L2:04, Karolinska University Hospital Solna, 171 76 Stockholm, Sweden; e-mail: khaleda.qazi@ki.se.