Abstract
Limited data are available describing donor adverse events (AEs) associated with filgrastim mobilized peripheral blood stem cell (PBSC) collections in unrelated volunteers. We report results in 2408 unrelated PBSC donors prospectively evaluated by the National Marrow Donor Program (NMDP) between 1999 and 2004. Female donors had higher rates of AEs, requiring central line placement more often (17% vs 4%, P < .001), experiencing more apheresis-related AEs (20% vs 7%, P < .001), more bone pain (odds ratio [OR] = 1.49), and higher rates of grades II-IV and III-IV CALGB AEs (OR = 2.22 and 2.32). Obese donors experienced more bone pain (obese vs normal, OR = 1.73) and heavy donors had higher rates of CALGB toxicities (> 95 kg vs < 70 kg, OR = 1.49). Six percent of donors experienced grade III-IV CALGB toxicities and 0.6% experienced toxicities that were considered serious and unexpected. Complete recovery is universal, however, and no late AEs attributable to donation have been identified. In conclusion, PBSC collection in unrelated donors is generally safe, but nearly all donors will experience bone pain, 1 in 4 will have significant headache, nausea, or citrate toxicity, and a small percentage will experience serious short-term adverse events. In addition, women and larger donors are at higher risk for donation-related AEs.
Introduction
Voluntary donation of bone marrow (BM) or peripheral blood stem cells (PBSCs) for hematopoietic cell transplantation is a well-established and accepted altruistic act, performed by thousands of related and unrelated donors throughout the world each year. Although donors generally recognize that donation is not risk-free, it is the responsibility of the transplant community to understand these risks, to pursue efforts that minimize risks, and to ensure that donors are fully informed
Over the past decade, a major shift has occurred from bone marrow to cytokine-mobilized PBSC collection as the dominant procedure for obtaining allogeneic hematopoietic cell grafts. The practice initially involved related donors, but in more recent years this method of collection has been extended to healthy unrelated donors. Although several studies have addressed safety considerations of the procedure for small cohorts of related donors,1-10 published unrelated donor safety experience is minimal,11-13 and large, comprehensive studies of PBSC donor safety in healthy unrelated donors have not been published. In addition, previous studies have not used standardized toxicity scales, making it difficult to compare the studies with each other and giving an incomplete understanding of the donor experience.
To address the imperative of fully ascertaining risk to a healthy unrelated donor of PBSCs, at the initiation of the National Marrow Donor Program (NMDP) PBSC collection program for transplants in 1999, donors were enrolled in a comprehensive study assessing donor adverse events (AEs), collection efficacy, and recipient outcomes. This paper reports detailed AE experiences of 2408 donors collected through April 2004, giving a much clearer picture of the PBSC donation experience.
Methods
Study cohort
This study included 2408 first-time stem cell donors who donated a PBSC product between July 1999 and April 2004. Informed consent was obtained from all donors in accordance with the Declaration of Helsinki for participation in an NMDP-sponsored and IRB-approved research protocol for manufacturing PBSC products. The NMDP protocol operated under an Investigational New Drug application accepted by the United States Food and Drug Administration (USFDA). All donors were evaluated to assess medical suitability, transplantation-transmissible infectious diseases, and contraindications (eg, pregnancy, autoimmune disease, history of thromboembolic disease) for receipt of filgrastim (rhG-CSF). All PBSC collections were performed according to the protocol after mobilization with filgrastim administered subcutaneously on 4 (only for recipients weighing < 35 kg) or 5 consecutive days at a daily dose of approximately 10 μg/kg. Administered filgrastim doses were rounded to some combination of 300-μg and 480-μg vials based on the donor's total body weight, so that protocol-defined targets ranged from 8.7 to 13.3 μg/kg per day. The protocol included provisions for filgrastim dose reductions in the presence of high-grade symptoms.
