Abstract
Patients with HIV-1 immune-related thrombocytopenia (HIV-1–ITP) have a unique Ab against platelet GPIIIa49-66 capable of inducing oxidative platelet fragmentation in the absence of complement. HIV-1–seropositive drug abusers are more prone to develop immune thrombocytopenia than non–drug abusers and have a higher coinfection with hepatitis C virus (HCV) than non–drug abusers (90% vs 30%). Molecular mimicry was sought by screening a phage peptide library with anti–GPIIIa49-66 antibody as bait for peptides sharing homology sequences with HCV. Several phage peptide clones had 70% homology with HCV protein. Sera from dually infected thrombocytopenic patients with HCV and HIV-ITP reacted strongly with 4 nonconserved peptides from HCV core envelope 1. Reactivity correlated inversely with platelet count (r2 = 0.7, P < .01). Ab raised against peptide PHC09 in GPIIIa−/− mice induced thrombocytopenia in wild-type mice. Affinity-purified IgG against PHC09 induced oxidative platelet fragmentation in vitro. Drug abusers dually infected with HCV and HIV-1 had a greater incidence and severity of thrombocytopenia as well as titer of anti–GPIIIa49-66/PHC09 Ab. NZB/W F1 mice injected with recombinant core envelope 1 developed Ab versus PHC09 and significantly decreased their platelet count (P < .001). Thus, HCV core envelope 1 can induce thrombocytopenia by molecular mimicry with GPIIIa49-66.
Introduction
Thrombocytopenic patients with early HIV-1 infection have a shortened platelet survival due to an autoantibody against an epitope on platelet surface integrin GPIIIa, GPIIIa49-66 (CAPESIEFPVSEARVLED).1-3 Their sera have increased immune complexes that contain platelet fragments as well as anti–GPIIIa49-66 Ab. The presence of anti–GPIIIa49-66 Ab correlates inversely with platelet count (r = − 0.71) and induces severe thrombocytopenia when injected into mice. This antibody is unique in that it induces complement-independent platelet fragmentation by oxidative platelet fragmentation due to the release of reactive oxygen species through activation of 12-lipoxygenase and NADPH oxidase.4-6
HIV-1 immune-related thrombocytopenia (HIV-1–ITP) is more frequent in drug abusers compared with non–drug abusers (37% vs 16% incidence, respectively), and more severe in HIV-1–seropositive drug abusers than non–drug abusers (platelet count < 10 × 109/L in 52% vs 9%, respectively).7,8 A striking feature of HIV-1 infection in drug abusers is the frequent coinfection with hepatitis C virus (HCV).9-13 The overall prevalence of HCV infection among HIV-1–infected individuals is 30% to 50%9 in non–drug abusers, with rates of coinfection as high as 90% in intravenous drug abusers.9-13 We asked whether coinfection with HCV facilitates ITP and, if so, what the mechanism would be. The presence of a relatively high-affinity immunodominant Ab against GPIIIa49-66 in HIV-1–ITP patients suggested antigen-driven B-cell clonal expansion. We therefore investigated whether coinfection of HCV in HIV-1–ITP patients enhances the likelihood of inducing anti–GPIIIa49-66 Ab due to molecular mimicry of hepatitis C with GPIIIa49-66, as we have shown for nef with HIV-1–ITP.14
Patients with HCV commonly develop immunologic thrombocytopenia (HCV-ITP) that correlates with severity of disease (eg, chronic active hepatitis, cirrhosis).15-17 The incidence of HCV-ITP in a series of 368 HCV Japanese patients with chronic persistent or chronic active hepatitis was 41%. The incidence of endemic HCV-ITP in 294 chronic patients was 10%, which increased to 32% with advanced liver disease.15 The frequency of B-cell production of anti–GPIIb-IIIa Ab was 27-fold greater than with control cells in 37 HCV-ITP patients with cirrhosis17 ; and an inverse correlation was found between platelet count and B-cell anti–GPIIb-IIIa Ab production in 51 patients with liver cirrhosis (73% with hepatitis C). This would suggest some degree of specificity. Like HIV-1-ITP, patients with HCV-ITP have increased serum immune complexes.16 We therefore reasoned that a second autoimmune disease with serum immune complex associated immunologic thrombocytopenia could also contain an anti–GPIIIa49-66 Ab capable of inducing oxidative platelet fragmentation—induced by molecular mimicry with an HCV peptide in addition to HIV nef peptide in HIV-1-ITP.15
In the present report, we demonstrate the following: (1) four HCV core-envelope peptides from a nonconservative region display molecular mimicry with GPIIIa49-66 by reactivity with anti–GPIIIa49-66 Ab. (2) The strongly reactive SAIHIRNASG peptide (PHC09) was examined more extensively. PHC09 injected into GPIIIa−/− mice induced an Ab capable of inducing oxidative platelet fragmentation in vitro and thrombocytopenia in vivo in wild-type mice. (3) Platelet counts of HIV-1 hepatitis C drug abusers correlate inversely with serum titer versus PHC09 (r2 = 0.7, n = 15, P < .01). (4) Injection of rHCV core envelope 1 protein into NZB/W F1 mice induces thrombocytopenia that correlates with murine anti-PCH09 Ab level. (5) Thrombocytopenic drug abusers dually infected with HIV-1 and hepatitis C have a greater incidence and titer of anti–GPIIIa49-66 Ab as well as greater incidence and severity of thrombocytopenia.
Methods
Human population
Coded stored frozen sera (sent to the clinical laboratory for platelet-Ab testing) were randomly obtained from thrombocytopenic intravenous drug abusers with both HCV and HIV infection, non–drug abuser hepatitis C patients, non–drug abuser HIV-ITP patients, and healthy control subjects. Liver chemistries (albumin, alkaline, phosphatase) were comparable in all 3 groups. These studies were approved by the New York University Medical Center Institutional Review Board.
