Abstract
Pleckstrin, the platelet and leukocyte C kinase substrate, is a prominent substrate of PKC in platelets, monocytes, macrophages, lymphocytes, and granulocytes. Pleckstrin accounts for 1% of the total protein in these cells, but it is best known for containing the 2 prototypic Pleckstrin homology, or PH, domains. Overexpressed pleckstrin can affect polyphosphoinositide second messenger–based signaling events; however, its true in vivo role has been unknown. Here, we describe mice containing a null mutation within the pleckstrin gene. Platelets lacking pleckstrin exhibit a marked defect in exocytosis of δ and α granules, αIIbβ3 activation, actin assembly, and aggregation after exposure to the PKC stimulant, PMA. Pleckstrin-null platelets aggregate normally in response to thrombin, but they fail to aggregate in response to thrombin in the presence of PI3K inhibitors, suggesting that a PI3K-dependent signaling pathway compensates for the loss of pleckstrin. Although pleckstrin-null platelets merged their granules in response to stimulation of PKC, they failed to empty their contents into the open canalicular system. This might be attributable to impaired actin assembly present in cells lacking pleckstrin. These data show that pleckstrin regulates the fusion of granules to the cell membrane and is an essential component of PKC-mediated exocytosis.
Introduction
Pleckstrin, the platelet and leukocyte C kinase substrate, is a prominent substrate of PKC in platelets, monocytes, macrophages, lymphocytes, and granulocytes.1 Pleckstrin accounts for 1% of the total protein in these cells, and it has long been used as a marker of activation in platelets and leukocytes.2-4 It is not found in erythrocytes, nor is it found in a wide range of nonhematopoietic cell lines.5-7 Pleckstrin has a paralog called pleckstrin-2 that is widely expressed and is not a phosphoprotein.8
Pleckstrin is perhaps best known for containing the 2 prototypic Pleckstrin homology, or PH, domains at its amino and carboxy-termini.5 PH domains are an approximately 100- to 120-amino acid module composed of a 7-stranded antiparallel β-sandwich that is closed at one corner by a C-terminal α helix.9-11 The SMART database indicates that PH domains are present in 271 human proteins,12 making them one of the most common domains in the deduced human proteome. Traditionally, PH domains are regarded as polyphosphoinositide-binding motifs.13-15 Polyphosphoinositide binding by the PH domain within a protein frequently localizes the protein to the cell membranes. In addition, some PH domains may interact with other targets such as the βγ subunits of heterotrimeric G proteins, serine/threonine phosphorylated proteins, and small GTPases.16,17 The structure of several PH domains complexed to polyphosphoinositides has been solved 18-20 (and others), confirming that there is a specific physical interaction between the inositol phosphate headgroup and the positively charged face of the PH domain.
Pleckstrin also contains an internal DEP domain.21 Originally, this was described in Dishevelled, Egl-10, and Pleckstrin (DEP). The DEP motif is found in 22 human and murine proteins, and this domain probably also mediates intermolecular interactions. Several targets for DEP domains have been proposed, and recent work by Ballon et al22 has shown that DEP domains of some proteins directly interact with the cytoplasmic tails of G-protein–coupled receptors. It is not known whether this is an universal feature of all DEP motifs.
Although the in vivo function of pleckstrin is unclear, heterologously expressed pleckstrin can affect second messenger–based signaling events mediated by phospholipase C, PI3Kγ, and inositol 5-phosphatases.23-26 Overexpression and microinjection studies suggest that pleckstrin is membrane localized, induces a shift of F-actin toward the cell cortex, and participates in the production of lamellipodia.27,28 Those studies of cultured cell lines further suggest that pleckstrin induces these cellular changes through a signaling pathway that involves phospholipids, integrins, and small GTP-binding proteins of the Rho family.29 These responses are tightly regulated by PKC-mediated phosphorylation of 3 residues in pleckstrin (Ser113, Thr114, and Ser117), located immediately after the amino-terminal PH domain.24 Although those overexpression studies represent useful starting points, further studies of platelets that lack pleckstrin are critical for a complete understanding of the contribution of this protein to platelet biology. Here, we describe the consequences of the introduction of a loss-of-function mutation within the murine pleckstrin gene.
Methods
Targeting of the murine pleckstrin gene
An 8.1-kb genomic DNA fragment, isolated from a 129SV agouti mouse strain library containing exon 3 of the pleckstrin gene, was used to generate the gene-targeting vector. This exon was replaced with a HPRT gene in the plasmid (Figure 1). The gene-targeting construct was transfected into E14TG2a embryonic stem (ES) cells by electroporation. After hypoxanthine and thymine (HAT) selection, Southern blotting EcoR1-digested genomic DNA was used to analyze ES cell clones for recombination within the pleckstrin gene. The probe was homologous to a sequence 5′ of the targeted region of the pleckstrin gene and was generated by polymerase chain reaction (PCR) amplification of genomic DNA using 5′-ggagaagcttgctgatag-3′ and 5′-aggaattgaagcggagac-3′ as primers.
