Abstract
Autologous hematopoietic cell transplantation (HCT) followed by nonmyeloablative allogeneic HCT (auto/alloHCT) provides cytoreduction and graft-versus-myeloma effects. We report on long-term outcomes of 102 patients with multiple myeloma who received auto/alloHCT with a median follow-up of 6.3 years. Treatment consisted of high-dose melphalan and autograft followed by 2-Gy total body irradiation, with or without fludarabine, and alloHCT from human leukocyte antigen-identical siblings. Postgrafting immunosuppressive agent was cyclosporine or tacrolimus and mycophenolate mofetil. Forty-two percent of patients developed grade 2 to 4 acute graft-versus-host disease (GVHD) and 74% extensive chronic GVHD. Five-year nonrelapse mortality after allografting was 18%, 95% related to GVHD or infections. Among 95 patients with detectable disease, 59 achieved complete remissions. Median time to progression was 5 years. Median overall survival (OS) was not reached. Median progression-free survival (PFS) was 3 years. Five-year OS and PFS were 64% and 36%, respectively. Seventy-three patients receiving autoHCT within 10 months from treatment initiation had 5-year OS of 69% and PFS of 37%. In multivariate analysis, β-2-microglobulin of more than 3.5 μg/mL at diagnosis and auto/alloHCT more than 10 months after treatment initiation correlated with shorter OS (P = .03 and P = .02) and PFS (P = .04 and P = .03), whereas Karnofsky scores less than 90% at allotransplantation correlated with shorter PFS only (P = .005). Long-term disease control and GVHD remain key issues.
Introduction
The optimal therapy for advanced-stage multiple myeloma (MM) remains poorly defined. Long-term remissions and possible cures have been described in patients who received allogeneic hematopoietic cell transplantation (HCT) after conventional high-dose conditioning regimens. In this setting, the addition of a graft-versus-myeloma (GVM) effect of donor T-cells may provide long-term disease control.1-4 Unfortunately, conventional allogeneic HCT has been associated with high nonrelapse mortality (NRM) and remains an option only for few selected patients.5-8
Reduced-intensity conditioning (RIC) regimens as preparation for allogeneic HCT have been associated with lower NRM while maintaining GVM effects. The European Group for Blood and Marrow Transplantation reported a comparison between 320 RIC and 196 myeloablative allogeneic HCT performed in 103 centers between 1998 and 2002. The 2 patient groups were significantly different. Patients treated with RIC were older (median age, 51 vs 45 years; P < .001), more often had progressive disease (28% vs 21%; P = .001), and were more likely to be treated with one or more prior autologous HCT (76% vs 11%; P < .001). This retrospective comparison confirmed that conventional allogeneic HCT was significantly associated with higher NRM compared with RIC (hazard ratio [HR], 1.87; confidence interval [CI] 95%, 1.2-2.8; P = .003). However, conventional allogeneic HCT was associated with a significantly lower relapse rate compared with RIC (HR, 0.51; CI 95%, 0.35-0.73; P < .001). T-cell depletion and the use of anti-CD52 antibody to control graft-versus-host disease (GVHD) were related to a higher relapse risk after RIC (HR, 2.3; P = .001 and HR 1.6; P = .03, respectively), probably because of inhibition of GVM effects.9
The use of RIC allogeneic HCT immediately after autologous HCT (auto/alloHCT) provides temporal separation between tumor reduction by high-dose chemotherapy and the GVM effect. Two preliminary studies showed NRM of 15% and 26% at 1 year after allografting, and high complete remission rates of 57% and 73%, respectively.10,11 These initial findings were both limited by small numbers of patients analyzed (52 and 17, respectively) and short follow-ups (median 18 and 13 months, respectively). However, the approach was safe and feasible for elderly patients and those with comorbidities who would otherwise have been excluded from conventional allogeneic transplantation protocols.
We previously published our experience on the use of auto/alloHCT for advanced stage MM in 52 patients with a median follow-up of 1.5 years after allografting.10 Here, we update and extend the study to include 105 patients enrolled in multicenter allogeneic transplantation protocols designed and coordinated by the Fred Hutchinson Cancer Research Center (FHCRC) with a median follow-up of 6.3 years after allografting.
Methods
Patients
Patient inclusion criteria for this analysis were (1) stage II or III MM at diagnosis or thereafter; (2) available human leukocyte antigen (HLA)–identical sibling donor; (3) programmed sequential treatment with conventional autologous HCT followed by nonmyeloablative auto/alloHCT; and (4) no prior autologous HCT.
