Abstract
One thousand two hundred twenty-two patients treated in the Rituximab with CHOP over age 60 years (RICOVER-60) trial were examined for central nervous system (CNS) disease developing during first-line therapy or after a complete or partial remission had been achieved. Patients received 6 or 8 courses of CHOP (cyclophosphamide, adriamycin, vincristine, prednisone) administered every 2 weeks (CHOP-14) with or without rituximab. CNS prophylaxis for patients with involvement of bone marrow, testes, upper neck, or head consisted of intrathecal (i.th.) methotrexate (days 1 and 5 of first 2 courses). Fifty-eight cases of lymphoma in the CNS were observed (36/609 patients in the CHOP-14 and 22/608 patients in the arituximab–CHOP-14 [R-CHOP–14] arm). The estimated 2-year incidence of CNS disease was 6.9% (confidence interval [CI] 4.5; 9.3) after CHOP-14 and 4.1% (CI 2.3; 5.9) after R-CHOP–14. R-CHOP reduced the relative risk for CNS disease to 0.58 (95% CI 0.3; 1.0, P = .046). Cox regression analysis identified “involvement of more than 1 extranodal site” and “B-symptoms” as significant risk factors for CNS disease. Patients treated with R-CHOP–14 did not show any benefit from i.th. methotrexate. We conclude that elderly patients with aggressive CD20-positive lymphoma show a significantly lower incidence of CNS disease if treated with R-CHOP–14 instead of CHOP-14. Intrathecal methotrexate has no role in preventing CNS disease for patients treated with combined immunochemotherapy (R-CHOP–14)—with the possible exception of patients with testicular involvement. The original clinical trials are registered on www.clinicaltrials.gov as NCT000052936.
Introduction
Involvement of the central nervous system (CNS) is a serious and mostly fatal complication of aggressive lymphoma. Because the incidence of CNS disease in diffuse large B-cell lymphoma (DLBCL) is low,1-6 prophylactic measures have been restricted to patients with disease characteristics empirically found to convey an increased risk of CNS disease. More recently, risk models have been developed derived from analyses of prospective studies.1,7-10 The efficacy of different forms of CNS prophylaxis has never formally been demonstrated.
Systemic therapy of CD20 expressing lymphomas has changed over recent years; in particular, shortening of the time interval between treatment cycles11,12 and the addition of rituximab to CHOP (cyclophosphamide, adriamycin, vincristine, prednisone)13,14 significantly improved outcome. It is not known how these changes in systemic therapy affect the frequency, clinical picture, and outcome of CNS events.
We analyzed CNS events occurring in elderly patients with aggressive lymphoma treated in the rituximab with CHOP over age 60 years (RICOVER-60) trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Our main objectives were to determine the impact of rituximab on CNS events, the consistency of recently identified risk factors, and the role of CNS prophylaxis in patients treated with chemoimmunotherapy.
Methods
Patients, diagnostic measures, and treatment
Elderly patients (1222; range in age, 61-80 years) with newly diagnosed aggressive B-cell lymphoma were randomized into the RICOVER-60 trial.15
Patients received 6 or 8 courses of CHOP every 2 weeks (CHOP-14) with or without 8 infusions of rituximab. Involved-field radiotherapy (36 Gy) was mandatory for patients with initial bulky disease (≥ 7.5 cm) and/or extranodal disease. Further details have recently been published.15
CNS disease
A lumbar puncture was required in patients with lymphoma manifestations in the head and neck and in patients with involvement of bone marrow (BM) or testes. Diagnostic procedures included evaluation of cytocentrifuge preparations as minimum requirement. Flow cytometry was performed in many instances. The diagnosis of CNS disease was based on the combination of typical CNS symptoms, radiologic findings, and the detection of lymphoma cells in the spinal fluid. CNS involvement with either parenchymal brain lesions, affection of the spinal cord, meningeosis lymphomatosa, or combinations thereof were counted. Whenever CNS disease was clinically suspected, imaging of the brain and/or the spine and a lumbar puncture were performed.