Mice
Female BALB/c and C57BL/6 mice were obtained from Taconic Farms (Germantown, NY). Integrin GPIIIa−/− knockout mice and NZB/W F1 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Animal work was approved by the New York University School of Medicine Animal Review Board.
Reagents
All reagents were obtained from Sigma-Aldrich (St Louis, MO) unless otherwise designated. 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) was obtained from Molecular Probes (Eugene, OR).
Peptides
Peptide GPIIIa49-66 (CAPESIEFPVSEARVLED), PHC09 (SAIHIRNASG), PHC07 (IFDPGAL), PHC01 (VDWQAPPGARS), PHC09 preceded by cysteine (CSAIHIRNASG), PHC09-H1 (SAYQVRNSSG), PHC09-H2 (SAYQVRNSTG), PHC09-H3 (SAYEVRNVSG), and irrelevant 10-mer peptide (GIGALFLGFL) were synthesized by Bio-Synthesis (Lewisville, TX).
Antibodies
Human anti–GPIIIa49-66 Ab and rabbit anti–GPIIIa49-66 Ab were obtained or produced in our laboratory as described previously.1,2 Mouse Ab against HCV peptides (PHC09) or mouse Ab against rHCV core envelope 1 were purified with protein A affinity chromatography as recommended by the manufacturer (Sigma-Aldrich, St Louis, MO).
Affinity purification of anti-PHC09 IgG
Serum IgG was isolated from HIV-1 drug abusers by protein A affinity chromatography. PHC09 preceded by cysteine (CSAIHIRNASG) was coupled to an affinity column with the heterobifunctional cross-linker sulfo-succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate as recommended by the manufacturer (Pierce Chemical, Rockford, IL). This again cross-links the resin with NH2-terminal cysteine of the peptide.
Screening of the phage display peptide library
Anti–GPIIIa49-66 Ab was incubated with 1012 to 1013 PhD-7 phage from a 7-mer linear peptide library obtained from New England Biolabs (Beverly, MA) in 2% nonfat milk in PBS at room temperature for 3 hours. Protein A– and alternating protein G–conjugated agarose beads were added overnight at 4°C. The beads were then centrifuged and washed 20 times with 0.1% Tween 20–PBS. Positive phages were eluted with trypsin-PBS by shaking for 10 minutes at room temperature, and eluted phage was titered and amplified as previously described.14 After the third round of panning, 30 clones were randomly selected for enzyme-linked immunosorbent assay (ELISA) and the positive clones sequenced. Phage peptide sequences were analyzed for sequence similarity to other proteins using the BLAST algorithm of the blast program and the database of the National Center for Biotechnology Information (NCBI).18
ELISA
Peptide (20 μg/mL) in 0.1 M sodium bicarbonate buffer (pH 9.6) was adsorbed to a 96-well ELISA plate at 4°C overnight, and blocked with blocking buffer (3% BSA in PBS–Tween [0.1%]) at room temperature for 2 hours. Primary antibody was added at room temperature for 1 hour, followed by horseradish peroxide (HRP)–conjugated secondary IgG for 1 hour at room temperature. Plates were washed 6 times with Tris-buffered saline 0.1% Tween 20 at each step in the procedure. The ABTS reagent used to develop the HRP color reaction at 405 nm was obtained from Pierce Chemical.
Production of anti-PHC09 Ab in GPIIIa−/− mice
GPIIIa−/− mice aged 6 to 8 weeks were immunized by intraperitoneal injection of 50 μg KLH-conjugated PHC09 with equal volume of mineral oil adjuvant for primary immunization. Booster injections were performed 3 times every 2 weeks. Immune sera were titered by ELISA on the PHC09 peptide. Positive sera were purified as described.
Assay of platelet particle formation
Gel-filtered murine or human platelets were isolated from EDTA-anticoagulated blood and labeled with anti–CD61-fluorescein isothiocyanate (FITC, human) or anti–CD41-FITC (murine) as previously described.3 Fluorescent-labeled platelets/particles were measured by flow cytometry using a FACScan (Becton Dickinson Immunocytometry Systems, Mountain View, CA). Gates were adjusted for platelets by exclusion of other blood cells. Fluorescent-labeled intact platelets were monitored in the right upper quadrant, with the y-axis measuring forward scatter and the x-axis measuring fluorescence. A shift in fluorescence from right upper quadrant to left upper quadrant and left lower quadrant reflected the percentage of platelet particle induction in 10 000 enumerated events.
Assay of platelet oxidation
Gel-filtered platelets were loaded with 10 μM DCFH-DA for 30 minutes at 37°C as described3 and challenged with platelet Ab. Intracellular DCFH is converted to a fluorescent form by hydrogen peroxide generated in this reaction. Oxidation was quantified by measuring the increase in mean fluorescence by flow cytometry.
Induction of passive thrombocytopenia in mice
Six-week-old female BALB/c mice were randomly divided into 4 groups (n = 4 per group). Purified control IgG, patient IgG, or mouse IgG against peptide PHC09 (50 μg of each) was injected intraperitoneally into Balb/c mice, and platelet counts were followed for 24 hours.
Determination of mouse platelet counts
Platelet counts were determined from 20-μL blood draw into Unopettes (no. 365855; Becton Dickinson Immunocytometry Systems), containing optimal anticoagulant concentration and diluent for quantitating platelet counts by phase-contrast microscopy.