One hundred five patients with MM fulfilling those criteria were sequentially enrolled at 10 centers on 4 FHCRC-coordinated multi-institutional protocols from August 1998 to August 2005. Patients proceeded to allogeneic HCT 40 to 180 days after autografting or whenever the following benchmarks were achieved: resolved mucositis with no need for intravenous hydration, renal and hepatic function returned to entry criteria, no need for intravenous antibiotics, and cytomegalovirus (CMV) antigen negative.
Clinical characteristics of the patients are shown in Table 1, and disease status at autografting is shown in Table 2. Twenty-one patients (20%) received more than one induction therapy line for refractory (n = 7) or relapsed disease after previous response (n = 14).
Inclusion criteria for allotransplantation were serum bilirubin less than twice normal, left ventricular ejection fraction more than 40%, creatinine clearance more than 40 mL/min, and Karnofsky performance status more than 60%. Written informed consent was obtained from all patients and donors in accordance with the Declaration of Helsinki on protocols approved by the institutional review boards of all participating institutions.
We retrospectively applied eligibility criteria for the ongoing Blood and Marrow Transplant Clinical Trial Network (BMT-CTN 0102) study in which double autologous (auto/auto) HCT is compared with auto/allo HCT. In our cohort, 76 patients fulfilled those criteria, having received autologous HCT within 10 months after treatment initiation, and we separately analyzed the outcome of this subgroup. Moreover, we evaluated the differences among these patients and the remaining 29 who had auto/auto HCT initiated more than 10 months after treatment initiation. The latter received more previous chemotherapy cycles (median 7 vs 4; P = .001) and were more often reinduced after failure of previous therapy (41% vs 12%; P = .001). There were no other significant differences in characteristics and disease status at diagnosis and at allografting among the 2 subgroups (data not shown).
Definition and assessment of prognostic factors at allografting
Serum albumin and β-2-microglobulin at diagnosis were available in 70 patients and used to calculate the International Staging Score (ISS).12
Comorbidities were assessed at allografting and described using an HCT-specific comorbidity index (HCT-CI).13 Performance status at allografting was described with the Karnofsky scale.14
Cytogenetic analyses were not planned at diagnosis or before allografting by protocol and were not routinely performed in all the participating centers during the years of enrollment of these patients. Nonetheless, we retrospectively collected data on conventional cytogenetic metaphase studies at autografting in 66 patients. Data on chromosome 13 deletion studied with fluorescence in situ hybridization (FISH) analysis for 13q14 were available in 18 patients at autografting (Table 1). Cytogenetic abnormalities evaluated at autografting with conventional karyotype were included as a covariate in the statistical analysis (Table 5).
Treatment
Autologous HCT.
Autologous granulocyte colony stimulating factor (G-CSF) mobilized peripheral blood mononuclear cells (G-PBMC) were harvested by leukapheresis after treatment with cyclophosphamide 3 to 4 g/m2 (day +1) and G-CSF 10 μg/kg subcutaneously (from day +3 through collection); 38 patients received additional paclitaxel (250 mg/m2 per day, day +2), and 25 received additional etoposide (200 mg/m2 per day; days +1, +2, +3) and dexamethasone (10 mg/day orally; days +1, +2, +3, +4). Two patients received G-CSF alone.
High-dose chemotherapy consisted of melphalan, 200 mg/m2 infused intravenously, at least 28 days after G-PBMC collection. Two patients received melphalan, 140 mg/m2, as a result of renal failure, and one patient with plasmablastic MM received 1,3-bis(2-chloroethyl)-1-nitroso-urea (BCNU) (300 mg/m2 [day −7], etoposide (200 mg/m2 per day [days −6, −5, −4, −3]), cytarabine (200 mg/m2 per day [days −6, −5, −4, −3]), and melphalan (140 mg/m2 [day −2]; BEAM regimen). No treatment for MM was given between autologous and allogeneic HCT.
Allogeneic HCT.
After recovery from autologous HCT, 102 patients proceeded to allotransplantation. Donors were HLA-identical siblings and received G-CSF 16 μg/kg per day from days −4 to 0 and leukaphereses on days −1 and 0. Donor G-PBMC harvested on day −1 were held overnight and infused with the day 0 collection.