The donation processes were facilitated by 73 donor centers and 96 apheresis centers. Donor centers managed donor medical evaluations, infectious disease marker testing, filgrastim administration, and data reporting. Apheresis centers performed collections, but in many cases assisted with filgrastim administration and data reporting. Donor data were collected at the time of predonation medical evaluation, before each filgrastim dose, and at the time of each apheresis procedure. Donors were further assessed 2 days after the final apheresis procedure, weekly until “full recovery,” and at 1 month, 6 months, and annually after donation. Donor symptoms were assessed using a subset of the Cancer and Leukemia Group B (CALGB) toxicity criteria. Symptoms assessed included allergy, anorexia, chills, fever, sweats, fatigue, headache, myalgia, nausea, vomiting, other flulike symptoms, local reactions, skin rash, pain, and infections. The Eastern Cooperative Oncology Group (ECOG) Performance Status was used to rate donor functional status.14 Adverse events associated with apheresis collections were reported in an open text field. Apheresis collections were targeted to process a given volume of donor blood based on recipient body weight (12 L, 18-20 L, or 24 L processed for recipients weighing < 35 kg, 35-70 kg, and > 70 kg, respectively). Early versions of the protocol (until version 7, September 2003) were biased toward 2 consecutive apheresis collections because the maximum single-sitting apheresis volume was set at 20 L.
End points
Donor hematologic recovery end points were the incidence of cytopenias after apheresis and at each follow-up time point as assessed by the change in white blood cell (WBC) counts, platelets, and hemoglobin. Donor organ toxicity end points were changes in renal and hepatic serum chemistries after apheresis and at each follow-up time point. Donor symptom-related end points were the incidence of bone pain, maximum severity of bone pain, maximum CALGB toxicity, and maximum level of ECOG Performance Status during mobilization and donation. A serious adverse event was defined as one that was fatal or immediately life threatening, or that caused or prolonged hospitalization, caused permanent disability, was a congenital anomaly, was a cancer, or was an overdose. An unexpected adverse event end point was met when an event possibly related to the administration of the study, study drug, or study product had not been identified in nature, severity, or frequency in the study protocol.
Statistical methods
The analysis quantified a variety of donor characteristics. Donor WBC, platelet, and hemoglobin recovery after donation were analyzed via paired t test. Logistic regression using the step-wise model selection method was used to evaluate risk factors that might have an influence on donor symptom-related end points. Factors considered were donor age, sex, race/ethnicity, weight, body mass index (BMI), cytomegalovirus (CMV) status, filgrastim dose, alkaline phosphatase at baseline, and the baseline values and the changes from baseline to preapheresis values of WBCs, platelets, hemoglobin, percentage of neutrophils, and percentage of mononuclear cells. The effects were estimated via odds ratios. Interactions were checked between all significant main effects, but none were found to be significant. Because of the large number of variables being tested in the models, a significance level of .01 was used in all multivariate analyses.
Results
Donor characteristics
Table 1 shows the characteristics of the 2408 donors analyzed for AEs associated with PBSC collection. Sixty percent of the donors were male and the large majority of donors were white. Two-thirds of the donors were younger than 40 years, with only 9% of donors older than 50 years. Of note, 70% of donors were considered overweight or obese with BMI of 25 kg/m2 or more or 30 kg/m2 or more, respectively. Male donors had higher BMI values than female donors with 22%, 47%, and 30% of male donors, respectively, considered normal, overweight, and obese compared with 42%, 31%, and 27% of female donors.
Two-thirds of the donors underwent 2 apheresis procedures, whereas the remaining donors completed their donations in a single day. Table 1 shows that 25% of the donors underwent large volume apheresis procedures (> 18 liters) on the first day of collection. Most of these large-volume procedures were planned, single-day collections, processing between 20 and 26 liters. The median duration of the first apheresis procedure was 3.6 hours (range, 1.3-8.9 hours).