Expression of recombinant HCV core envelope 1 in E coli
HCV core envelope 1 C-histidine tag cDNA encoding 24 amino acids (167-191) of the core and 160 amino acids of the envelope (192-350) and containing the PHC09 sequence was cloned into expression vector PET29a (Novagen, Madison, WI). PHC09 is composed of amino acids 190 to 199 that contain the 2 C-terminal amino acids of the core and the 8 N-terminal amino acids of the envelope. Escherichia coli BL21 (DE3) PlysS cells were transformed with the pET29a-HCV core-envelope 1 plasmid cultured in LB medium (Bacto-tryptone [1%; Difco Laboratories, Detroit, MI], Bacto-yeast extract [0.5%; Difco Laboratories], NaCl [1%], pH 7.0]) containing carbenicillin (50 μg/mL) and chloramphenicol (34 μg/mL) with shaking at 37°C until the OD (600 nm) was 0.4 to 0.6. Expression was induced by the addition of 1 mM IPTG at 37°C for 4 hours with shaking, and purified by Ni-NTA agarose resin. The protein was dialyzed in PBS, and the purity verified with 12% sodium dodecyl sulfide–polyacrylamide gel electrophoresis (SDS-PAGE).
Immunodeficiency animal model
Two animal models were used. (1) NZB/W F1 mouse model: NZB/W F1 mice were directly challenged with rHCV core envelope 1 protein containing the corresponding PHC09 sequence, or scrambled peptide (25 μg/mouse, every 10 days for 9 injections). Platelet counts and antibody titer versus PHC09 were monitored after every injection. (2) BALB/c mouse model: BALB/c mice were immunosuppressed, as described19 with an initial 6 mg cyclophosphamide/mouse (Mead Johnson, Princeton, NJ) and maintained with additional doses of 2 mg/mouse every week. BALB/c mice were challenged with control or rHCV core envelope 1 protein (25 μg/mouse) every 10 days for 9 injections after the total leukocyte count reached less than 2.5 × 109/L. Platelet counts and antibody titers versus PHC09 were monitored as above.
Results
Identification and characterization of selected phage clones
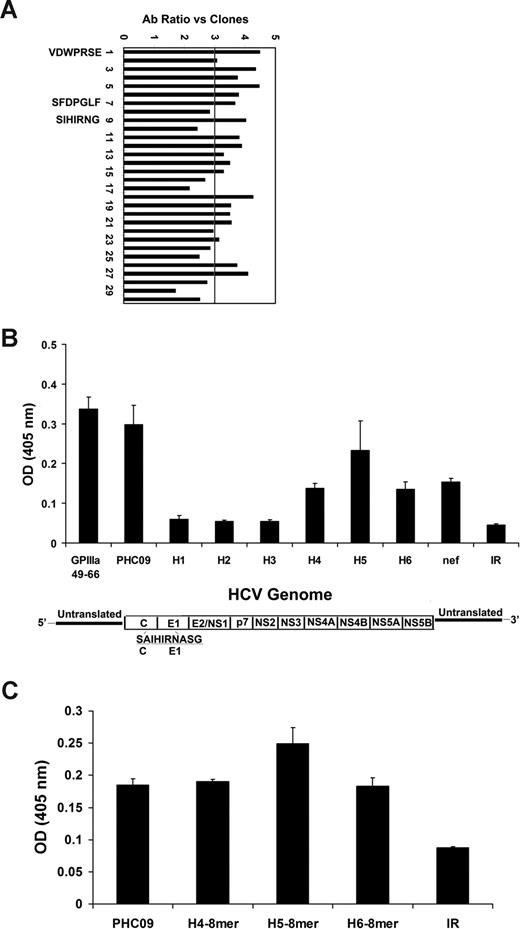
To test the hypothesis that an HCV epitope contributes to the generation of anti–GPIIIa49-66 Ab, we screened a filamentous phage display 7-mer peptide library using anti–GPIIIa49-66 antibody as bait, and focused on peptides sharing homology sequences with HCV protein. Thirty phage clones that reacted with anti–GPIIIa49-66 Ab by ELISA were randomly selected. Clones with nonspecific cross-reactivity to BSA were also screened by ELISA. Twenty clones reacted positively with anti–GPIIIa49-66 Ab with high OD value and low cross-reactivity with BSA (ratio > 3; Figure 1A). Three positive clones (PHC09 [SIHIRNG], PHC07 [SFDPGLF], and PHC01 [VDWPRSE]) showed sequence homology with HCV protein. Clone PHC09 was in a nonconserved core envelope 1 region of hepatitis C virus. We also searched the database for other nonconserved clones with homology for PCH09. Six such clones were found; PHC09-H1-H6, PHC09-H5, and H6 had the highest homology for PCH09 (Table 1). These clones were synthesized as 10 mers and were compared for reactivity with GPIIIa49-66, PHC09, conserved PHC01 and PHC07, and nef. Figure 1B demonstrates the strongest Ab reactivity with PHC09 and PHC09-H5. Table 1 also records the HIV genotype. Note that PHC09 is a relatively rare genotype (HCV6) in the United States (1.5%), whereas PHC09-H1 to PHC09-H-6 are more common HCVla or HCV1b genotypes (38% and 36%, respectively).
Panning of PhD 7-phage peptide library. (A) Positive clonal analysis for phages following the third round of panning. Clones were subjected to ELISA for binding to anti–GPIIIa49-66 Ab. BSA is used as control antigen. Clones that gave an anti–GPIIIa49-66 Ab/BSA OD ratio more than 3 were designated positive. Sequence analysis is given for positive peptides analyzed by BLAST algorithm of the Blast program and database of the National Center for Biotechnology Information (NCBI). Three clones are molecular mimics with close sequence similarity to HCV proteins. PHC09 is a nonconserved peptide in the core-envelope 1 region. (B) Anti–GPIIIa49-66 reactivity with various 10-mer peptides: GPIIIa49-66, PHC09, homologous peptides (H1-H6) of PHC09, HIV-nef, and irrelevant peptide (IR). (C) Anti–GPIIIa49-66 reactivity with various 8-mer peptides. SEM is given. HCV genome map is provided.