Nonmyeloablative conditioning consisted in all patients of 2 Gy total body irradiation (TBI) at 7 cGy/min by linear accelerator or cobalt on day 0. Twenty-seven patients received additional fludarabine (30 mg/m2) on days −4, −3, and −2.
Donor G-PBMCs were infused after TBI. Postgrafting immunosuppression included mycophenolate mofetil (MMF; 15 mg/kg orally twice a day from the evening of day 0 until day +27) and cyclosporine (CSP; 5.0-6.25 mg/kg orally twice a day from day −3 to day +56 or +80 and then tapered) or tacrolimus (0.06 mg/kg orally twice a day from day −3 to day +56, and then tapered).10,15 Patients received standard supportive care and antimicrobial prophylaxis while CMV reactivation was monitored and treated with ganciclovir.
Donor chimerism was assessed 28, 56, 180, and 360 days after allogeneic HCT on peripheral blood T cells, granulocytes, and unfractionated marrow using FISH analysis in sex-mismatched pairs and polymerase chain reaction analyses of polymorphic microsatellite regions in sex-matched pairs.16
Disease responses
Disease responses were defined according to the International Uniform Response Criteria for multiple myeloma.17 Complete remission (CR) required absence of monoclonal protein in serum and urine by protein electrophoresis and by immunofixation, less than 5% plasma cells in marrow aspirates, absence of clonal disease by flow cytometry, and no increase in osteolytic lesions. Very good partial remission (VGPR) was defined as detection of serum monoclonal protein and/or Bence-Jones proteinuria by immunofixation but not by electrophoresis or at least 90% reduction in Bence-Jones proteinuria with excretion less than 100 mg/24 hours, and no increase in size or number of osteolytic lesions. Partial remission (PR) was defined as more than 50% reduction in the levels of serum monoclonal protein, more than 90% reduction in Bence-Jones proteinuria with excretion less than 200 mg/24 hours, and no increase in size or number of osteolytic lesions. Patients with less than a PR after induction chemotherapy or autografting were considered refractory, whereas the disease was considered stable if no response meeting the criteria of CR, VGPR, or PR was observed after allografting. Progressive disease was considered an increase in serum monoclonal proteins or urine light chains of at least 25% in patients with refractory or stable disease, whereas relapse was considered as the reappearance of bone marrow infiltration; serum monoclonal immunoglobulin or urine light chains or new bone lesions in patients in previous CR; or a 25% increase in any disease marker for patients in prior PR. Patients were evaluated for disease once before autologous conditioning and once before nonmyeloablative conditioning to estimate baseline levels of disease activity before each transplantation. Disease responses were assessed 56, 84, and 180 days after allotransplantation and thereafter at 6-month intervals or according to clinical status.
Toxicities and GVHD evaluation
Regimen-related toxicities during the first 100 days after allotransplantation were evaluated according to National Cancer Institute (NCI) Common Terminology Criteria version 2 (CTC v2.0) grading system (http://ctep.cancer.gov/reporting/ctc.html). Grading and treatment of acute and chronic GVHD were performed according to established criteria.18 The date of discontinuation of all immunosuppressive therapy for surviving patients was obtained by chart review and contact with participating centers.
End points
Primary end points of the study were evaluation of overall and progression-free survivals after nonmyeloablative allotransplantation. Secondary end points were assessment of disease responses, evaluation of NRM, and incidence of acute and chronic GVHD.
Statistical methods
Overall and progression-free survival were estimated by the Kaplan-Meier method. Cumulative incidences of relapse, NRM, and chronic GVHD were estimated by methods described previously.19 Prevalence of chronic GVHD was estimated according to the method of Pepe et al.20 Cox regression analysis was used for univariate and multivariate analysis of risk factors for mortality, relapse, and NRM, with relapse and NRM considered competing risks. Acute and chronic GVHD were considered as time-dependent covariates in such analyses.
Results
Autologous HCT
Toxicities and infections.
Most patients experienced mucositis, nausea and vomiting, and diarrhea and required a median of 7 (range, 0-38) days' hospitalization after high-dose melphalan. The median numbers of days of neutrophil counts less than 500/μL and platelet counts less than 2 × 104/μL were 8 (range, 0-21) and 3 (range, 0-38), respectively. Patients received a median of 1 transfusion (range, 0-21) of red blood cells (RBCs) and 1 transfusion (range, 0-130) of platelets (PTLs). Fifty-seven documented infections were reported: 35 bacterial, 13 viral (including 6 CMV reactivation and 1 CMV infection), and 9 fungal. Two patients, both with rapidly progressive myeloma, died after developing CMV pneumonitis and disseminated fungal infection, 31 and 163 days after autologous HCT, respectively.