Five recombinant clones were microinjected into blastocysts, and chimeras were generated. These chimeras then were backcrossed with C57Bl/6J mice to produce heterozygotes. Genotyping of mice was done by Southern blotting as described in the previous paragraph, or by PCR of genomic DNA. The PCR used a sense primer (5′-atgcaaacaacagtcagtgaggaaagaggg-3′) that was homologous to a region 5′ of the targeted region and 2 antisense primers. One antisense primer was homologous to HPRT (5′-cattgctcagcggtgctgtccatctgcacg-3′), and the second antisense primer (5′-gaacccaagcgtctctctcctccag-3′) was homologous to exon 3 of the murine pleckstrin gene. Finally, interbreeding of heterozygous siblings yielded animals homozygous for the desired mutation, ie, mice lacking pleckstrin. All studies comparing pleckstrin+/+ with pleckstrin−/− mice were done with animals that were littermates. The animals were maintained in a pathogen-free environment. Approval for the use of mice in this study was obtained from the University of Pennsylvania.
Platelet aggregation and ATP secretion
Blood was drawn from the vena cava of anesthetized mice into a Corning 15-mL centrifuge tube (Corning, Corning, NY) containing sodium citrate diluted to a final concentration of 0.042% (wt/vol). The blood was centrifuged at 200g for 7 minutes at room temperature, and platelet-rich plasma (PRP) was removed. PRP was incubated with 1 μM prostaglandin E1 for 20 minutes at room temperature before it was centrifuged at 800g for 10 minutes. The pelleted platelets were resuspended at a density of 2.5 × 108 platelets/mL in HEPES-Tyrode buffer pH 7.4 (134 mM NaCl, 3 mM KCl, 0.3 mM NaH2PO4, 12 mM NaHCO3, 2 mM MgCl2, 5 mM HEPES, 5 mM glucose, 0.35% [wt/vol] bovine albumin). Platelets were kept at 37°C throughout all experiments. Changes in light transmission and secretion of ATP were recorded simultaneously by using a Chronolog Dual-Channel Lumi-Aggregometer (Chrono-Log, Havertown, PA). Aliquots (0.25 mL) of platelet suspensions were preincubated at 37°C for 2 minutes and then stimulated with different agonists. Platelet ATP secretion was measured by the luciferin/luciferase reaction, after adding Chrono-lume no. 395 (Chrono-Log) to the aggregometer cuvette.
Measurement of P-selectin surface expression and Jon/A binding
Prewarmed washed platelets were diluted to a density of 5 × 106 and then stimulated at 37°C with 300 nM PMA or 0.1 unit/mL thrombin in the presence of P-selectin-PE (BD PharMingen, San Jose, CA) or PE-conjugated Jon/A according to the manufacturer's protocol. The samples were analyzed on a FACSCalibur (BD PharMingen) using FlowJo software (TreeStar, Ashland, OR).
Immunoblotting
Murine (1.5 × 108) platelets were lysed in NuPAGE LDS Sample Buffer (Invitrogen, Carlsbad, CA) and were then fractionated by electrophoresis on a 4% to 12% Bis-Tris gel. After transfer to polyvinylidene fluoride membranes (Invitrogen), blots were incubated with anti–mouse pleckstrin antibody as per protocols recommended by the manufacturer (BD Biosciences, San Jose, CA.) HRP-conjugated anti–mouse IgG was used as the secondary antibody, and signals were detected with enhanced chemiluminescence (ECL; GE Healthcare, Little Chalfont, United Kingdom).
Platelet actin assembly
Washed platelets were resuspended at a concentration of 107/mL in HEPES-Tyrode buffer, pH 7.4. After prewarming for 5 minutes at 37°C, they were stimulated with 300 nM PMA. The platelets were fixed with 0.55 volume of 10% paraformaldehyde for 3 minutes after PMA stimulation. The fixed cells were permeabilized and stained with 0.1 volume of 1% Triton X-100 containing 45 μM Alexa Fluor (633-phalloidin) at room temperature. Phalloidin binding was measured by flow cytometry (FACSCalibur), and data were analyzed with FlowJo software (TreeStar).
Results
Targeting of the murine pleckstrin gene
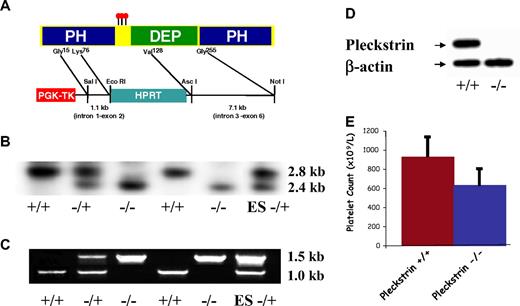
To elucidate pleckstrin function in vivo, we introduced a loss of function mutation into the murine pleckstrin gene within E14TG2a ES cells. We targeted the third of the 10 exons in the pleckstrin gene, which includes the coding region for the pleckstrin phosphorylation sites, as well as the key C-terminal α helix of the amino PH domain (Figure 1A). Five independent ES lines that were identified by both PCR and Southern blotting were microinjected into blastocysts. Three of these lines produced chimeric mice that yielded heterozygotes. Heterozygous pleckstrin+/− mice were intercrossed, and the expected numbers of pleckstrin+/− and pleckstrin−/− offspring were produced (not shown). Southern blotting of genomic DNA (Figure 1B), PCR of pleckstrin transcripts (Figure 1C), and sequence analysis (not shown) from targeted mice confirmed the deletion of the third exon. Immunoblotting with a monoclonal antibody that recognizes pleckstrin protein outside of the targeted region showed the complete absence of this protein in pleckstrin−/− platelets (Figure 1D).
