Abstract
In paroxysmal nocturnal hemoglobinuria (PNH) hemolytic anemia is due mainly to deficiency of the complement regulator CD59 on the surface of red blood cells (RBCs). Eculizumab, an antibody that targets complement fraction 5 (C5), has proven highly effective in abolishing complement-mediated intravascular hemolysis in PNH; however, the hematologic benefit varies considerably among patients. In the aim to understand the basis for this variable response, we have investigated by flow cytometry the binding of complement fraction 3 (C3) on RBCs from PNH patients before and during eculizumab treatment. There was no evidence of C3 on RBCs of untreated PNH patients; by contrast, in all patients on eculizumab (n = 41) a substantial fraction of RBCs had C3 bound on their surface, and this was entirely restricted to RBCs with the PNH phenotype (CD59−). The proportion of C3+ RBCs correlated significantly with the reticulocyte count and with the hematologic response to eculizumab. In 3 patients in whom 51Cr labeling of RBCs was carried out while on eculizumab, we have demonstrated reduced RBC half-life in vivo, with excess 51Cr uptake in spleen and in liver. Binding of C3 by PNH RBCs may constitute an additional disease mechanism in PNH, strongly enhanced by eculizumab treatment and producing a variable degree of extravascular hemolysis.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a hematologic disorder characterized by the clonal expansion of one or a few hematopoietic stem cells that are incapable of glycosylphosphatidylinositol (GPI)–anchor biosynthesis, due to an acquired somatic mutation in the phosphatidylinositol glycan class A (PIG-A) gene.1-6 Affected progeny cells are deficient in all GPI-anchored surface proteins, including the complement regulators CD55 and CD59.7-9 Thus, PNH red blood cells (RBCs) are exquisitely vulnerable to activated complement, and particularly to the membrane attack complex (MAC),10,11 resulting in chronic intravascular hemolysis with recurrent exacerbations, and consequent anemia.
Eculizumab (Soliris; Alexion Pharmaceuticals, Cheshire, CT) is a humanized monoclonal antibody against complement fraction 5 (C5), which inhibits MAC formation.12 Eculizumab has proven highly beneficial in the treatment of transfusion-dependent PNH patients.13-15 In a placebo-controlled phase 3 trial, eculizumab led to a marked decrease in transfusion requirement, and improvement in anemia, fatigue, pain, shortness of breath, and QoL measures.15 These data were confirmed in 2 subsequent studies,16,17 the last one also suggesting that eculizumab may reduce the occurrence of thromboembolic events.17
In the face of such gratifying clinical results, it is clear that not all patients respond equally to the treatment. In some patients there is only little improvement of anemia, and some still require blood transfusion at times, with signs of persistent hemolysis (reticulocytosis, elevated unconjugated bilirubin).15,16 In this work, we have investigated the notion that in patients with suboptimal hematologic response to eculizumab there may be extravascular hemolysis mediated by complement effector mechanisms other than MAC.15 Based on flow cytometry analysis of complement fraction 3 (C3) on RBCs, we provide evidence of selective C3 opsonization of GPI-negative red cells, the extent of which tends to correlate with the clinical response to eculizumab, and may be the manifestation of a novel phenomenon in the pathophysiology of PNH.
Methods
Patients
The study was conducted in 56 Italian PNH patients (Table 1); biologic samples were collected by venipuncture according to standard procedures, after informed consent was obtained in accordance with the Declaration of Helsinki as approved within the study protocol by the Institutional Review Board at the Federico II University of Naples. Twenty-eight patients were studied at diagnosis, before any treatment; 13 of them were retested while on eculizumab. An additional 28 patients were tested when they were already receiving eculizumab. Several patients were analyzed repeatedly during the treatment; 5 patients were studied bimonthly during the first 3 months of treatment. As controls, we collected samples from 5 cold agglutinin disease (CAD) patients (positive controls) as well as from 10 healthy subjects (negative controls).