AEs associated with filgrastim
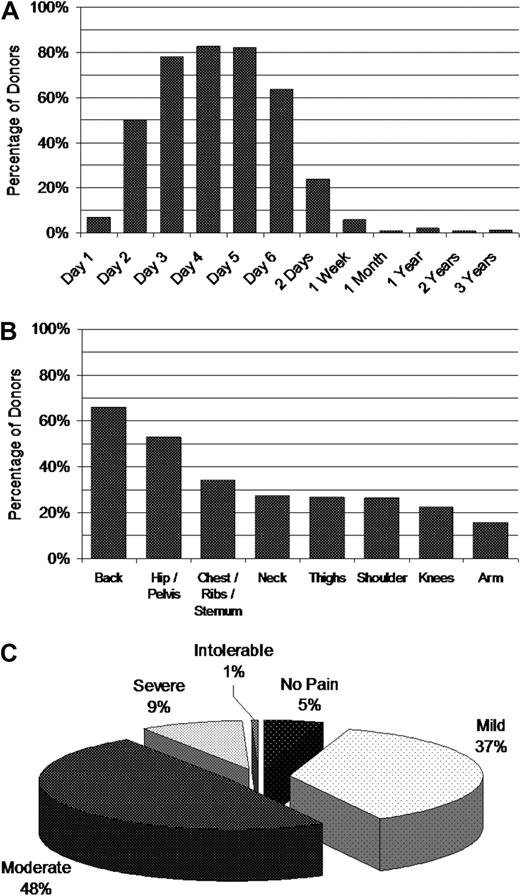
As expected, the large majority of donors experienced bone pain, peaking on Day 4 of mobilization after just 3 doses of filgrastim (Figure 1A). Fifty percent reported bone pain after a single dose of filgrastim. Bone pain appeared most often in the axial skeleton with smaller percentages reported in other bones (Figure 1B). Most donors reporting pain received pain medications, usually acetaminophen or ibuprofen, with approximately 65% and 30% reporting some pain relief or complete pain relief, respectively. Nine percent of donors experienced severe bone pain with 1% percent considering the pain “intolerable” (Figure 1C). Pain decreased significantly after filgrastim administration was stopped, but was still reported in 24% and 6% of donors at 2 days and 1 week, respectively, after the final apheresis collection.
Incidence of bone pain. Pain symptoms were evaluated before administration of filgrastim each day and at each follow-up after donation. (A) Percentage of PBSC donors who experienced bone pain. (B) Site of bone pain frequency on Day 4. (C) Frequency of highest severity of bone pain during mobilization and collection.
Incidence of bone pain. Pain symptoms were evaluated before administration of filgrastim each day and at each follow-up after donation. (A) Percentage of PBSC donors who experienced bone pain. (B) Site of bone pain frequency on Day 4. (C) Frequency of highest severity of bone pain during mobilization and collection.
Logistic regression was performed to identify factors predicting for higher intensity bone pain versus lower intensity (moderate, severe, or intolerable vs mild or absent). Only donor sex predicted for intensity of bone pain (female vs male OR = 1.39; 95% CI, 1.18-1.64; P < .001). Risk factors for the incidence of bone pain on Day 4 of filgrastim administration were female sex, CMV-seronegative status, being obese, and having a low percentage of neutrophils before the first filgrastim dose (Table 2). Although these risk factors were highly significant statistically, the clinical relevance is low because the actual incidence of bone pain within the risk groups differed by only a few percentage points.