CNS prophylaxis
CNS prophylaxis was mandatory for patients with infiltration of BM and testes or lymphoma manifestation in the upper neck or head including sinuses, orbita, oral cavity, tongue, and salivary glands. Prophylaxis consisted of intrathecal (i.th.) methotrexate (MTX; 15 mg) followed by folinic acid rescue on days 1 and 5 of the first 2 cycles.
Statistical analysis
Patients with first recurrence of aggressive lymphoma to the CNS after achieving complete remission (CR), uncertain CR (CRu), or partial remission (PR), and patients with spread of disease to the CNS during first-line therapy were included. The primary end point was time to CNS disease defined as time from randomization to disease progression in the CNS, treatment failure with CNS involvement at the end of therapy, or CNS relapse after CR/CRu. Secondary endpoints were survival after CNS disease and time to meningeosis. Survival was defined as time from the diagnosis of CNS disease until death from any cause. Time to meningeosis was defined as time from randomization to disease progression with meningeosis, treatment failure with meningeosis, or relapse with meningeosis. Patients with parenchymal disease were censored. Time to CNS disease, time to meningeosis, and survival were estimated according to the method of Kaplan and Meier.16 Estimators at 2 years are given with 95% confidence limits. For univariate analyses log-rank tests were performed, P values less than .05 were considered significant. All factors with P less than .1 were included in the multivariate analysis. To identify prognostic factors for CNS disease, we used the proportional hazard model. We proceeded in a stepwise approach for including single factors as suggested by Collet.17 The strength of prognostic factors was estimated by determining relative risks and the corresponding 95% confidence intervals (CIs). All calculations were made in SPSS/PC+ version 11.5 (SPSS, Chicago, IL).
Results
A total of 1222 elderly patients (median age 68 years) with CD20-positive, aggressive B-cell lymphomas were eligible for this analysis. Three hundred seven (305) patients were treated with 6 (8) courses of CHOP-14; 306 (304) patients received 6 (8) courses of rituximab-CHOP-14 (R-CHOP–14). Five patients with CNS involvement at diagnosis were excluded for this analysis. Patient and disease characteristics of the remaining 1217 patients are listed in Table 1.
Fifty-eight patients (4.8%) developed CNS disease. The site of CNS involvement, the remission status after first-line therapy, and the extent of disease (isolated CNS or combined with systemic disease) are shown in Table 2.
Two-thirds of the patients (n = 38) with CNS disease showed intracerebral or intraspinal involvement. An isolated meningeosis lymphomatosa was diagnosed in 15 patients (25.9%). Five patients experienced a combined parenchymal and meningeal relapse. In 20 patients (34.5%), CNS disease occurred after a complete remission had been achieved, and 2 patients had an unknown response at the end of therapy. In the remaining 36 patients (62.0%), however, CNS disease was diagnosed together with a PR or—more frequently—with progressive disease.
Overall, 24 patients (41.4%) experienced a CNS event while concurrently diagnosed with systemic disease. Simultaneous CNS and systemic disease was observed more often in patients with parenchymal disease (47.4%) than in patients with meningeosis (26.7%).
The median time interval between diagnosis and CNS disease was 8 months (range: 1-39), median survival after CNS disease was only 2.5 months.
The role of rituximab
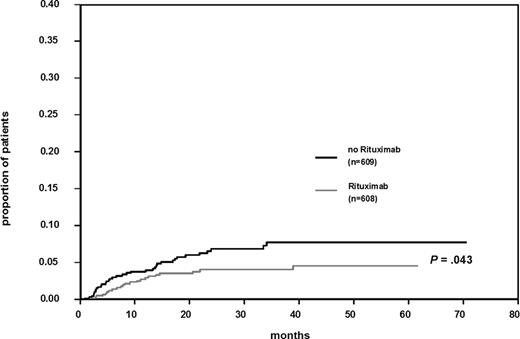
Twenty-two of 608 patients (3.6%) treated with R-CHOP–14, and 36 of 609 patients (5.9%) not given rituximab experienced a CNS event. Figure 1 shows the Kaplan-Meier estimates for time to CNS disease. The difference for patients treated with or without rituximab is significant (log-rank test, P = .043).The estimated 2-year incidence of CNS disease was 6.9% (CI 4.5; 9.3) after CHOP-14 and 4.1% (CI 2.3; 5.9) after R-CHOP–14. Patients treated with R-CHOP–14 showed a relative risk (RR) for CNS disease of 0.58 (95% CI 0.3; 1.0, P = .046).