Panning of PhD 7-phage peptide library. (A) Positive clonal analysis for phages following the third round of panning. Clones were subjected to ELISA for binding to anti–GPIIIa49-66 Ab. BSA is used as control antigen. Clones that gave an anti–GPIIIa49-66 Ab/BSA OD ratio more than 3 were designated positive. Sequence analysis is given for positive peptides analyzed by BLAST algorithm of the Blast program and database of the National Center for Biotechnology Information (NCBI). Three clones are molecular mimics with close sequence similarity to HCV proteins. PHC09 is a nonconserved peptide in the core-envelope 1 region. (B) Anti–GPIIIa49-66 reactivity with various 10-mer peptides: GPIIIa49-66, PHC09, homologous peptides (H1-H6) of PHC09, HIV-nef, and irrelevant peptide (IR). (C) Anti–GPIIIa49-66 reactivity with various 8-mer peptides. SEM is given. HCV genome map is provided.
Reactivity of Ab with a modified epitope
Since the core envelope 1 protein is partially cleaved during virus secretion from the infected cell with removal of 2 amino acids from the core, we tested the remaining 8-mer peptide from 3 different HCV isolates. All 3 had similar or greater activity than the PHC09 10-mer peptide (Figure 1C).
Cross-reactivity of HCV-ITP, HIV-ITP, and HCV-HIV-ITP sera with GPIIIa49-66, PHC09, PHC09-H5, PHC07, and nef
Since HIV-1/HCV-ITP drug abusers have a higher incidence and severity of ITP, we reasoned that they might have a greater incidence of anti–GPIIIa49-66 Ab secondary to molecular mimicry with HCV peptides as well as recently reported HIV-nef.14 Figure 2A through C demonstrate considerably increased Ab reactivity versus GPIIIa49-66 in 12 of 15 HIV/HCV-ITP drug abusers (Figure 2C) compared with 2 of 15 HCV-ITP patients (Figure 2A; P < .001) and 6 of 15 HIV-ITP patients (Figure 2B; P < .05, χ2 analysis). Similar differences were noted for the other 4 molecular mimicry HCV and nef Ags in the 3 groups of patients (Table 2). Of further interest was the presence of Ab to all 4 Ags in 7 of 15 HIV/HCV-ITP patients compared with 1 HCV-ITP patient and 1 HIV-ITP patient (P = .019). A greater severity of thrombocytopenia, less than 30 × 109/L (Figure 2D), was also noted in 8 of 15 HIV/HCV-ITP drug abusers compared with 2 HCV-ITP (P < .025) and 3 HIV-ITP (P = .06) patients. Thus, the greater incidence and severity of thrombocytopenia in HIV-1/HCV-ITP drug abusers could be explained on the basis of molecular mimicry of GPIIIa49-66 with the 4 nonconserved HCV peptides and HIV-1 nef.
Serum reactivity to GPIIIa49-66 mimicry peptide and thrombocytopenia in HCV, HIV, and HIV/HCV-ITP patients. Serum samples were collected from immunologic thrombocytopenia patients (15 HCV-ITP [A], 15 HIV-ITP [B], and 15 HIV/HCV-ITP intravenous drug abusers [C]). Serum reactivity to GPIIIa49-66 and its mimicry peptides HCV (PHC09, PHC09-H5, and PHC07) as well as HIV-1 peptide nef and irrelevant peptide (IR) was measured by ELISA with patient sera. SEM is given. Horizontal lines refer to 2 SDs. The severity of thrombocytopenia was evaluated by platelet count (D). Horizontal bar is an arbitrary 30 × 109/L cut-off.
Serum reactivity to GPIIIa49-66 mimicry peptide and thrombocytopenia in HCV, HIV, and HIV/HCV-ITP patients. Serum samples were collected from immunologic thrombocytopenia patients (15 HCV-ITP [A], 15 HIV-ITP [B], and 15 HIV/HCV-ITP intravenous drug abusers [C]). Serum reactivity to GPIIIa49-66 and its mimicry peptides HCV (PHC09, PHC09-H5, and PHC07) as well as HIV-1 peptide nef and irrelevant peptide (IR) was measured by ELISA with patient sera. SEM is given. Horizontal lines refer to 2 SDs. The severity of thrombocytopenia was evaluated by platelet count (D). Horizontal bar is an arbitrary 30 × 109/L cut-off.
In this regard, it is of interest to cite the work of Yong and Wang20 who used an antihuman HCV core polyclonal Ab to pan a phage peptide library for hepatitis C epitope mapping. An analysis of their data reveals the presence of a dominant linear epitope at residues 19 to 25 of the N-terminal end. This peptide, PQXV(I)XFP, has 86% homology with platelet GPIIIa51-57 (PESIEFP), which resides within the GPIIIa49-66 peptide, confirming our observations.
Clinical association of HCV peptide–specific antibodies and thrombocytopenia
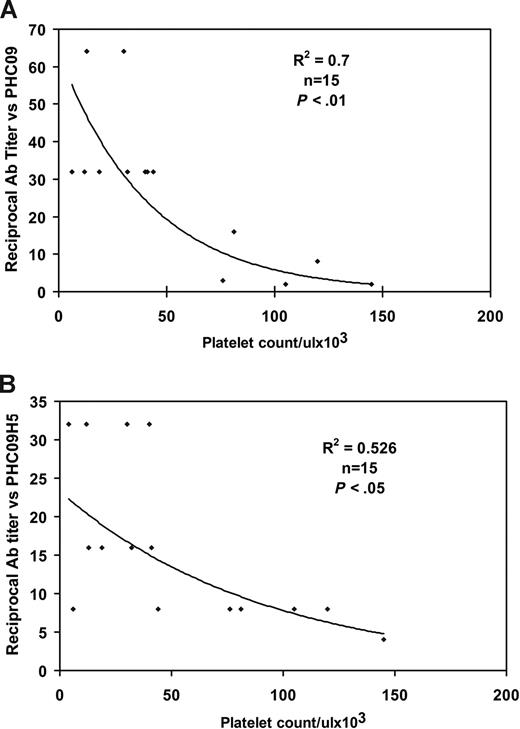
To determine the clinical association of HCV peptide and thrombocytopenia in patients, we obtained 15 serum samples from thrombocytopenic HIV-1/HCV-infected drug abusers and looked for a correlation between their platelet count and titer versus the 3 HCV peptides. Figure 3A and B demonstrates an inverse correlation between patient platelet count and Ab titer versus the PHC09 peptide (r2 = 0.7, P < .01) and the PHC09-H5 peptide (r2 = 0.53, P < .05). Since Ab versus PHC09 provided the best correlation (Figure 3A) and highest titers (Figure 2A-C), we focused on anti-PHC09 Ab located in HCV core envelope 1.