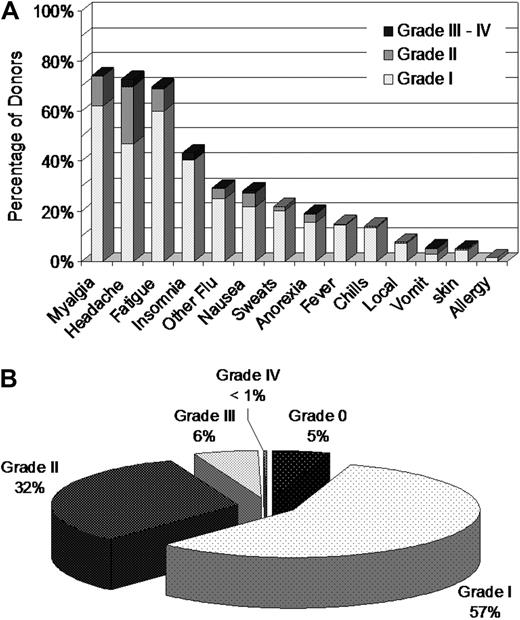
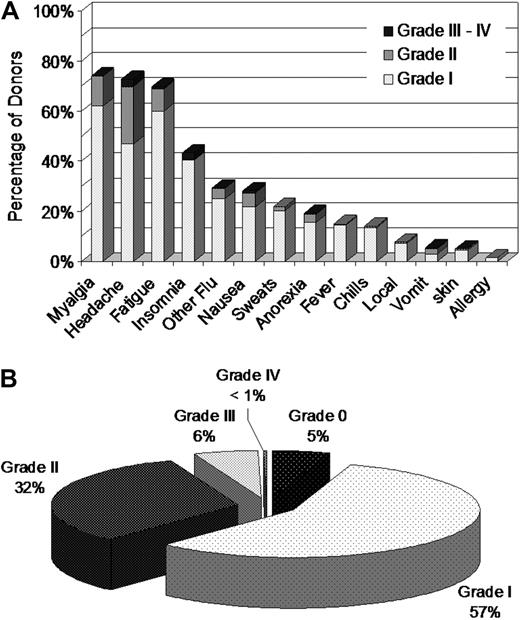
Donors were assessed for additional symptoms using abbreviated CALGB toxicity criteria. More than 70% of donors reported some degree of myalgia, headache, or fatigue, with smaller percentages reporting insomnia, nausea, fever, emesis, or other symptoms (Figure 2A). Zero or I was the maximum grade of all CALGB AEs reported by 62% of donors (Figure 2B). Six percent of donors experienced at least one grade III to IV toxicity and 38% experienced at least one grade II to IV toxicity. After bone pain and myalgia, headache was the most commonly reported symptom with 26% of donors experiencing headache of grade II or higher at some point during mobilization and collection (Figure 2A). Symptoms disappeared almost completely by 1 week after donation, with the exception of slight persisting increases in fatigue, headache, and myalgia (1 week after donation vs baseline: 7.9% vs 0.8%, 4.9% vs 2.5%, and 3.7% vs 2.1%, respectively). Rates of headache and myalgia returned to baseline by the 1-month assessment, but a few donors continued to report fatigue (3.1% at 1 month vs a baseline of 0.8%).
Assessment of donor symptoms using the Abbreviated CALGB Toxicity Criteria. Abbreviated CALGB Toxicity Criteria were evaluated before administration of filgrastim each day. (A) Frequency of highest CALGB score reported by PBSC donors during mobilization and collection. (B) Frequency of highest CALGB score across all symptoms reported by PBSC donors during mobilization and collection.
Assessment of donor symptoms using the Abbreviated CALGB Toxicity Criteria. Abbreviated CALGB Toxicity Criteria were evaluated before administration of filgrastim each day. (A) Frequency of highest CALGB score reported by PBSC donors during mobilization and collection. (B) Frequency of highest CALGB score across all symptoms reported by PBSC donors during mobilization and collection.
The maximum CALGB score across all symptoms during the process of PBSC mobilization and apheresis collection was analyzed using logistic regression. The analysis outcome was a maximum CALGB score of II or higher. Regression results are shown in Table 3. Female donors and very heavy donors experienced more grade II or higher CALGB toxicity symptoms during mobilization and donation. Similar associations were found when the cohort was analyzed for grade III or higher toxicities. Sex distribution analysis of grade III-IV CALGB toxicities showed an increase in rates of nausea, vomiting, and anorexia in female donors; whereas headache, insomnia, fatigue, and other severe toxicities were comparable between the males and females.