Pleckstrin targeting. (A) Recombination into the pleckstrin gene results in the deletion of the nucleotides corresponding to amino acids 76 through 128 encoded within exon 3. This removes the distal portion of a β sheet, and the critical α helix from the first PH domain. (B) Southern blotting of mouse genomic DNA. Southern blotting shows a 2.8-kb wild-type band, and a 2.4-kb targeted band. (C) PCR of genomic DNA with a sense primer homologous to a region 5′ of the targeted region and antisense primers homologous to either HPRT or exon 3 of the pleckstrin gene. (D) Antipleckstrin immunoblot of platelet lysates showing complete loss of pleckstrin protein in mice homozygous for the recombinant gene. (E) The mean and SD of platelet counts derived from 8 pleckstrin-null mice and 8 wild-type littermates are shown.
Pleckstrin targeting. (A) Recombination into the pleckstrin gene results in the deletion of the nucleotides corresponding to amino acids 76 through 128 encoded within exon 3. This removes the distal portion of a β sheet, and the critical α helix from the first PH domain. (B) Southern blotting of mouse genomic DNA. Southern blotting shows a 2.8-kb wild-type band, and a 2.4-kb targeted band. (C) PCR of genomic DNA with a sense primer homologous to a region 5′ of the targeted region and antisense primers homologous to either HPRT or exon 3 of the pleckstrin gene. (D) Antipleckstrin immunoblot of platelet lysates showing complete loss of pleckstrin protein in mice homozygous for the recombinant gene. (E) The mean and SD of platelet counts derived from 8 pleckstrin-null mice and 8 wild-type littermates are shown.
Pleckstrin is a critical effector for PKC-mediated platelet activation
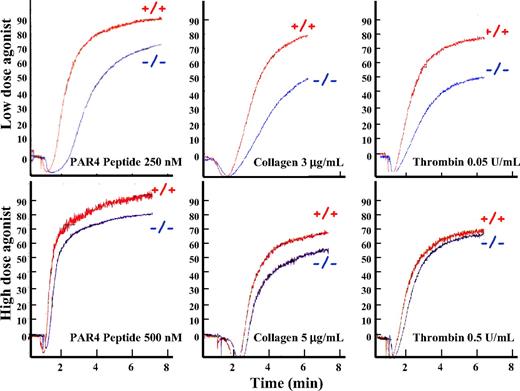
Mice lacking pleckstrin appeared to develop normally and had normal complete blood counts, except for mild thrombocytopenia (Figure 1E.) To determine whether pleckstrin-null platelets have a functional defect, we analyzed the ability of washed platelets to aggregate in response to agonists. Platelets lacking pleckstrin changed shape and aggregated normally in response to high concentrations of the thrombin receptor (PAR4) agonist peptide, collagen, or thrombin (Figure 2 bottom). Stimulation of platelets with lower doses of these same agonists showed a mild defect of aggregation in pleckstrin-null platelets compared with platelets derived from wild-type littermates (Figure 2 top). These data argue that pleckstrin−/− platelets have defective aggregation that is apparent under physiologic activation conditions.
Pleckstrin-null platelets have a PKC-mediated aggregation defect. Washed murine platelets lacking pleckstrin were analyzed after agonist stimulation in a Lumi-Aggregometer. The y-axis shows the relative aggregation, and the x-axis shows time in minutes. Platelets lacking pleckstrin have nearly normal aggregation in response to high doses of a peptide agonist of PAR4 (the dominant murine thrombin receptor), collagen, and thrombin (bottom panels). In contrast, lower doses of these agonists show that pleckstrin-null platelets have a mild aggregation defect (top). Results are representative of 6 experiments.
Pleckstrin-null platelets have a PKC-mediated aggregation defect. Washed murine platelets lacking pleckstrin were analyzed after agonist stimulation in a Lumi-Aggregometer. The y-axis shows the relative aggregation, and the x-axis shows time in minutes. Platelets lacking pleckstrin have nearly normal aggregation in response to high doses of a peptide agonist of PAR4 (the dominant murine thrombin receptor), collagen, and thrombin (bottom panels). In contrast, lower doses of these agonists show that pleckstrin-null platelets have a mild aggregation defect (top). Results are representative of 6 experiments.