Inverse correlation between anti-HCV peptide Ab titer and thrombocytopenia. (A) Anti-HCV peptide (PHC09). (B) Anti-HCV peptide (PHC09-H5). Serum titer to HCV peptides was measured by ELISA with serial dilutions of sera.
Inverse correlation between anti-HCV peptide Ab titer and thrombocytopenia. (A) Anti-HCV peptide (PHC09). (B) Anti-HCV peptide (PHC09-H5). Serum titer to HCV peptides was measured by ELISA with serial dilutions of sera.
Effect of the HCV peptide–specific antibodies on Ab-induced oxidative platelet fragmentation
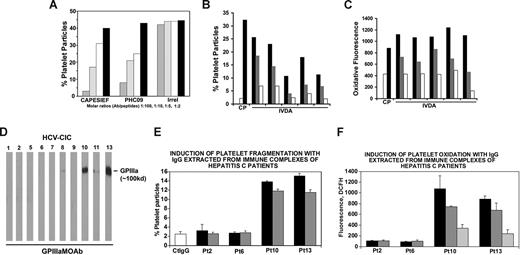
To assess the potential effect of HCV peptide on Ab-induced platelet fragmentation, peptide PHC09 (SAIHIRNASG) was then tested for its ability to inhibit affinity-purified patient GPIIIa49-66 Ab-induced platelet fragmentation in vitro. PHC09 significantly inhibited Ab-induced human platelet fragmentation at a (IC50) molar Ab/peptide ratio of 1:5 (similar to GPIIIa49-66 peptide CAPESIEFPVSEARVLED), whereas irrelevant peptide had no effect at 1:100 (Figure 4A).
Inhibition of anti–GPIIIa49-66 Ab-induced oxidative platelet fragmentation by HCV peptides. (A) Effect of HCV peptides on anti–GPIIIa49-66 platelet reactivity. Patient IgG was preincubated with different peptides prior to incubation with gel-filtered platelets. CAPESIEF refers to positive control (CAPESIEFPVSEARVLED). Irrel refers to irrelevant peptide.  ,
,  , □, ■ refer to molar Ab/peptide ratios of 1:100, 1:10, 1:5, and 1:2, respectively. Effect of IgG against HCV peptide (PHC09) on (B) platelet fragmentation and (C) oxidation. Affinity-purified IgG from 5 HIV-1/HCV-ITP patients with high reciprocal Ab titer to PHC09-induced platelet fragmentation (B) and oxidation (C) in vitro. C (□) indicates control human IgG; P (■), HIV-1-ITP patient IgG; and IVDA horizontal line, affinity purified anti-HCV peptide PHC09 IgG from dually infected drug abusers. The ■,
, □, ■ refer to molar Ab/peptide ratios of 1:100, 1:10, 1:5, and 1:2, respectively. Effect of IgG against HCV peptide (PHC09) on (B) platelet fragmentation and (C) oxidation. Affinity-purified IgG from 5 HIV-1/HCV-ITP patients with high reciprocal Ab titer to PHC09-induced platelet fragmentation (B) and oxidation (C) in vitro. C (□) indicates control human IgG; P (■), HIV-1-ITP patient IgG; and IVDA horizontal line, affinity purified anti-HCV peptide PHC09 IgG from dually infected drug abusers. The ■,  , □ refer to doubling dilutions of isolated IVDA IgG (25, 12.5, and 6.25 μg/mL, respectively). (D) Presence of platelet fragments in patients with HCV-ITP. Immunoblots of serum immune complexes run on SDS-PAGE and immunoblotted with anti–GPIIIa49-66. (E) Effect of HCV IgG isolated from HCV immune complexes on (E) platelet fragmentation and (F) platelet DCFH oxidation. Pt refers to patient. ■,
, □ refer to doubling dilutions of isolated IVDA IgG (25, 12.5, and 6.25 μg/mL, respectively). (D) Presence of platelet fragments in patients with HCV-ITP. Immunoblots of serum immune complexes run on SDS-PAGE and immunoblotted with anti–GPIIIa49-66. (E) Effect of HCV IgG isolated from HCV immune complexes on (E) platelet fragmentation and (F) platelet DCFH oxidation. Pt refers to patient. ■,  ,
,  refer to serial 1:2 dilutions of IgG, starting at 50 μg/mL. SEM is given.
refer to serial 1:2 dilutions of IgG, starting at 50 μg/mL. SEM is given.