AEs associated with venous access and apheresis
If the PBSC product could not be collected using peripheral veins, a central venous line was inserted. Figure 3A provides information on central venous access. On the first day of collection, 166 females (17% of female donors) required a central line, whereas only 61 males (4% of male donors) required one (P < .001). There were 22 females (3% of female donors on the second day of donation) who did not have a central line on the first day but required one on the second day of donation, compared with only 4 males (0.4% of male donors on the second day of donation; P < .001). Central lines were placed with comparable frequencies in the internal jugular, subclavian, and femoral veins.
Adverse events associated with access and apheresis. (A) Percentage of PBSC donors who required central venous access, by sex. (B) Percentage of PBSC donors who reported adverse events as a result of donation, by sex.
Adverse events associated with access and apheresis. (A) Percentage of PBSC donors who required central venous access, by sex. (B) Percentage of PBSC donors who reported adverse events as a result of donation, by sex.
Apheresis-related AEs were reported by 20% of females and 7% of males on the first day of donation (P < .001), and by 10% of females and 4% of males on the second day of donation (P < .001, Figure 3B). Fifty-one percent of these AEs were complications of citrate administration (tingling, numbness, carpal-pedal spasm, etc) with 20% of donors who experienced AEs reporting nausea and 22% reporting problems with access (intravenous lines infiltrated, multiple intravenous line placement attempts, hematomas, poor flow, etc). Other apheresis-related AEs occurred more rarely (1%–6% of the reported events) and consisted of pain (headache, back, and chest pain), chills, hypertension or hypotension, allergic reactions, fatigue, or syncope.
Blood counts and chemistries
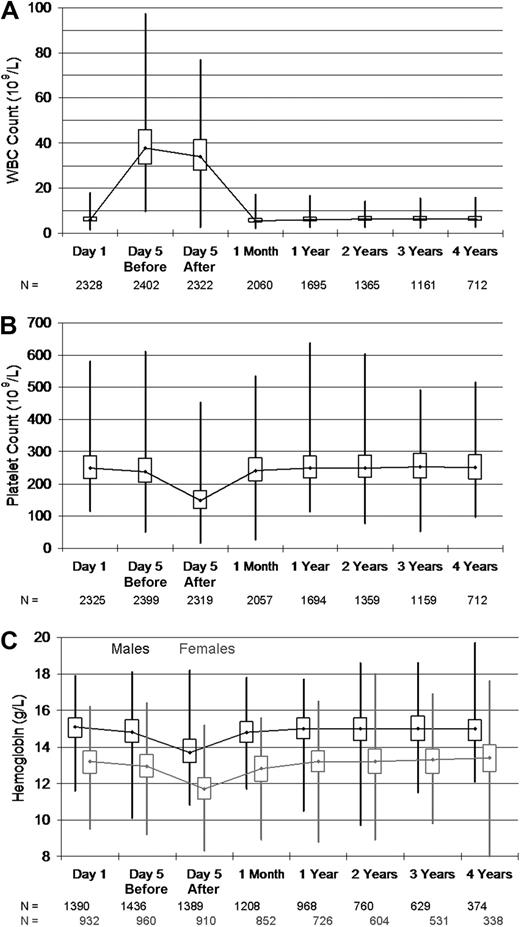
The results of univariate analyses of changes in WBCs, platelets, and hemoglobin are shown in Table 4 and illustrated in Figure 4A through C. As expected, donor WBCs rose sharply during mobilization and donation as a result of filgrastim injection, and then declined below baseline at 1 month after donation (P < .001). At yearly follow-up, 1 to 4 years after donation, donor WBC levels were slightly elevated compared with baseline values (mean increase, 0.12-0.29 × 109/L). Although statistically significant WBC changes were observed at 1 month and annual follow-up time points, absolute differences were clinically irrelevant. Donor platelet and hemoglobin levels declined modestly by Day 5, before apheresis during filgrastim administration. Major changes in hemoglobin and platelets accompanied each apheresis collection procedure. At 1 month after donation, platelet and hemoglobin levels rebounded but still remained below baseline (P < .001). No clinically relevant changes in hemoglobin and platelet levels were observed during annual follow-up.