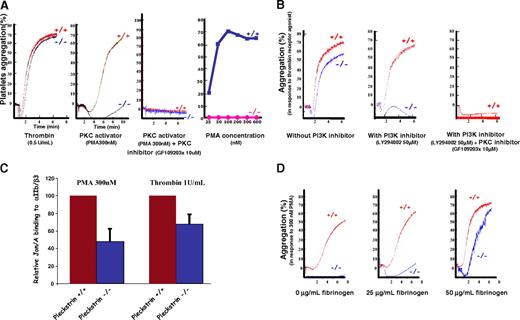
Previous tissue culture studies argue that pleckstrin is regulated by PKC phosphorylation. We therefore reasoned that a functional defect induced by the loss of pleckstrin might be most apparent in cells stimulated by direct pharmacologic activators of PKC, such as phorbol esters (PMA). Indeed, washed pleckstrin-null platelets exhibited a marked aggregation defect in response to 300 nM PMA, a dose of PMA that induced full aggregation in platelets derived from wild-type littermates (Figure 3A second graph from left). The ability of various PMA doses to induce aggregation in pleckstrin-null and wild-type platelets is shown in Figure 3A far right. Pleckstrin-null platelets failed to aggregate even when exposed to doses of PMA that were approximately 20-fold greater than the half-maximal effective concentration (EC50) for wild-type platelets. This shows that pleckstrin is a critical effector of PKC-mediated signaling pathways that lead to platelet activation.
PKC-mediated aggregation is absent in pleckstrin-null platelets. (A) In contrast to their response to thrombin, washed pleckstrin-null platelets completely fail to aggregate in response to all doses of the PKC stimulant, PMA (25-600 nM). The third tracing from the left shows that the effect of PMA on platelet aggregation is completely ablated by the PKC inhibitor, GF109203x. The dose response was derived from 3 experiments. (B) The presence of a PI3K inhibitor completely eliminates the ability of pleckstrin-null platelets to aggregate in response to the peptide agonist of PAR4 (250 μM). Inhibition of wild-type platelets with both a PI3K inhibitor (LY294002) and PKC inhibitor (GF109203x) resulted in a total loss of aggregation in response to 250 μM of the PAR4 peptide agonist. The results are representative of 3 experiments. (C) Loss of pleckstrin also impairs the binding of Jon/A, an antibody that only recognizes the activated form of αIIbβ3. The mean and SD are derived from 6 experiments with PMA (P < .001) and 10 experiments with thrombin (P < .001). (D) The addition of exogenous fibrinogen reverts the aggregation defect of washed platelets lacking pleckstrin in response to 300 nM PMA. These results are representative of 3 experiments.
PKC-mediated aggregation is absent in pleckstrin-null platelets. (A) In contrast to their response to thrombin, washed pleckstrin-null platelets completely fail to aggregate in response to all doses of the PKC stimulant, PMA (25-600 nM). The third tracing from the left shows that the effect of PMA on platelet aggregation is completely ablated by the PKC inhibitor, GF109203x. The dose response was derived from 3 experiments. (B) The presence of a PI3K inhibitor completely eliminates the ability of pleckstrin-null platelets to aggregate in response to the peptide agonist of PAR4 (250 μM). Inhibition of wild-type platelets with both a PI3K inhibitor (LY294002) and PKC inhibitor (GF109203x) resulted in a total loss of aggregation in response to 250 μM of the PAR4 peptide agonist. The results are representative of 3 experiments. (C) Loss of pleckstrin also impairs the binding of Jon/A, an antibody that only recognizes the activated form of αIIbβ3. The mean and SD are derived from 6 experiments with PMA (P < .001) and 10 experiments with thrombin (P < .001). (D) The addition of exogenous fibrinogen reverts the aggregation defect of washed platelets lacking pleckstrin in response to 300 nM PMA. These results are representative of 3 experiments.
Role of PI3K
It is clear from Figure 3A that washed pleckstrin-null platelets do not aggregate in response to PKC activation, yet they are capable of aggregating in response to upstream signals induced by thrombin. This finding suggests that another PKC-independent pathway must compensate for the loss of pleckstrin in the response to stimulation by extracellular agonists. We hypothesized that this alternative pathway might also use PtdIns(4,5)P2-dependent second messengers. Because phosphorylation of PtdIns(4,5)P2 by PI3K generates second messengers that also contribute to platelet aggregation,30-32 we further hypothesized that PI3K might compensate for the loss of pleckstrin. In support of this hypothesis, washed pleckstrin-null platelets were much more sensitive than wild-type platelets to the selective PI3K inhibitor, LY294002 (Figure 3B). A similar effect was seen when a pharmacologically distinct PI3K inhibitor, wortmannin, was used instead of LY294002 (not shown). Moreover, wild-type platelets aggregate efficiently in the presence of either LY294002 or GF109203X (a PKC antagonist) alone, but they fail to aggregate in the presence of both inhibitors together (Figure 3B far right).