Allogeneic HCT
The median time between autologous and allogeneic HCT was 69 (range, 40-281) days. Four patients received their planned allotransplants more than 180 days after autologous HCT to fulfill the recovery criteria. Three patients who underwent autologous HCT within 10 months of initiation of treatment for MM did not receive their allotransplants because of rapidly progressive disease and death from infections 1 and 4 months after high-dose melphalan (n = 2) and for insurance reasons (n = 1). This latter patient later received his allotransplantation off protocol.
Engraftment.
All 102 allografted patients had sustained engraftment. On day +28, medians of 90%, 95%, and 95% of peripheral blood T cells, granulocytes, and nucleated marrow cells, respectively, were of donor origin. This increased to medians of 96% to 100% on day +84. One patient with 25% donor T-cell chimerism on day +84 received, on day +117, pentostatin (1 mg/m2), followed by donor leukocyte infusion (DLI) of 1 × 107 CD3 cells/kg, on day +119. He subsequently evolved to 70% T-cell chimerism, 120 days after DLI.
Regimen-related toxicities, GVHD, and infections.
The nonmyeloablative low-dose TBI was generally well tolerated. Fifteen percent of patients experienced neutropenia and 8% thrombocytopenia with median periods of neutrophil counts less than 500/μL and platelet counts less than 2 × 104/μL of 5 (range, 2-19) days and 1 (range, 1-3) days, respectively. Eight percent of patients required transfusions for a median of 2 (range, 1-39) RBCs and 3 (range, 2-32) PTLs, respectively. During the first 100 days after transplantation, 31% of patients had transient increases in serum creatinine levels (22% grade 2 and 9% grade 3 toxicity), 20% had transient hyperbilirubinemia and increases in serum transaminases not related to GVHD (10% grade 2 and 10% grade 3), 12% had hypertension and sinus tachycardia (3% grade 2 and 9% grade 3), 1% had sinus bradycardia (grade 4), 12% had nausea and vomiting (6% grade 2 and 6% grade 3), 2% had anorexia requiring parenteral nutrition (grade 4), 8% had neurologic changes such as tremors and confusion (4% grade 2 and 4% grade 3) and hallucination and seizures, and 1% had stupor and failure to thrive (grade 4). Most of these adverse events were transitory and probably due to CSP or tacrolimus. During the first 100 days after allografting, 81% of patients were treated entirely in the outpatient setting, whereas 19% required hospitalization for a median of 5 (range, 2-42) days.
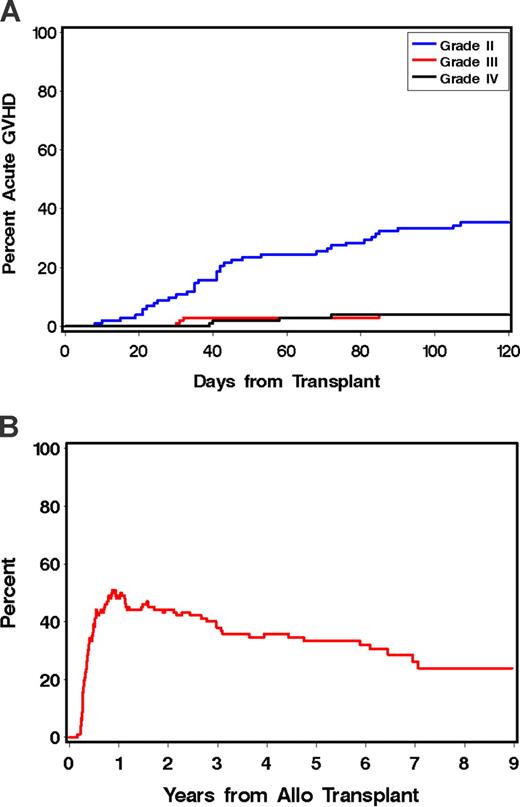
Forty-three patients (42%) developed grades 2 to 4 acute GVHD at a median of 42 (range, 8-107) days after allotransplantation. This was grade 2 in 34 patients, grade 3 in 4 patients, and grade 4 in 5 patients (Figure 1A). The sites involved in GVHD were skin, gut, and liver (n = 12), skin and gut (n = 9), skin and liver (n = 5), and skin only (n = 4). In most patients, GVHD responded either to resumption of CSP, MMF, or treatment with methylprednisolone (at 1-2 mg/kg per day with subsequent taper).