Box and whiskers plot of blood counts showing the maximum, upper quartile, median, lower quartile, and minimum values obtained from PBSC donors on the first day of injection (Day 1), before and after donation (Day 5 before and Day 5 after), and during follow-up after donation. (A) Donor white blood cell counts. (B) Donor platelet counts. (C) Donor hemoglobin levels, by sex.
Box and whiskers plot of blood counts showing the maximum, upper quartile, median, lower quartile, and minimum values obtained from PBSC donors on the first day of injection (Day 1), before and after donation (Day 5 before and Day 5 after), and during follow-up after donation. (A) Donor white blood cell counts. (B) Donor platelet counts. (C) Donor hemoglobin levels, by sex.
We evaluated the extremes of WBC, platelet, and hemoglobin changes, because these values could put donors at higher risk for stroke, bleeding, or complications of anemia. Although nearly a third of donors experienced a WBCs above 50 × 109/L, less than 1% of donors exceeded a higher risk level of 75 × 109/L. No episodes of thrombosis or stroke were reported. After 2 days of collection, nearly 40% of donors had platelet counts below 100 × 109/L, with approximately 2% of donors going below 50 × 109/L and a single donor going below 20 × 109/L. Males and females experienced similar declines in hematocrit values accompanying apheresis, but female donors often declined to values below 30% (5% and 9% occurrence after the first and second apheresis procedure, respectively). Very rarely, donors persisted with low platelet or hemoglobin levels at 1 month after donation. Of note, platelet drops were larger with high versus standard volume apheresis procedures (−105 × 109/L vs −83 × 109/L, P < .001).
Donors had screening blood chemistries drawn fewer than 30 days before their procedures. A comparison of screening levels with values at 1 month, and 1, 2, and 3 years after donation showed no clinically relevant changes in chemistries analyzed (BUN, creatinine, alkaline phosphatase, and total bilirubin; data not shown).
Performance status
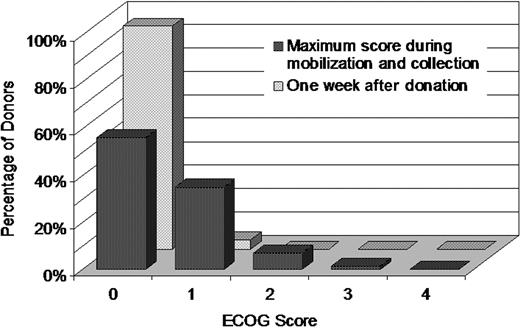
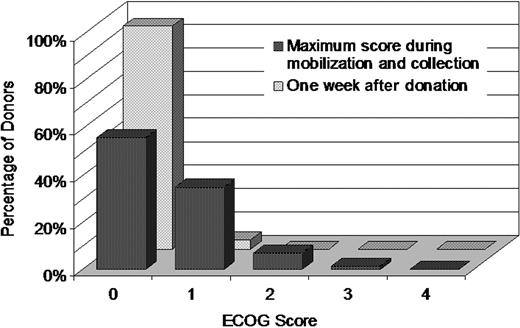
Donors' functional state was rated using ECOG Performance Status. Figure 5 shows the maximum ECOG score during the course of PBSC mobilization and donation. The majority of donors reported an ECOG performance score of zero, with approximately 70% reporting a zero grade on Day 4 of mobilization and both days of donation. ECOG scores quickly returned to normal with 93% reporting a score of 0 on Day 2 of mobilization, 95% at 1 week after donation, and almost 100% at the remaining time points. Fifty-six percent of donors had an ECOG score of zero throughout the mobilization and donation process. Eight percent had a score of 2 or higher at some point during mobilization and collection.
Frequencies of PBSC donor's highest ECOG score during mobilization and collection and of donor's ECOG score at 1 week after donation. The donor's ECOG Performance Status was rated before administration of filgrastim each day and at each follow-up after donation.