These data argue that parallel pathways contribute to platelet activation in response to extracellular stimuli. Either PKC or PI3K-mediated signals alone are sufficient for thrombin-induced aggregation, so that the inhibition of only one pathway does not block aggregation. However, aggregation is blocked by the simultaneous inhibition of both pathways. In addition to platelet aggregation, we also analyzed agonist-induced activation of αIIbβ3 by flow cytometry to assess the activation-dependent binding of Jon/A. As shown in Figure 3C, platelets lacking pleckstrin had a defect in αIIbβ3 activation.
Pleckstrin is a critical component of platelet exocytosis
Previous studies that used pharmacologic inhibitors have suggested that PKC is a critical component of platelet secretion. We reasoned that pleckstrin might be the mediator of this PKC-induced secretion. Because the experiments shown in Figures 2 and 3A and B used washed platelets, there was no extracellular fibrinogen to support aggregation unless the platelets secreted the components of their α granules. To determine whether the aggregation defects of pleckstrin-null platelets was due to their inability to secrete fibrinogen, we analyzed whether the addition of exogenous fibrinogen could revert the aggregation defect in pleckstrin-null platelets. We found that the addition of fibrinogen to washed platelets corrected the aggregation defect in platelets lacking pleckstrin (Figure 3D). Except for a small delay in the initiation of aggregation, the addition of extracellular fibrinogen was able to completely revert the PMA-induced aggregation defect caused by loss of pleckstrin. These data suggest that defective exocytosis of fibrinogen is the principal cause for impaired PKC-mediated aggregation in pleckstrin-null platelets.
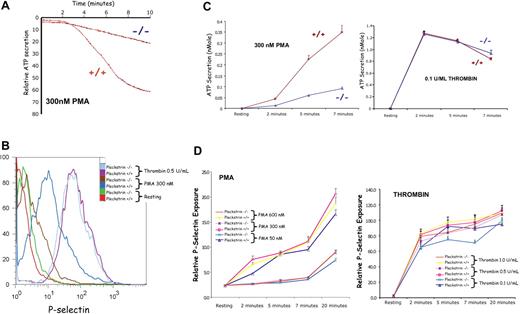
The ability of PKC to induce secretion of α and δ granules in pleckstrin-null platelets was analyzed. (Figure 4) δ Granule secretion was measured by a lucifer/luciferase assay (Figure 4A), and α granule secretion was quantified by surface expression of CD62P, P-selectin (Figure 4B). Platelets lacking pleckstrin had an approximately equal deficit in exocytosis of both α and dense granules after PMA stimulation (Figure 4C,D left). As shown in Figure 4D, the deficit was striking even at time points as late as 20 minutes after stimulation (Figure 4D left). This suggests that a biochemical step common to the release of both types of storage granules is affected by the loss of pleckstrin. However, there was no impairment of α or δ granule exocytosis in pleckstrin-null platelets after thrombin stimulation (Figure 4C,D right). This indicates that critical components of the soluble NSF attachment protein receptor (SNARE)–soluble NSF attachment protein (SNAP) exocytosis machinery within the genetically modified platelets are intact. It further suggests that the defect in pleckstrin-null platelets is limited to the pathway that links PKC activation with exocytosis.
PKC-mediated exocytosis is impaired in platelets lacking pleckstrin. PMA-induced exocytosis was analyzed biochemically (A,C), as well as by flow cytometry (B,D). The effect of the pleckstrin loss on function mutation on the δ (dense) granule secretion was analyzed in a Lumi-Aggregometer (Chrono-Log). (A) A representative tracing is shown, and pooled results showing the mean and SD derived from 5 experiments for PMA and 4 experiments with thrombin are plotted (C). Platelets lacking pleckstrin had a consistent deficit in dense granule section (P < .01 for all time points). The flow histogram (B) shows the relative surface exposure of P-selectin as a marker of α granule fusion with the cell membrane. The pooled analysis derived from 4 experiments is shown (D). For all doses and time points, paired analysis showed a P < .05.
PKC-mediated exocytosis is impaired in platelets lacking pleckstrin. PMA-induced exocytosis was analyzed biochemically (A,C), as well as by flow cytometry (B,D). The effect of the pleckstrin loss on function mutation on the δ (dense) granule secretion was analyzed in a Lumi-Aggregometer (Chrono-Log). (A) A representative tracing is shown, and pooled results showing the mean and SD derived from 5 experiments for PMA and 4 experiments with thrombin are plotted (C). Platelets lacking pleckstrin had a consistent deficit in dense granule section (P < .01 for all time points). The flow histogram (B) shows the relative surface exposure of P-selectin as a marker of α granule fusion with the cell membrane. The pooled analysis derived from 4 experiments is shown (D). For all doses and time points, paired analysis showed a P < .05.