Acute and chronic GVHD in patients receiving tandem auto/allo HCT. (A) Cumulative incidence of grade 2 to 4 acute GVHD; grade 2 (blue line), grade 3 (red line); grade 4 (black line). (B) Prevalence of chronic GVHD.
Acute and chronic GVHD in patients receiving tandem auto/allo HCT. (A) Cumulative incidence of grade 2 to 4 acute GVHD; grade 2 (blue line), grade 3 (red line); grade 4 (black line). (B) Prevalence of chronic GVHD.
Seventy-four patients (74%) developed chronic extensive GVHD at a median of 167 (range, 90-830) days after transplantation. The estimated percentage of patients remaining on immunosuppressive therapy at 1, 2, and 5 years after transplantation was 49%, 44%, and 33%, respectively (Figure 1B). All documented infections and viral reactivations after allografting are described in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Disease response.
Disease responses after autologous and allogeneic HCT are summarized in Table 2, and patient disease status at each phase of treatment is shown in Table 3.
Among 83 patients who had detectable disease before allografting, 47 (57%) obtained CR at a median of 208 (range, 28-773) days after allografting. Achieving CR was not significantly associated with chronic GVHD (HR, 0.60; CI, 95% 0.3-1.3; P = .18).
Relapse.
Fifty percent of patients who completed the treatment (51/102) had disease progression or relapse after allografting. The median time to relapse after allotransplantation was 5 years (Figure 2B). In particular, 47% of patients in CR (31/66), 54% of patients in VGPR (6/11), and 53% of patients in PR (10/19) eventually relapsed.
Kaplan-Maier estimates of OS and PFS in patients receiving tandem auto/allo HCT. (A) OS (blue lines) in 102 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 73 patients who received autoHCT within 10 months from treatment beginning (dotted line) and PFS (red lines) in 102 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 73 patients who received autoHCT within 10 months from treatment beginning (dotted line). (B) Cumulative incidence of disease progression or relapse (blue lines) in 102 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 73 patients who received autoHCT within 10 months from treatment beginning (dotted line) and NRM (red lines) in 102 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 73 patients who received autoHCT within 10 months from treatment beginning (dotted line). (C-E) Kaplan Maier estimates of PFS. (C) Disease status at autoHCT: CR/VGPR (blue line, n = 26), PR (red line, n = 50), < PR (green line, n = 26). (D) Disease status at alloHCT: CR/VGPR (blue line, n = 43), PR (red line, n = 41), < PR (green line, n = 18). (E) Karnofsky performance status at alloHCT: more than 90% (red line, n = 66), less than 90% (blue line, n = 33). (F) Overall survival from disease relapse or progression in 51 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 36 patients who received autoHCT within 10 months from treatment beginning (dotted line).
Kaplan-Maier estimates of OS and PFS in patients receiving tandem auto/allo HCT. (A) OS (blue lines) in 102 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 73 patients who received autoHCT within 10 months from treatment beginning (dotted line) and PFS (red lines) in 102 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 73 patients who received autoHCT within 10 months from treatment beginning (dotted line). (B) Cumulative incidence of disease progression or relapse (blue lines) in 102 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 73 patients who received autoHCT within 10 months from treatment beginning (dotted line) and NRM (red lines) in 102 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 73 patients who received autoHCT within 10 months from treatment beginning (dotted line). (C-E) Kaplan Maier estimates of PFS. (C) Disease status at autoHCT: CR/VGPR (blue line, n = 26), PR (red line, n = 50), < PR (green line, n = 26). (D) Disease status at alloHCT: CR/VGPR (blue line, n = 43), PR (red line, n = 41), < PR (green line, n = 18). (E) Karnofsky performance status at alloHCT: more than 90% (red line, n = 66), less than 90% (blue line, n = 33). (F) Overall survival from disease relapse or progression in 51 patients who completed the tandem auto/alloHCT (solid line) and in the subgroup of 36 patients who received autoHCT within 10 months from treatment beginning (dotted line).
β-2-Microglobulin level more than 3.5 μg/mL at diagnosis, Karnofsky performance status (KPS) less than 90% at allografting, and receiving autologous HCT more than 10 months after treatment initiation were risk factors for disease relapse after allografting by univariate and by multivariate analysis. Achieving less than PR at allografting was significantly related to relapse by univariate analysis but not multivariate analysis (Tables 5 and 6).