Cumulative risk of CNS disease in patients treated with and without rituximab together with chemotherapy.
Cumulative risk of CNS disease in patients treated with and without rituximab together with chemotherapy.
In patients given R-CHOP–14, parenchymal CNS disease accounted for 50% of CNS events compared with 75% in patients receiving CHOP only. Conversely, the percentage of patients with meningeosis increased from 16.7% of CNS events in patients receiving CHOP-14 to 40.9% in patients treated with R-CHOP–14. Only 6 of 22 R-CHOP–14 patients showed simultaneous systemic disease (27.3%) compared with 50% in patients given CHOP.
Risk factor analysis
By univariate analysis (Table 3) an increased risk for CNS disease was associated with involvement of more than one extranodal site, presence of B-symptoms, impaired Eastern Cooperative Oncology Group (ECOG) performance status, BM infiltration, elevated lactate dehydrogenase (LDH), and advanced stage. Also patients with an intermediate/high- or high-risk International Prognostic Index (IPI) had a significantly increased risk for CNS disease. The number of treatment courses (6 or 8) did not influence the risk of CNS disease, while the addition of rituximab significantly reduced the risk.
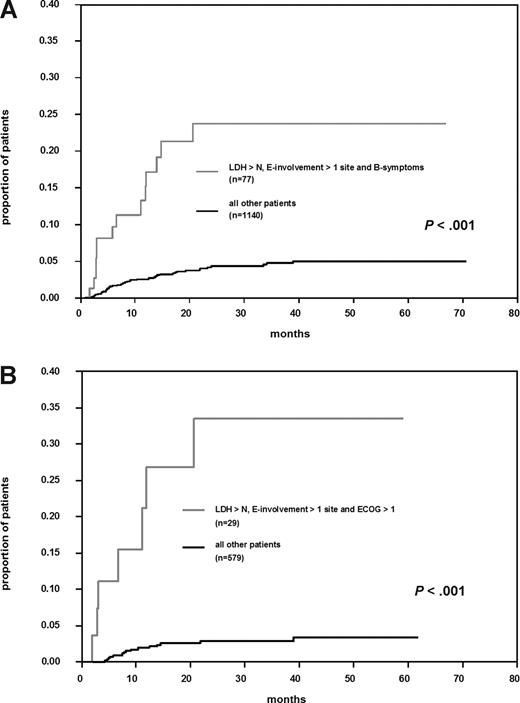
Multivariate Cox regression analysis identified “involvement of more than one extranodal site” and “presence of B-symptoms” as significant independent prognostic factors. “Elevated LDH” increased the RR (1.5) but was not significant (Table 4). Seventy-seven patients (6.3%) with these factors had a significantly increased risk for CNS disease compared with patients without this profile (P < .001; Figure 2A). Their cumulative risk of CNS disease was 23.8% (CI 12.4; 35.2) at 2 years, about 6-fold the incidence rate observed in other patients. Including rituximab into the Cox regression model did not change the results. “Involvement of more than one extranodal site” and “presence of B-symptoms” remained significant and a trend for “elevated LDH” to increase the risk of CNS involvement was observed. Rituximab decreased the risk for CNS disease (RR = 0.5; P = .026).