Frequencies of PBSC donor's highest ECOG score during mobilization and collection and of donor's ECOG score at 1 week after donation. The donor's ECOG Performance Status was rated before administration of filgrastim each day and at each follow-up after donation.
The maximum ECOG score during the process of PBSC mobilization and apheresis collection was analyzed using logistic regression. The analysis outcome was a maximum ECOG score of 2 or higher. Only 1 risk factor, donor sex, was found to significantly affect the outcome. Compared with male donors, females had an increased frequency of ECOG score of 2 or more (OR = 2.13; 95% CI, 1.59-2.86; P < .001).
Unexpected serious adverse events and long-term toxicities
We conducted an analysis of serious or unexpected events in this cohort of 2408 donors. Fifteen events occurred, for a rate of 0.6%, and are described in Table 5. The majority of these events were symptoms that were serious enough to warrant hospital admission for observation. The events were most often associated with filgrastim therapy (severe headache, nausea) or apheresis (local bleeding, citrate toxicity). Three donors were hospitalized for more worrisome symptoms of severe chest or back pain. Full cardiac evaluations were performed and none of these donors experienced a cardiac event. Risk factor and donor characteristic analysis did not reveal associations between these rare adverse events.
Reported malignancies in donors
We reviewed NMDP follow-up data regarding the incidence of development of malignancies in this cohort of 2408 donors. Annual attempts at follow-up were made for all donors (median follow-up, 49 months; range, 2 days to 99 months). No cases of acute myelogenous leukemia or myelodysplasia were reported. Twenty-five nonhematologic cancers of various types occurred along with one case of chronic lymphocytic leukemia (Table 6). Comparison of the incidence of these cancers to expected rates according to the SEER database showed no evidence of increased cancer risk in the donor cohort.
Discussion
Over the past several years, increasing numbers of donors have been receiving cytokines (almost invariably filgrastim) for several days followed by harvest of circulating marrow stem cells by the process of leukapheresis. Several small- to moderate-sized experiences have been published describing PBSC donor outcomes, focusing mainly on related donors.4,7,10,11,15-17 Short-term toxicities associated with PBSC donation reported previously generally occurred because of either (1) filgrastim administration (local reactions, pain, flulike symptoms), (2) complications associated with placement of a central venous catheter when peripheral access is inadequate (infection, bleeding, pneumothorax), and/or (3) problems with leukapheresis (bleeding secondary to anticoagulation, hypocalcemia due to acid citrate dextrose [ACD] use, etc). This study significantly expands on previously published work by (1) including a very large number of prospectively enrolled unrelated donors, (2) reporting long-term normalization of hematologic and chemistry laboratory values, (3) describing the donor experience in more detail by CALGB (comparable with CTCAE scales) and ECOG scores, (4) providing more detailed data regarding the donor perceptions of pain, (5) more fully characterizing line placement– and apheresis-related toxicities, and (6) identifying by multivariate analysis specific populations at higher risk for toxicities.
This paper confirms previous studies that have shown PBSC collection to be generally safe for adult donors, but prospective donors should be carefully educated about the measurable risk of significant acute toxicities they may encounter. Donors in this study did not experience the rare, filgrastim-related event of splenic rupture (incidence likely 1/5-10 000),18-21 and no donors experienced fatal complications (incidence worldwide estimated at 1/10 000).22 The association of filgrastim administration with an increase in myeloid leukemia and/or myelodysplasia in patients with a few diseases such as congenital neutropenia (Kostmann syndrome),23,24 along with anecdotal reports of myeloid leukemia occurring in related PBSC donors,25 has raised concern about long-term risks of filgrastim administration to healthy donors. Myeloid leukemia or myelodysplasia was not detected in long-term follow-up of donors on this study, and, aside from a clinically insignificant increase in WBCs, blood counts of donors followed in this cohort normalized and remained normal years later. Although theoretic concerns have been raised by some in the medical community about filgrastim contributing to an increased risk of myeloid leukemia/myelodysplasia in healthy donors, this study supports preliminary evidence presented in several small prospective and larger retrospective studies that have yet to detect in increase in filgrastim-treated donors going on to develop myeloid leukemia/myelodysplasia.26-30 That said, this analysis is not adequate to fully address this concern, and follow-up for hematologic malignancies is ongoing. A large, prospective donor safety study is needed to answer this question. Currently, the NMDP has an ongoing study that will address in a more definitive manner the theoretic concern of whether filgrastim causes an increase in risk of leukemia in unrelated donors. The Donor Health and Safety Subcommittee of the Center for International Blood and Marrow Transplant Research (CIBMTR) and several national donor registries are performing studies aimed at addressing this concern in related donors.