Inhibition of anti–GPIIIa49-66 Ab-induced oxidative platelet fragmentation by HCV peptides. (A) Effect of HCV peptides on anti–GPIIIa49-66 platelet reactivity. Patient IgG was preincubated with different peptides prior to incubation with gel-filtered platelets. CAPESIEF refers to positive control (CAPESIEFPVSEARVLED). Irrel refers to irrelevant peptide.  ,
,  , □, ■ refer to molar Ab/peptide ratios of 1:100, 1:10, 1:5, and 1:2, respectively. Effect of IgG against HCV peptide (PHC09) on (B) platelet fragmentation and (C) oxidation. Affinity-purified IgG from 5 HIV-1/HCV-ITP patients with high reciprocal Ab titer to PHC09-induced platelet fragmentation (B) and oxidation (C) in vitro. C (□) indicates control human IgG; P (■), HIV-1-ITP patient IgG; and IVDA horizontal line, affinity purified anti-HCV peptide PHC09 IgG from dually infected drug abusers. The ■,
, □, ■ refer to molar Ab/peptide ratios of 1:100, 1:10, 1:5, and 1:2, respectively. Effect of IgG against HCV peptide (PHC09) on (B) platelet fragmentation and (C) oxidation. Affinity-purified IgG from 5 HIV-1/HCV-ITP patients with high reciprocal Ab titer to PHC09-induced platelet fragmentation (B) and oxidation (C) in vitro. C (□) indicates control human IgG; P (■), HIV-1-ITP patient IgG; and IVDA horizontal line, affinity purified anti-HCV peptide PHC09 IgG from dually infected drug abusers. The ■,  , □ refer to doubling dilutions of isolated IVDA IgG (25, 12.5, and 6.25 μg/mL, respectively). (D) Presence of platelet fragments in patients with HCV-ITP. Immunoblots of serum immune complexes run on SDS-PAGE and immunoblotted with anti–GPIIIa49-66. (E) Effect of HCV IgG isolated from HCV immune complexes on (E) platelet fragmentation and (F) platelet DCFH oxidation. Pt refers to patient. ■,
, □ refer to doubling dilutions of isolated IVDA IgG (25, 12.5, and 6.25 μg/mL, respectively). (D) Presence of platelet fragments in patients with HCV-ITP. Immunoblots of serum immune complexes run on SDS-PAGE and immunoblotted with anti–GPIIIa49-66. (E) Effect of HCV IgG isolated from HCV immune complexes on (E) platelet fragmentation and (F) platelet DCFH oxidation. Pt refers to patient. ■,  ,
,  refer to serial 1:2 dilutions of IgG, starting at 50 μg/mL. SEM is given.
refer to serial 1:2 dilutions of IgG, starting at 50 μg/mL. SEM is given.
Induction of oxidative platelet fragmentation with Ab
We next affinity-purified IgG with a PHC09 affinity column and confirmed that (similar to anti–GPIIIa49-66) anti-PHC09 IgG induces oxidative platelet fragmentation in vitro (Figure 4B,C). We therefore looked for platelet fragments in the immune complexes of 10 HCV-ITP patients by immunoblot. They were found in patient nos. 10 and 13 (Figure 4D). We next isolated IgG from the serum immune complexes of patients nos. 10 and 13 as well as patients nos. 2 and 6 who had undetectable platelet fragments. Both positive patients tested (nos. 10 and 13) had immune complex IgG capable of oxidatively fragmenting platelets, whereas the 2 control patients (nos. 2 and 6) were unreactive (Figure 4E,F).
Effect of mouse Ab against HCV peptide on induction of thrombocytopenia in mice
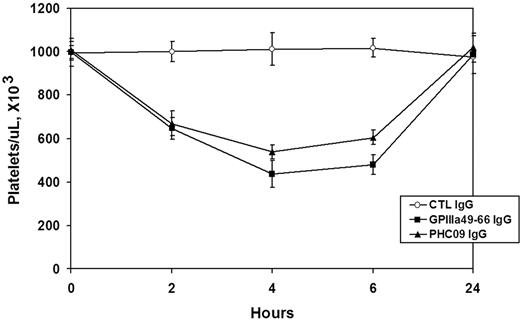
The in vivo relevance of this peptide was further tested by raising an Ab against the KLH-conjugated peptide in GPIIIa−/− KO mice, which do not recognize the peptide as self, avoiding the problem of mouse tolerance. The mouse serum Ab against PHC09 was shown to bind to GPIIIa49-66 (data not shown). Affinity-purified mouse anti-PHC09 IgG, mouse control IgG, and rabbit anti–GPIIIa49-66 IgG (50 μg/mouse, n = 4/group) were then injected intraperitoneally into Balb/c mice, and platelet counts followed for 24 hours. Figure 5 demonstrates that whereas mouse control IgG had no effect, rabbit anti–GPIIIa49-66 IgG or mouse anti-PHC09 IgG induced thrombocytopenia.
Effect of Ab against HCV peptide on induction of thrombocytopenia in Balb/C mice. Purified control mouse IgG (Ctl), rabbit GPIIIa49-66 IgG, and mouse IgG against PHC09 (50 μg) were injected intraperitoneally into Balb/C mice, and platelet counts followed for 24 hours. n = 4. SEM is given.
Effect of Ab against HCV peptide on induction of thrombocytopenia in Balb/C mice. Purified control mouse IgG (Ctl), rabbit GPIIIa49-66 IgG, and mouse IgG against PHC09 (50 μg) were injected intraperitoneally into Balb/C mice, and platelet counts followed for 24 hours. n = 4. SEM is given.