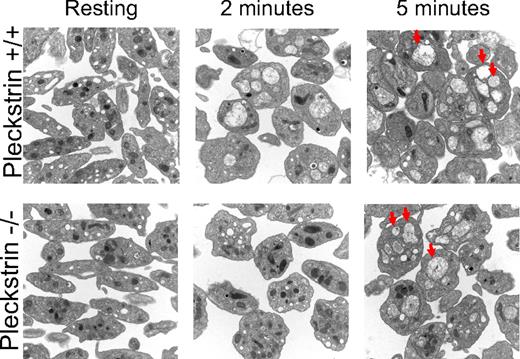
This exocytosis defect was examined in greater detail with the use of high-resolution electron microscopy of PMA-stimulated platelets. Before stimulation, pleckstrin-null platelets contained as many α and dense granules as wild-type platelets (Figure 5 top and bottom left). After 2 minutes of stimulation with PMA, wild-type platelets fused their α and δ granules (Figure 5 top middle). In contrast, platelets lacking pleckstrin failed to merge their granules at that time point (Figure 5 bottom middle). It is notable that after stimulation with PMA for 5 minutes, the pleckstrin-null platelets had merged their granules as much as wild-type platelets (Figure 5 top and bottom right). Despite ultimately coalescing their α and δ granules after 5 minutes of stimulation with PMA, platelets lacking pleckstrin still fail to adequately merge their granule membrane proteins with the cell surface membrane (Figure 4D), empty the contents of their δ granules into the extracellular environment (Figure 4C), or secrete the contents of their α granules to support aggregation (Figure 3A,D). This indicates that pleckstrin is required for a cellular step that occurs after granule-to-granule fusion but before granule-to-cell membrane fusion.
Loss of pleckstrin allows granule-to-granule fusion. Morphologies of platelet granules were analyzed in resting platelets and in platelets exposed to PMA for 2 or 5 minutes. Platelets derived from wild-type or pleckstrin-null platelets appeared identical under basal conditions. Wild-type platelets coalesced their granules more rapidly than platelets lacking pleckstrin (compare images in middle column.) However, wild-type and pleckstrin-null platelets appeared identical after 5 minutes of stimulation, with both genotypes of platelets having numerous merged granules (several examples indicated by arrowheads.) This shows that loss of pleckstrin impairs the efficiency of granule-to-granule fusion but does not prevent it. Shown are 20 000× magnifications captured with an FEI Tecnai T12 electron microscope operated at 80-kV accelerating voltage.
Loss of pleckstrin allows granule-to-granule fusion. Morphologies of platelet granules were analyzed in resting platelets and in platelets exposed to PMA for 2 or 5 minutes. Platelets derived from wild-type or pleckstrin-null platelets appeared identical under basal conditions. Wild-type platelets coalesced their granules more rapidly than platelets lacking pleckstrin (compare images in middle column.) However, wild-type and pleckstrin-null platelets appeared identical after 5 minutes of stimulation, with both genotypes of platelets having numerous merged granules (several examples indicated by arrowheads.) This shows that loss of pleckstrin impairs the efficiency of granule-to-granule fusion but does not prevent it. Shown are 20 000× magnifications captured with an FEI Tecnai T12 electron microscope operated at 80-kV accelerating voltage.
Pleckstrin and actin assembly
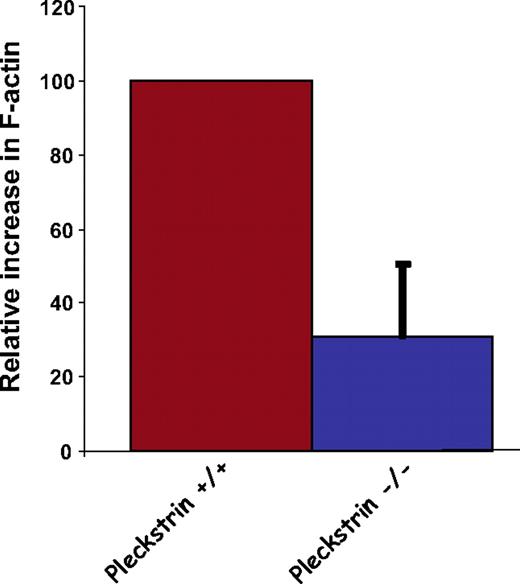
Previous work in neuronal cells and platelets have suggested that reassembly of the actin cytoskeleton is required for exocytosis.33-35 Heterologous expression studies show that pleckstrin can reorganize the actin cytoskeleton.27,28,36 Because the available evidence suggests that disassembly of F-actin facilitates exocytosis in platelets, it is possible that pleckstrin indirectly links PKC with exocytosis by facilitating F-actin reorganization. Therefore, we analyzed the ability of pleckstrin-null platelets to increase their levels of F-actin in response to PMA. As shown in Figure 6, platelets lacking pleckstrin showed a significant deficit in assembly of actin. Although this does not prove that pleckstrin contributes to platelet exocytosis through a pathway dependent on actin dynamics, the actin assembly defect in pleckstrin-null platelets is consistent with this hypothesis.