Because the data provided in this paper represent the practice of a large number of centers (73 donor centers and 96 apheresis facilities), the study provides a baseline for what would be considered expected rates of toxicity associated with filgrastim-mobilized PBSC collection. Understanding that increased rates of toxicity occur with female and heavier donors should allow centers to monitor and treat pain and other side effects in these populations more effectively. The expected toxicity baseline provided by this study must be qualified, however. NMDP donors must be between ages 18 and 60 years and are unrelated to recipients. NMDP has specific guidelines for donor screening that may declare donors ineligible that would be considered acceptable by some centers if they were related to the recipient. In the setting of related donors, pediatric donors, and donors older than 60 years, the observations made in this study may not be valid. These donor groups have specific issues (size in pediatrics and increased risk of end-organ dysfunction and cancer in adults older than 60 years) that may put them at increased risk for toxicities associated with donation. Studies aimed at comprehensive assessment of early and late donation-related toxicities in related pediatric and adult donors, especially donors older than 60 years, are warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This project has been supported by funding from the National Marrow Donor Program and the Health Resources and Services Administration contract nos. 240-97-0036 and 231-02-0007 to the National Marrow Donor Program.
The CIBMTR is supported by Public Health Service Grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Resources and Services Administration (DHHS); and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen; anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US; Baxter International; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene; CellGenix; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex; CytoTherm; DOR BioPharma; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals; European Group for Blood and Marrow Transplantation; Gambro BCT; Gamida Cell; Genzyme; Histogenetics; HKS Medical Information Systems; Hospira; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery; Merck & Company; The Medical College of Wisconsin; MGI Pharma; Michigan Community Blood Centers; Millennium Pharmaceuticals; Miller Pharmacal Group; Milliman USA; Miltenyi Biotec; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics; Otsuka Pharmaceutical Development & Commercialization; Pall Life Sciences; PDL BioPharma; Pfizer; Pharmion; Saladax Biomedical; Schering Plough; Society for Healthcare Epidemiology of America; StemCyte; StemSoft Software; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS; Vidacare; Vion Pharmaceuticals; ViraCor Laboratories; ViroPharma; and Wellpoint.
The views expressed in this article do not reflect the official policy or position of the Health Resources and Services Administration, the National Marrow Donor Program, the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the US government.
National Institutes of Health
Authorship
Contribution: M.A.P. had primary responsibility for study design, data analysis and interpretation, and paper writing, and had primary responsibility for the entire paper as an accurate and verifiable report; P.C. had primary responsibility for data file preparation, data analysis and interpretation, and paper writing; J.P.K. participated in study design and data analysis and interpretation; S.K. participated in data file preparation and data analysis; J.P.M. and R.J.K. participated in data file preparation, data interpretation, and paper writing; J.D.R., S.F.L., and P.A. participated in data interpretation and paper writing; M.D.H. participated in data file preparation; B.R.L. participated in study design and data analysis and interpretation; M.M.H. participated in study design, data interpretation, and paper writing; and D.L.C. had responsibility for study design, data file preparation, data analysis and interpretation, and paper writing, and had responsibility for the entire paper as an accurate and verifiable report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Pulsipher, University of Utah School of Medicine, Division of Hematology/Blood and Marrow Transplant, 30 North 1900 East, Room 5C402, Salt Lake City, UT 84132-2408; e-mail: michael.pulsipher@hsc.utah.edu.