Cumulative risk of CNS disease in “high-risk” patients. (A) All patients with elevated lactate dehydrogenase (LDH), involvement of > 1 extranodal site, and presence of B-symptoms versus all other patients. (B) Patients treated with R-CHOP–14 and elevated LDH, involvement of > 1 extranodal site, and Eastern Cooperative Oncology Group performance status > 1 versus all other patients treated with R-CHOP–14.
Cumulative risk of CNS disease in “high-risk” patients. (A) All patients with elevated lactate dehydrogenase (LDH), involvement of > 1 extranodal site, and presence of B-symptoms versus all other patients. (B) Patients treated with R-CHOP–14 and elevated LDH, involvement of > 1 extranodal site, and Eastern Cooperative Oncology Group performance status > 1 versus all other patients treated with R-CHOP–14.
Because treatment of DLBCL now regularly includes rituximab, we repeated the risk factor analysis, this time considering only patients treated with R-CHOP. Involvement of more than one extranodal site and elevated LDH were significant; presence of B-symptoms was replaced by ECOG-performance status. This risk group (4.8% of patients treated with R-CHOP–14) showed a probability for CNS events at 2 years of 33.5% (CI 12%; 55%) compared with 2.8% (CI 1%; 4%) in other patients given R-CHOP–14 (Figure 2B).
Intrathecal prophylaxis
Two hundred seventy-three patients (22.4%) received i.th. MTX at least during one cycle of chemotherapy, and 202 patients (16.6%) received 4 i.th. injections, but only 11 of 58 patients (19.0%) with a CNS event had received i.th. MTX. Unfortunately, patients with involvement of the upper neck could not exactly be evaluated for protocol violation because the case report forms failed to precisely define “upper neck.” One hundred twenty of 210 patients (57.1%) with involvement of testes, BM, or head received i.th. MTX as per protocol, whereas 90 patients (42.9%) were not treated. The high number of protocol violations was unexpected. Treating physicians obviously were not convinced that BM involvement (57.5% protocol violations) or involvement of sites adjacent to the skull (40.0% protocol violations) put patients at sufficiently high risk for CNS disease to require i.th. MTX. Protocol violations for testicular involvement were only 16.7%. The high number of protocol violations gave us the unique opportunity to analyze the efficacy of i.th. MTX in high-risk populations treated with R-CHOP–14 or CHOP-14. Overall, the percentage of CNS events in prophylaxed patients was slightly lower (2.5%) than in patients without CNS prophylaxis (4.4%), but this difference was not significant. In univariate analysis of patients with involvement of testes, BM, or any site adjacent to the skull, the risk of CNS disease was higher for patients without i.th. prophylaxis—if they had not received rituximab. In contrast, when therapy included rituximab, the risk for CNS relapse was significantly lower regardless whether i.th. MTX had been administered or not (Figure 3). The proportional hazard model with an interaction term between rituximab and prophylaxis adjusted for the IPI-factors confirmed a relevant interaction (RR = 6.1). Thus, no effect of i.th. MTX on any type of CNS event was detectable when modern immunochemotherapy including rituximab was administered.
Cumulative risk of CNS disease in patients with testes, bone marrow, or head involvement dependent on intrathecal prophylaxis and rituximab application.
Cumulative risk of CNS disease in patients with testes, bone marrow, or head involvement dependent on intrathecal prophylaxis and rituximab application.
Discussion
In patients with non-Hodgkin lymphoma, CNS relapse was reported in 0% to 39% depending on the histologic subtypes represented in the study population.3-6,18,19 If patients with DLBCL and treated with CHOP or CHOP-like regimens with or without CNS prophylaxis were considered, CNS events were in the range of 4% to 7%.1,2,9,10
The French Groupe d'Etude des Lymphomes de l'Adulte (GELA) demonstrated a reduction of CNS events to 2.2% in patients treated with ACVBP (doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone) and both i.th. (15 mg) and systemic treatment (2 courses of 3 g/m2) with MTX.7 The efficacy of this strategy was confirmed in a randomized study comparing ACVBP followed by i.th./systemic MTX with CHOP.20 We reported a low rate of CNS events (2.2%) in 1693 patients treated on previous DSHNHL protocols8 ; in elderly patients (60-75 years) the percentage of CNS events was slightly higher (2.8%). Patients had been treated with CHOP every 2 or 3 weeks and half of them had received CHOE(toposide)P.12 As etoposide crosses the blood/brain barrier and CHOEP patients had a significantly lower incidence of CNS events (RR = 0.4), we concluded that etoposide may have contributed to the lower number of CNS events.