Loss of pleckstrin impairs actin assembly. After stimulation of washed murine platelets with 300 nM PMA, platelets were fixed, permeabilized, and stained with fluorescent phalloidin. Flow cytometry was used to quantitate phalloidin binding in 100 000 cells, and analysis was performed using FlowJo software. Shown is the mean plus or minus SEM for 5 experiments (P < .015).
Loss of pleckstrin impairs actin assembly. After stimulation of washed murine platelets with 300 nM PMA, platelets were fixed, permeabilized, and stained with fluorescent phalloidin. Flow cytometry was used to quantitate phalloidin binding in 100 000 cells, and analysis was performed using FlowJo software. Shown is the mean plus or minus SEM for 5 experiments (P < .015).
Discussion
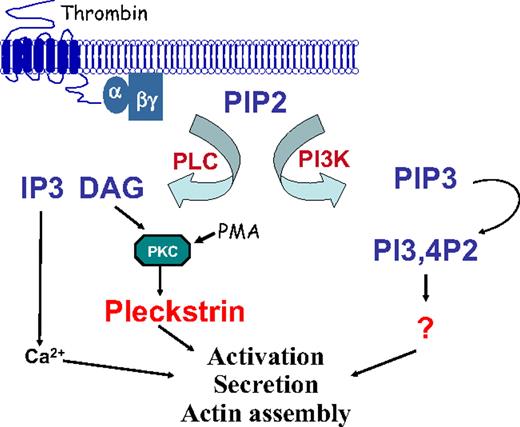
Although pleckstrin phosphorylation has long been used as a marker for protein kinase C activation in platelets, until now its true in vivo function has been unknown. Our results show a previously unrecognized association between pleckstrin and PKC-mediated exocytosis. This is consistent with work published by Haslam and Lynham37 that showed that, although phosphorylation of pleckstrin coincided with platelet secretion, phosphorylation did not correlate with aggregation that occurred in the absence of platelet secretion. Our data also show that thrombin-induced platelet secretion can be mediated by 1 of 2 parallel pathways. The first involves PKC and pleckstrin, and the second involves PI3K. A model of these signaling pathways is shown in Figure 7. These observations raise several questions about the nature of the mechanism by which pleckstrin mediates PKC-directed exocytosis and the generality of this function for pleckstrin in other hematopoietic cells.
Model of signaling by pleckstrin leading to platelet activation. Stimulation of an agonist receptor leads to the activation of PI3K and PLC. These 2 enzymes participate in alternative signaling pathways, leading to platelet aggregation. Activation of PLC leads to the production of DAG, PKC activation, and pleckstrin phosphorylation. Once phosphorylated, pleckstrin binds to the cell membrane and contributes to integrin activation, actin assembly, and exocytosis. An alternative pathway to platelet activation involving PI3K is pleckstrin-independent.
Model of signaling by pleckstrin leading to platelet activation. Stimulation of an agonist receptor leads to the activation of PI3K and PLC. These 2 enzymes participate in alternative signaling pathways, leading to platelet aggregation. Activation of PLC leads to the production of DAG, PKC activation, and pleckstrin phosphorylation. Once phosphorylated, pleckstrin binds to the cell membrane and contributes to integrin activation, actin assembly, and exocytosis. An alternative pathway to platelet activation involving PI3K is pleckstrin-independent.
Although pleckstrin contributes to the PKC-induced release of both α and dense granules, the electron microscopy studies argue that pleckstrin is not required for all aspects of vesicle exocytosis. During PKC-mediated platelet activation, storage granules redistribute, merge together, and finally fuse with the open canalicular system.38 Pleckstrin is not required for vesicle trafficking or granule-to-granule fusion, but it appears to be specifically required for the granule fusion with the plasma membrane. Pleckstrin could mediate this process by functioning as a docking protein or by directly regulating the SNARE-SNAP machinery. Alternatively, pleckstrin's effect on exocytosis might be indirect, mediated, for example, by pleckstrin-disassembling components of the actin cytoskeleton, and thus allowing granule release.
Does pleckstrin control exocytosis by functioning as a docking protein?
Pleckstrin is almost solely composed of 2 PH domains and a DEP domain. Because neither motif has enzymatic activity, the function of pleckstrin most probably involves multiple intermolecular interactions mediated by these domains. One possibility is that pleckstrin becomes anchored to particular regions of the membrane via its PH domains, and, once localized to those regions, pleckstrin via its DEP domain recruits another protein that is a critical effector for platelet exocytosis. In other words, pleckstrin might act as a docking protein for this putative exocytotic effector.
Does pleckstrin control exocytosis through phosphoinositide binding?
Another intriguing possibility is that pleckstrin sequesters phosphoinositides in a PKC-regulated manner, and this is required for exocytosis. Several other PKC substrates, including GAP43, MARCKS, and CAP23, also bind phospholipids and affect the actin cytoskeleton. Note that dense granule secretion from permeabilized platelets is inhibited by peptides that prevent the phosphorylation of MARCKS,39,40 which is believed to reversibly cluster PtdIns(4,5)P2. There is evidence that GAP43, MARCKS, and CAP23 all codistribute with PtdIns(4,5)P2, potentially promoting its retention or clustering or both.41,42 These 3 proteins might constrain the diffusion of PtdIns(4,5)P2within the plasma membrane, possibly concentrating it within discrete microdomains of the cell membrane in a manner that is required for the control of exocytosis or actin organization.