Pathological effect of rHCV core envelope 1 on mouse platelet counts
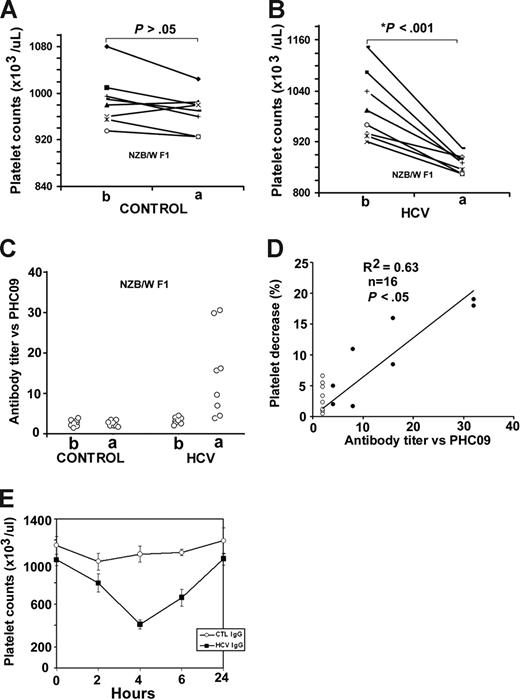
Since PHC09-SAIHIRNASG is a molecular mimic of GPIIIa49-66 and located in HCV core envelope 1, we investigated its in vivo pathological effect on the mouse platelet count. Autoimmune SLE NZB/W F1 mice and immunosuppressed BALB/c mice were challenged with multiple rHCV core-envelope 1 injections. Platelet counts decreased in 8 of 8 NZB/W F1-treated mice (P = .001; mean platelet count of 1.003 ± 0.029 vs 0.87 ± 0.074 × 109/L; Figure 6A,B), whereas no decrease in platelet count was noted in the comparison groups immunized with control protein or rHCV core envelope 1 peptide in BALB/c immunosuppressed mice (data not shown). In addition, the serum titer to peptide PHC09 was elevated in NZB/W F1 mice immunized with rHCV core envelope 1, whereas the control group had no significant effect (Figure 6C). The platelet decrease correlated inversely with serum titer versus PHC09 (r2 = 0.63, n = 16, P < .05) (Figure 6D).
Effect of recombinant HCV core envelope 1 injection on mouse platelet counts. NZB/W F1 mice were challenged with multiple control (A) or rHCV core envelope 1 (B) injections. At 90 days, the platelet count had decreased in 8 of 8 NZB/W F1 mice (x-axis: b and a refer to before and after immunization, respectively; n = 8, P < .001) (B). No decrease in platelet count was found in the comparison group immunized with control protein (A). The serum titer to peptide PHC09 was elevated in NZB/W F1 mice immunized with rHCV core envelope 1 (HCV; C), whereas the control protein (control) had no effect. (D) Correlation between percentage platelet count decrease and serum titer versus PHC09 in NZB/W F1 mice. Percentage platelet decrease correlated inversely with serum titer versus PHC09 (r2 = 0.63, n = 16, P < .05). · and ○ refer to NZB/W F1 mice immunized with rHCV core envelope 1 or control protein, respectively. (E) In vivo induction of thrombocytopenia in mice injected with affinity-purified anti-PHC09 IgG derived from NZB/W F1 mice immunized with rHCV core envelope 1. Purified control mouse IgG (Ctl IgG), and mouse IgG against PHC09 (HCV IgG; 25 μg/mouse) were injected intraperitoneally into Balb/c mice (n = 4 per group). SEM is given.
Effect of recombinant HCV core envelope 1 injection on mouse platelet counts. NZB/W F1 mice were challenged with multiple control (A) or rHCV core envelope 1 (B) injections. At 90 days, the platelet count had decreased in 8 of 8 NZB/W F1 mice (x-axis: b and a refer to before and after immunization, respectively; n = 8, P < .001) (B). No decrease in platelet count was found in the comparison group immunized with control protein (A). The serum titer to peptide PHC09 was elevated in NZB/W F1 mice immunized with rHCV core envelope 1 (HCV; C), whereas the control protein (control) had no effect. (D) Correlation between percentage platelet count decrease and serum titer versus PHC09 in NZB/W F1 mice. Percentage platelet decrease correlated inversely with serum titer versus PHC09 (r2 = 0.63, n = 16, P < .05). · and ○ refer to NZB/W F1 mice immunized with rHCV core envelope 1 or control protein, respectively. (E) In vivo induction of thrombocytopenia in mice injected with affinity-purified anti-PHC09 IgG derived from NZB/W F1 mice immunized with rHCV core envelope 1. Purified control mouse IgG (Ctl IgG), and mouse IgG against PHC09 (HCV IgG; 25 μg/mouse) were injected intraperitoneally into Balb/c mice (n = 4 per group). SEM is given.
Since platelet counts represent an equilibrium between platelet production and destruction, we reasoned that the modest decrease in platelet count could have been dampened by a compensatory increase in platelet production by the challenged bone marrow. We therefore affinity-purified the serum IgG with PHC09, to increase its relative concentration. Figure 6E demonstrates a more pronounced thrombocytopenia (60% decrease in platelet count compared with control IgG).
Discussion
Our current data clearly demonstrate that the HCV core envelope 1 can induce functionally active anti–GPIIIa49-66 Ab by mimicking platelet GPIIIa49-66 as antigen and inducing immune thrombocytopenia in NZB/W F1 mice. Anti–HCV core envelope 1 Ab correlates inversely with platelet counts in HIV-1 drug abusers with HCV infection. To our knowledge, this is the first report that an HCV epitope can induce anti–GPIIIa49-66 Ab by molecular mimicry.
We now describe a second immune complex–associated platelet immunologic disorder (HCV-ITP) in which a unique Ab is developed against GPIIIa49-66 capable of inducing oxidative platelet fragmentation. It is now apparent that this new pathologic mechanism of platelet destruction is not unique for HIV-1–ITP and could possibly be associated with other immune complex–associated disorders in which serum complexes may contain platelet fragments and anti–GPIIIa49-66 Ab.