Cytogenetic abnormalities or more than one course of induction therapy did not significantly affect relapse or progression after allografting by univariate analysis (Table 5). In the subgroup of 73 patients who received their autologous HCT within 10 months from treatment initiation, median time to relapse after allotransplant was 5.3 years (Figure 2B).
NRM.
NRM was 1% at day +100 and 11%, 14%, and 18% at 1, 2, and 5 years after allografting, respectively (Figure 2B). GVHD and infections were responsible for 18 of 19 nonrelapse-related deaths. Acute grade 2 to 4 GVHD was significantly associated with NRM by univariate and multivariate analyses but not chronic extensive GVHD (Tables 5 and 6). However, chronic extensive GVHD and infectious complications accounted for 10 deaths, 3 of which occurred more than 3 years after allografting in patients who had achieved disease remission. Comorbidities (HCT-CI ≥ 2), poor performance status (KPS < 90%) at allotransplantation, and age more than 60 years at allografting were not associated with higher risk of NRM (Table 5).
Overall and progression-free survivals.
After a median follow-up of 6.3 years after allografting (range 2-9), 60 of 102 (59%) patients survived and 33 of 102 (32%) are in remission. The causes of death for the 39 patients who completed the treatment are described in Table 4. Median overall survival (OS) has not been reached, whereas median progression-free survival (PFS) was 3 years. Five-year estimated OS and PFS were 64% and 36%, respectively (Figure 2A).
By univariate analysis, mortality was significantly associated with β-2-microglobulin levels greater than 3.5 μg/mL at diagnosis, KPS less than 90% at allografting, achieving less than PR at allografting, auto/alloHCT more than 10 months after treatment initiation, and more than one induction regimen before auto/alloHCT (Table 5). By multivariate analysis, only β-2-microglobulin levels greater than 3.5 μg/mL at diagnosis and auto/alloHCT more than 10 months after treatment initiation were risk factors for mortality; other factors did not reach statistical significance (Table 6).
By univariate analysis, shorter PFS was significantly related to β-2-microglobulin levels greater than 3.5 μg/mL at diagnosis, achieving less than PR at allografting, auto/alloHCT more than 10 months after treatment initiation, KPS less than 90% at allografting, and acute grade 2 to 4 GVHD (Table 5; Figure 2E). By multivariate analysis, β-2-microglobulin levels greater than 3.5 μg/mL at diagnosis, auto/alloHCT more than 10 months after treatment initiation, and KPS less than 90% at allografting maintained significant associations with shorter PFS (Table 6).
In 83 patients who achieved CR after allografting, the achievement of CR, evaluated as a time-dependent covariate, was associated with lower risk of overall and progression related mortality (HR, 0.17; CI 95% 0.1-0.4; P < .001 and HR, 0.50; CI, 95% 0.3-0.9; P = .02, respectively). In the subgroup of 73 patients who received their auto/alloHCT within 10 months from treatment initiation, median OS was not reached, whereas median PFS was 3.9 years. Five-year estimated OS and PFS were 69% and 37%, respectively (Figure 2A).
Salvage therapy and survival after disease progression or relapse.
Data on salvage therapy were available for 45 of 51 patients who had disease progression or relapse after allotransplantation. Treatment was initiated a median of 2 (range, 0.2-6.6) years after allografting. Treatments included thalidomide (n = 12), bortezomib (n = 10), bortezomib and thalidomide (n = 3), and conventional chemotherapy (n = 11). Nine patients received donor lymphocyte infusion (DLI) after a median of 41 (range, 3-81) months after allografting. Four had additional chemotherapy before DLI. After DLI, transient responses were observed in 2 patients who received additional chemotherapy, but all patients eventually developed progressive disease.
After a median follow-up of 2.7 (range, 0.5-5.8) years from the time of relapse/progression, the median duration of survival after relapse was 3.7 years (Figure 2F). Patients with chronic GVHD documented before relapse tended to have a lower mortality rate after relapse (HR, 0.47; CI 95% 0.2-1.1; P = .10). In the subgroup of 73 patients who received their auto/alloHCT within 10 months from treatment initiation, median overall survival after relapse was not reached and 5-year estimated survival after relapse was 57% (Figure 2F).