In the RICOVER-60 trial, relapse to the CNS occurred in 58 patients (4.8%); the estimated 2-year incidence of CNS events was 6.9% in patients treated with CHOP-14 and 4.1% in patients with R-CHOP–14 (P = .043). The only other paper that compared CNS events occurring in elderly patients treated with or without rituximab and CHOP-21 reported an incidence of CNS disease of 5.3% with no significant difference between patient groups.21 An abstract from British Columbia reported a frequency of 9% compared with 5% (P = .202) of CNS relapses in patients treated with CHOP or R-CHOP, respectively.22 Thus, currently available information indicates that treatment with R-CHOP results in a moderate decrease of CNS events. Also, the presentation of CNS disease in patients treated with R-CHOP seems to change with more patients experiencing isolated meningeal disease—a manifestation more amenable to therapy than parenchymal CNS disease.
Animal experiments have shown that the levels of rituximab measured in the cerebrospinal fluid (CSF) are distinctly lower (0.1-1.7%) than the corresponding serum levels.23 A phase 1 study demonstrated that i.th. administration of rituximab by Ommaya reservoir is feasible, safe, and efficacious.24 There is evidence that patients with CNS lymphoma have a disrupted blood/brain barrier and therefore also may benefit from intravenous rituximab.25,26
Whether the prophylactic effect of rituximab on CNS disease represents a direct effect to the CNS remains elusive. We consider it more likely that the reduction of CNS events in the rituximab arms of the RICOVER-60 trial reflects the improved systemic efficacy of R-CHOP–14.
Data from GELA and the DSHNHL suggest that administration of cytotoxic agents crossing the blood/brain barrier should be more effective in reducing CNS events than the addition of rituximab to chemotherapy alone. A study from the Royal Marsden Hospital (London, United Kingdom) reported an exquisitely low incidence of CNS relapse (1.1%) in patients who to large parts had received both etoposide and rituximab.27 We did not see any CNS event in patients treated with rituximab in the phase 2 MegaCHOEP studies. On the other hand, a small phase 2 study indicates that CNS relapses continue to occur with R-CHOEP–14.28
The analysis of risk factors for CNS events in our study confirmed previous observations that involvement of more than one extranodal site and elevated LDH are independent predictors for CNS disease.1,7-9 The presence of B-symptoms was an additional independent risk factor when all patients were considered. Restricting the analysis to patients treated with R-CHOP–14 gave similar results (although the ECOG performance status replaced the presence of B-symptoms as risk factor). This model identified 4.8% of patients treated with R-CHOP–14 as “high risk” showing an estimated risk of CNS disease of 33.5%.
Incidences of CNS events have been calculated and risk factors determined to answer the most important practical question: which patient should receive prophylaxis to reduce the risk of CNS disease? Unfortunately, no study has been able to properly address this question. While anecdotal reports suggested benefit,29 other studies do not support the notion that i.th. MTX reduces CNS disease in DLBCL.30 Accordingly, current practice of CNS prophylaxis varies widely. While investigators agree that prophylactic treatment of all patients is not justified, the risk groups receiving prophylaxis differ. Involvement of paranasal sinuses, testes, orbital cavity, BM, but also of bone, breast, or blood have been named to trigger prophylaxis; risk factors (LDH, number of extranodal sites, B-symptoms) delineated from prospective studies still seem less accepted.31,32 There is no valid information if prophylactic measures other than i.th. MTX are more effective in any of these settings.