Attempts to induce thrombocytopenia by injection of HCV peptide PHC09 into immunosuppressed wild-type mice were unsuccessful—either due to immune tolerance (mouse GPIIIa has 83% homology with human GPIIIa21 ) or cyclophosphamide-induced inhibition of anti–GPIIIa49-66 Ab production. However, we were able to induce anti–GPIIIa49-66 Ab and resultant thrombocytopenia in 8 of 8 autoimmune NZB/W F1 mice that correlated inversely with their platelet count. This is likely due to impairment of immune surveillance in these mice with polyclonal B-lymphocyte hyperactivity to self-antigen that parallels the B-cell hyperactivity in early HIV-1 infection.22
Studies by others have demonstrated that the HCV core protein 1 is cleaved by signal peptidases between residues 191 and 192 to generate the retained E1 envelope.23,24 PHC09 contains 10 amino acids (SAIHIRNASG). The major (8 amino acids) molecular mimicry sequence (IHIRNASG) is located in the retained HCV 1 envelope region, which is likely to retain the epitope specificity. Indeed, a human monoclonal Ab (H111) derived from a hepatitis C donor infected with HCV genotype 1b reacts with the N-terminal 192 to 202 amino acids of E1 from 12 different E1 proteins obtained from virus isolates of genotypes 1a, 1b, 2b, and 3a.25 PHC09-H5, the epitope analyzed in our study, has homology with 20 different E1 isolates, with the candidate linear epitope containing the critical amino acids—RN-SG-Y-. Thus PHC09-H5 appears to be a common hepatitis C virus epitope.
Of interest were our observations on the greater incidence and severity of ITP in drug abusers coinfected with HIV-1 and HCV compared with HCV-ITP or HIV-ITP. The increased anti–GPIIIa49-66 Ab incidence, titer, and cross-reactivity with molecular mimotopes from HCV (core envelope protein 1) as well as HIV-1 (nef) provide an explanation for the clinically observed greater incidence and severity of dually infected drug abusers. This is likely due to HIV-induced dysregulation of immune function in which spontaneous activation of B cells and immunoglobulin production has been reported.26,27 It is suggested that molecular mimicry of HCV or HIV-1 peptide with other epitopes on the platelet or megakaryocyte surface might also play a role in the induction of HCV/HIV-ITP thrombocytopenia.
Ab against PHC09-H5 was found in 6 of 15 HCV-ITP and 8 of 15 HIV-HCV-ITP patients. Ab was not detected in the other approximately 7 patients (∼50%), suggesting that molecular mimicry could be dependent upon the fine specificity of the specific mimotope in the nonconserved region, or that other mechanisms can be invoked in these patients. This could vary and is dependent upon the ability of the hepatitis C virus envelope to avoid host immune surveillance. Nevertheless, it is now apparent that at least some patients with HCV-ITP may develop their ITP due to molecular mimicry. This is supported by our ability to induce immunologic thrombocytopenia in NZB/WF1 mice with a core-envelope construct. It is well known that thrombocytopenia increases with severity of disease—and has been claimed that this may be due to liver disease rather than Ab. We consider this debatable because of patients whom we have studied with severe liver cirrhosis who had abundant IgG on their platelets as well as in their immune complexes.16
It is of interest that both HCV and HIV-1 each contain different structural elements that mimic peptide sequences in GPIIIa49-66 and that both viruses are capable of inducing GPIIIa49-66–specific antibodies capable of causing thrombocytopenia. A possible explanation is that both the HCV and HIV-1 mimicry epitopes are in nonconserved highly mutated regions of the 2 viruses, and that mimicry of host proteins serves to help viruses escape host immune surveillance. Therefore the mutations of these mimicry regions are likely to contribute to the autoimmunity in general, and in our study, thrombocytopenia more specifically. It is also likely that coinfection of HCV and HIV-1 increases the chances of molecular mimicry.
In summary, these findings demonstrate the potential of HCV or other pathogens to induce antiplatelet Ab by molecular mimicry—and suggests that platelet membrane GPIIIa49-66 may be a common immunodominant target for these pathogens, particularly HIV-1/HCV for the induction of immunologic thrombocytopenia.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (Bethesda, MD) grants K01 DA020816 (Z.L.), HL 13336, and DAO-04315 (S.K.), and New York University Center for AIDS Research Grant 30-1-7649 (Z.L.).
National Institutes of Health
Authorship
Contribution: W.Z. performed and analyzed most of the experiments; M.A.N. performed the platelet fragmentation experiment; W.B. sug-gested the HCV genotype experiments; Z.L. designed the phage display experiments; and S.K. guided the project and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simon Karpatkin or Zongdong Li, Department of Medicine, New York University School of Medicine, 550 1st Ave, New York, NY 10016; e-mail: simon.karpatkin@med.nyu.edu or Liz04@med.nyu.edu.

![Figure 2. Serum reactivity to GPIIIa49-66 mimicry peptide and thrombocytopenia in HCV, HIV, and HIV/HCV-ITP patients. Serum samples were collected from immunologic thrombocytopenia patients (15 HCV-ITP [A], 15 HIV-ITP [B], and 15 HIV/HCV-ITP intravenous drug abusers [C]). Serum reactivity to GPIIIa49-66 and its mimicry peptides HCV (PHC09, PHC09-H5, and PHC07) as well as HIV-1 peptide nef and irrelevant peptide (IR) was measured by ELISA with patient sera. SEM is given. Horizontal lines refer to 2 SDs. The severity of thrombocytopenia was evaluated by platelet count (D). Horizontal bar is an arbitrary 30 × 109/L cut-off.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/17/10.1182_blood-2008-09-181073/4/m_zh80060930850002.jpeg?Expires=1765918604&Signature=yEeLTiH7GhZTmMRisShuG-FilJGNInBvnwPa0-BkAI7E7VEQU3luUqLs6iRQ5m3YOWb-L3gefNt2BG8j3rT307-gOeovBbyZ37btWY5dlIwKvL5v6kw5dm6df0DfPmjV4mSLu2CARqVRIkGaFokUaZ2MB3P0r7WY8mY9y9tjSFBEIfVw6UtM~Pq7JiTrJxlQ1Q6Ak2SSWbw3dN~iK9NZlDtfCQYAVpx1oK3ucRoAK8wlyCFUjN7b6WKdps6PKyN6WAHc26PJdsmshrYolTDrmRlqgF4~qkp8sJm3Kj0MGpQFAvehqBHMkdgHUpNBnCleri6khocwjxn8zhjof9moEg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)