Discussion
Consolidation with 1 or 2 cycles of high-dose chemotherapy and autologous HCT has been considered optimal treatment for advanced stage MM for patients younger than 65 years of age. However, despite the superiority of autologous HCT in some but not all trials compared with conventional chemotherapy, this approach is not curative and most patients eventually relapse.8,21-23
Up-front treatment with single versus double autologous HCT has been compared in 5 randomized studies.24-28 The largest trial, the IFM 94 study, consisting of 399 patients with a median follow-up of 6.2 years from randomization, showed a significant benefit of double autologous HCT compared with single autologous HCT. Median PFS and OS were 2.5 and 4.8 years, respectively, for double autologous HCT versus 2.1 and 4 years, respectively, for single autologous HCT.25 However, patients who had CR or very good PR after the first autologous HCT had no improvements in outcomes after a second autologous HCT, suggesting that additional high-dose melphalan does not eliminate the malignant clone and different approaches are needed.25
Tandem autologous HCT with new agents such as thalidomide and bortezomib as induction, consolidation, and maintenance therapy has been used by the Arkansas group protocols. Barlogie and coworkers29 reported 5-year estimate OS and event-free survival (EFS) of 66% and 51%, respectively, in the Total Therapy 2 trial, and the results of the Total Therapy 3 trial, incorporating bortezomib in up-front therapy, were also encouraging: patients maintained sustained CRs.30
Nonmyeloablative allotransplantation after autologous HCT (tandem auto/alloHCT) as consolidation of initial therapy may provide additional graft-versus-myeloma activity. Tandem auto/alloHCT has been compared with double autologous HCT in nonrandomized trials by 3 groups, and prospective trials are ongoing.31-33 Garban and colleagues31 reported high-risk patients with elevated β-2-microglobulin levels and chromosome 13 deletion, as determined by FISH analysis. The allogeneic HCT conditioning regimen consisted of busulfan, fludarabine, and antithymocyte globulin. In this study, similar median PFS durations were observed in the auto/alloHCT group compared with the double autologous HCT group (2.6 vs 2.9 years; P = .35), and longer median OS durations were seen in the double autologous HCT group (2.9 vs 3.9 years; P = .07). The study by Bruno et al32 compared double autologous HCT versus tandem auto/alloHCT in MM, with the treatment assignment based on the availability of HLA-identical sibling donors. The conditioning regimen for allogeneic HCT was with 2-Gy TBI.10,15 In this study, a significant advantage was observed for the tandem auto/alloHCT group (median OS, not reached vs 4.8 years [P = .03] and median PFS, 3.6 vs 2.7 years [P = .07], respectively). Finally, a small study by Rosiñol et al33 compared tandem autologous HCT with autografting followed by RIC and allogeneic transplantation. In this study, patients with MM failing to achieve at least near-complete remission after a first autologous HCT received either a second autologous HCT or a RIC allogeneic HCT based on the availability of a HLA-identical sibling donor. Allogeneic HCT conditioning was with fludarabine and melphalan. They observed no statistical difference in PFS and OS between double autologous HCT and auto/alloHCT (median, 1.9 vs 1.6 years [P = .4] and median, 4.8 years vs not reached [P = .9]). Nonetheless, they reported a trend for a longer time to relapse after RIC allogeneic HCT than after second autologous HCT (median, 2.6 years vs not reached; P = .08).
Here, we report on the largest series of patients with MM treated with auto/allo HCT with the longest follow-up published to date. The overall results were encouraging, with a median OS not reached and estimated OS at 5 years of 64%. Estimated PFS at 5 years was 36%. In particular, the subgroup of patients treated within 10 months from initiation of therapy had 5-year estimated OS and PFS of 69% and 37%, respectively.
Attal et al reported in the IFM 94 study 5-year estimated OS values of 40% and 47% and 5-year estimated PFS values of 18% and 26% in the single and double autologous HCT groups, respectively.25 Comparison of these results is problematic, because we analyzed patients not uniformly treated at diagnosis and we used the day of allotransplantation for calculating overall and progression-free survivals, whereas Attal et al calculated survivals on an intention-to-treat basis from the day of randomization.
We observed that, after auto/alloHCT, patients seemed to have a continuous risk of relapse. This may be different from the possible plateau observed with high-dose allogeneic HCT in younger patients.5-8
Performance score at allografting was a major risk factor for relapse. This might be because MM, with associated osteolytic lesions, anemia, and renal impairment, had profound effects on patient well-being and levels of activity. Thus, Karnofsky scores might have reflected disease activity. Other studies have reported on the impact of performance status on OS34-37 and on PFS38,39 in patients with MM.