Given the ability of PH domains to bind phosphoinositides, pleckstrin might also bind and sequester PtdIns(4,5)P2. In platelets and leukocytes, pleckstrin represents 1% of total cellular protein. The abundance of pleckstrin would facilitate its ability to bind a significant amount of PtdIns(4,5)P2. As with MARCKS, it is possible that phosphorylation regulates the binding of pleckstrin to PtdIns(4,5)P2, so that clustering of this phospholipid by pleckstrin could be reversible and therefore able to be regulated.
Does pleckstrin affect the formation of the SNARE complex?
SNARE proteins are docking proteins that are present on the inner face of the cell membrane, as well as on the outer face of vesicular membranes. The fusion of the vesicular membrane with the cell membrane requires the formation of a core complex created by the binding of SNARE proteins on the opposing membranes. SNAPs facilitate this process. Blocking antibodies directed against several SNAREs and SNAPs impair secretion of α and dense granules in permeabilized platelets.43-45 Studies of transgenic mice indicate that the SNARE protein, VAMP-8, contributes to both α granule and dense granule secretion.46 However, loss of VAMP-8 does not cause a defect specific to PKC-mediated exocytosis similar to the phenotype seen in mice lacking pleckstrin. Because thrombin-mediated exocytosis is normal in pleckstrin-null platelets, the SNARE-SNAP complex must be intact in these cells. Thus, it is more likely that pleckstrin influences a regulator of SNARE-SNAP machinery rather than the complex itself. PKC-mediated phosphorylation of SNAP-23 is normal in platelets lacking pleckstrin (not shown). It is remarkable that the loss of pleckstrin does not affect granule-granule fusion but blocks granule-membrane fusion. This suggests that pleckstrin is required for a highly specific fusion step in the process of exocytosis. To date, no individual component of the SNARE-SNAP machinery has been shown to be critical for one specific fusion event.
Is the cytoskeleton the link between pleckstrin and exocytosis?
Several studies indicate that cytoskeletal regulation plays a central role in controlling platelet exocytosis. For example, blocking actin polymerization with cytochalasin enhances the release of platelet-dense granules,33-35 suggesting that actin contributes to the regulation of platelet exocytosis, which is analogous to the contribution of actin to neuronal cell exocytosis.47 Other indications that the cytoskeleton has a role in platelet exocytosis include the observation that preventing myosin light chain phosphorylation impairs platelet secretion48,49 and that by adding recombinant scinderin (a calcium-dependent F-actin severing protein) to permeabilized platelets enhances dense granule exocytosis.50 As mentioned earlier, MARCKS also contributes to platelet secretion39,40 and binds actin in addition to phosphoinositides. It should also be noted that platelet microtubules are not involved in PKC-induced exocytosis, because this process is unaffected by taxol and vincristine51,52
As shown in Figure 6, loss of pleckstrin results in defective actin assembly. This shows that pleckstrin contributes to actin dynamics within platelets. Therefore, it is possible that pleckstrin regulates PKC-mediated platelet exocytosis through a pathway dependent on actin.
What is the role of pleckstrin in other hematopoietic cells?
In addition to its expression in platelets, pleckstrin is also an abundant protein within leukocytes. Brumell et al53 have shown that phosphorylated pleckstrin associates with the phagosomal membrane on ingestion of IgG-opsonized particles in monocyte/macrophage cell lines. Analogously, Brumell et al54 showed that the stimulation of neutrophils with chemoattractants induces the PKC-mediated phosphorylation of pleckstrin and induce its interaction with membranes or with the cytoskeleton or both. Although pleckstrin did not colocalize with granules with resting neutrophils, to our knowledge there are no data that addresses whether pleckstrin migrates toward these organelles in activated cells. It is notable that pleckstrin is 1% of the total cellular protein in lymphocytes, monocytes, and granulocytes. Determining its true in vivo function within these cells is an area of active investigation in our laboratory.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported by funding from the National Heart, Lung, and Blood Institute (Bethesda, MD; HL073289, HL083392, and HL40387; C.S.A.).
National Institutes of Health
Authorship
Contribution: L.L. and Y.W. designed and performed research, analyzed data, and wrote the paper; M.F. and E.W.S. performed research, analyzed data, and wrote the paper; J.C., J.D., and M.A.L. analyzed data and wrote the paper; and C.S.A. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charles S. Abrams, Hematology-Oncology Division, University of Pennsylvania School of Medicine, 421 Curie Blvd, Biomedical Research Bldg II/III #912, Philadelphia, PA 19104; e-mail: abrams@mail.med.upenn.edu.
References
Author notes
*L.L. and Y.W. contributed equally to this study.