Of note, all recommendations for prophylaxis originate from the pre-rituximab era. As hardly any patient with B-cell lymphoma today is treated without rituximab, the question of CNS prophylaxis needs reconsideration. With the RICOVER-60 trial, we for the first time demonstrate that rituximab significantly reduces the number of CNS events and that the nature of CNS disease is changing. In line with current practice, i.th. MTX was able to reduce the incidence of CNS disease if patients were treated with CHOP only. In contrast, patients with high risk for CNS disease (involvement of BM, testes, head, or adjacent lymph nodes), but treated with rituximab and CHOP-14, failed to show any benefit from i.th. MTX. There are caveats to this observation, as close to half of these patients were not given i.th. MTX by choice of their treating physicians. This uncontrolled treatment pattern, however, might even strengthen our conclusion, because i.th. MTX was omitted preferentially in patients with “soft” evidence for its efficacy (patients with BM involvement or sites adjacent to the skull). No difference between patients with or without i.th. prophylaxis were found. Because 83.3% of patients with testicular involvement received i.th. MTX, we cannot rule out that prophylaxis was effective in this subgroup. The necessity of i.th. MTX administration in patients with testicular involvement has repeatedly been emphasized.33 Overall, in patients treated with R-CHOP–14 and involvement of BM, testes, head, or adjacent lymph nodes, the estimated 2-year incidence of CNS disease was low (3.5% [CI 0.0; 7.4]), regardless whether i.th. MTX was administered or not, and it is highly questionable if this would justify prophylaxis in any risk group. This holds particularly true because many patients were not in CR at the time of CNS progression and/or showed systemic relapse. Such patients will be doing poor anyway. For the 4.8% of patients treated with R-CHOP–14 who belonged to the high-risk group as defined by our COX regression analysis (involvement of >1 extranodal site, elevated LDH, poor ECOG performance status), the probability of CNS disease seems substantial (33.5% at 2 years). We still hesitate to recommend i.th. MTX to this or any other “high-risk” population, because 3 of 7 patients (43%) belonging to our high-risk group had received i.th. MTX but still relapsed to the CNS, shedding doubt on the efficacy of i.th. MTX in general. Whether other prophylactic measures are more effective and should be offered to high-risk patients cannot be answered by our study. In any case, between 4 and 6 patients would need CNS prophylaxis to prevent one CNS relapse.
We conclude that the addition of rituximab to CHOP reduces the risk of CNS disease in elderly patients with DLBCL. Patients with involvement of more than one extranodal site, elevated LDH, and poor performance status remain at risk for CNS events also if rituximab is added to chemotherapy. I.th. MTX significantly reduced CNS events in patients with involvement of BM, testes, head, or adjacent lymph nodes if no rituximab was administered. In patients treated with R-CHOP–14, the incidence of CNS disease overall and in this risk group was low; i.th. MTX failed to reduce the risk with the possible exception of patients with testicular involvement.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Deutsche Krebshilfe for funding the RICOVER-60 trial and all colleagues and patients for participating in the study. The DSHNHL is a member of the Kompetenznetz Maligne Lymphome, a research network supported by the German Ministry for Science and Research.
Authorship
Contribution: V.B. and N.S. wrote the report; S.Z. and M.L. did the statistical analysis; N.S., M.L., and M.P. designed the study; and M.P. was chairman of the RICOVER-60 study.
For a complete list the DSHNHL Study Group participants, see the Appendix, available on the Blood website (see the Supplemental Materials link at the top of the online article).
Conflict-of-interest disclosure: M.P. is a member of the Roche and Eli Lilly advisory boards. N.S. and M.L. have received research support from Roche. All other authors declare no competing financial interests.
Correspondence: Norbert Schmitz, Department of Hematology and Stem Cell Transplantation, Asklepios Hospital St Georg, Lohmühlenstrasse 5, D-20099 Hamburg, Germany; e-mail: n.schmitz@asklepios.com.
References
Author notes
*V.B. and N.S. contributed equally to this manuscript.