In this series, cytogenetic studies were not performed at specific time points and with uniform methods. Within this limitation, the data did not show an effect of cytogenetics on relapse risk, supporting the notion that allogeneic transplantation might overcome bad prognostic genetic features of the MM clone. A recent study on the impact of specific cytogenetic abnormalities, evaluated with FISH, on survival after allogeneic transplantation in MM, showed that del(17p13) was the only studied cytogenetic feature with a significant negative impact on PFS, whereas other abnormalities, such as translocation t(4;14), might have been overcome by the allotransplant.40 Ongoing studies, such as the Blood and Marrow Transplant Clinical Trial Network (BMT-CTN 0102) prospective study comparing autologous-autologous with autologous-allogeneic, with planned FISH and conventional cytogenetics analysis at diagnosis and at allografting, will clarify the role of these cellular features in the allotransplantation setting and potentially give key indications for risk-adapted strategies.
In contrast to what was reported for a larger group of patients with various diseases and donor type given nonmyeloablative conditioning allografting,41 in our series, poor performance status and presence of comorbidities were not related to an increased risk of NRM. In addition, being older than 60 years of age at allografting was not related to an increased risk of NRM, confirming the safety of the nonmyeloablative conditioning.
GVHD was the main cause of NRM. The cumulative incidence of chronic extensive GVHD was 74%. This rate was comparable with what was observed in patients with a variety of hematologic malignancies who received the same nonmyeloablative HCT conditioning.42 Long-term systemic therapy was needed to control GVHD over time, with 25% of current patients reported to still be on immunosuppression 7 years after allografting. In this study, we were not able to associate clinical GVHD with protection from relapse. However, we observed prolonged survival after relapse. Of 51 patients who experienced relapse or progression after auto/alloHCT, the probability of survival 2 years after relapse was 65% and 76% for the subgroup of patient who received the autologous HCT within 10 months from primary therapy. Attal et al reported that, with median follow-ups of 2.4 and 2.5 years after relapse in the single and double autologous HCT group, respectively, the probability of survival 2 years after relapse was only 36% in both groups.25 It is possible that our patients benefited from effective salvage treatments, as other studies have reported,43-46 or that ongoing GVM effects continued to modulate disease activities.
In summary, auto/alloHCT is a treatment option for patients with advanced stage MM, and we observed encouraging results, particularly in patients who received autologous HCT within 10 months from primary therapy. Nonetheless, long-term disease control remains an issue. Results of large prospective studies, such as the BMT-CTN 0102 trial, are needed to evaluate the outcome of auto/alloHCT compared with double autologous HCT. The addition of novel agents (eg, thalidomide, bortezomib, and lenalidomide) as induction or postgrafting therapy, acting with GVM effects against disease-specific antigens, might further improve the outcome.47-49
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Michelle Bouvier, RN, for study management; Jennifer Freese and Gresford Thomas for assistance with data retrieval; and Helen Crawford, Bonnie Larson, and Sue Carbonneau for assistance with manuscript preparation. We also thank the patients who participated in these protocols and the nurses and staff who cared for them.
This work was supported by grants CA78902, CA15704, and HL088021 from the National Institutes of Health, Bethesda, MD; M.R. was supported by Progetto Vita Vitae, Comitato Regionale Piemontese Gigi Ghirotti and by a postdoctoral research fellowship from the American-Italian Cancer Foundation.
National Institutes of Health
Authorship
Contribution: M.R., B.M.S., R.F.S., and D.G.M. conceived and designed the study; F.S., J.A.S., B.B., T.L., E.D.A., P.A.M., M.A.P., P.H., R.T.M., T.C., B.M.S., and D.G.M. provided study materials or patients; M.R., B.S., B.M.S., and D.G.M. collected and assembled the data; M.R., B.S., B.M.S., R.F.S., and D.G.M. analyzed and interpreted the data; M.R., B.S., B.M.S., R.F.S., and D.G.M. wrote the manuscript; and M.R., B.S., F.S., J.A.S., B.B., T.L., E.D.A., P.A.M., M.A.P., P.H., R.T.M., T.C., F.R.A., M.L.S., W.B., B.M.S., R.F.S., and D.G.M. approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David G. Maloney, MD, PhD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, PO Box 19024, Seattle, WA 98109; e-mail: dmaloney@fhcrc.org.