Characteristics of PNH patients studied while on eculizumab
| UPN . | Sex . | TE . | Tx/y . | Hb . | ARC . | LDH . | T Bil . | % PNH RBCs . | % C3+ PNH RBCs . | Hematologic response . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | During . | Before . | During . | Before . | During . | Before . | During . | Before . | During . | ||||||
| 6 | F | 2 | 0 | 9.6 | 13.0 | 100 | 120 | 743 | 239 | 14 | 19 | 55.2 | 3.3 | Optimal | |
| 7 | M | 4 | 0 | 5.0 | 14.0 | 150 | 130 | 2282 | 339 | 22 | 26 | 62.7 | 34.6 | Optimal | |
| 8 | M | BC | 12 | 0 | 9.1 | 11.8 | 90 | 90 | 1264 | 255 | 106 | 250 | 19.5 | 0.5 | Optimal |
| 9 | M | 6 | 0 | 8.0 | 13.2 | 212 | 312 | 1934 | 266 | 22 | 28 | 78.0 | 21.3 | Optimal | |
| 14 | M | BC | 12 | 0 | 11.0 | 13.3 | 42 | 201 | 489 | 170 | 47.4 | 54.1 | 82.0 | 0.6 | Optimal |
| 16 | M | 9 | 0 | 7.5 | 12.9 | 234 | 179 | 1216 | 192 | 10.6 | 36.7 | 98.0 | 34.1 | Optimal | |
| 19 | F | 9 | 0 | 6.5 | 12.0 | 94 | 147 | 1365 | 374 | 26.3 | 19.1 | 33.0 | 8.3 | Optimal | |
| 21 | M | 22 | 0 | 7.5 | 13.0 | 290 | 150 | 5311 | 200 | 180.8 | 129.4 | 96.1 | 55.2 | Optimal | |
| 23 | M | 10 | 0 | 8.5 | 11.5 | 155 | 130 | 1306 | 335† | 28 | 26 | 37.6 | 22.6 | Optimal | |
| 25 | M | 48 | 0 | 10.1 | 12.9 | 176 | 55 | 1524 | 201 | 33 | 14 | 12.0 | 1.7 | Optimal | |
| 26 | F | 12 | 0 | 8.5 | 11.5 | 126 | 126 | 1800 | 259 | 28 | 26 | 78.7 | 17.0 | Optimal | |
| 34 | F | DVT | 2 | 0 | 7.9 | 11.0 | 130 | 140 | 3915 | 529 | 11 | 16 | 76.0 | 15.8 | Optimal |
| 36 | M | CVA, PVT | 0 | 0 | 10.0 | 12.8 | 130 | 54 | 680 | 225 | 26 | 30 | 64.5 | 20.2 | Optimal |
| 40 | F | 3 | 0 | 7.0 | 11.0 | 97 | 120 | 1392 | 177 | 46 | 40 | 48.0 | 21.9 | Optimal | |
| 41 | M | 0 | 0 | 9.0 | 11.7 | 241 | 285 | 2100 | 250 | 29 | 19 | 54.0 | 59.3 | Optimal | |
| 3 | F | 11 | 0 | 8.0 | 10.6 | 240 | 310 | 2051 | 228 | 24 | 24 | 61.0 | 33.6 | Major | |
| 4 | F | CVA | 2 | 0 | 8.7 | 10.0 | 160 | 340 | 2145 | 460 | 25 | 21 | 65.0 | 21.5 | Major |
| 5 | F | 2 | 0 | 8.1 | 9.3 | 240 | 230 | 1438 | 969† | 15 | 27 | 68.0 | 10.3 | Major | |
| 10 | F | 11 | 0 | 7.0 | 10.5 | 60 | 130 | 2545 | 209 | 41 | 16 | 91.0 | 52.7 | Major | |
| 13 | M | 4 | 0 | 10.5 | 9.9 | 51 | 204 | 1986 | 232 | 25.2 | 41.9 | 88.0 | 61.4 | Major | |
| 15 | F | 10 | 0 | 7.8 | 10.9 | 202 | 274 | 1170 | 211 | 30.8 | 20.9 | 50.0 | 44.4 | Major | |
| 17 | M | 0 | 0 | 8.0 | 10.0 | 198 | 299 | 3968 | 860† | 57.4 | 73.5 | 88.7 | 16.6 | Major | |
| 20 | M | Yes | 48 | 0 | 7.5 | 10.0 | 150 | 226 | 7190 | 296 | 73.0 | 47.9 | 98.5 | 58.2 | Major |
| 22 | M | 0 | 0 | 7.5 | 10.0 | 140 | 185 | 1216 | 356† | 16 | 18 | 60.0 | 43.3 | Major | |
| 27 | F | 0 | 0 | 9.3 | 10.5 | 230 | 139 | 727 | 250 | 14 | 16 | 82.0 | 8.5 | Major | |
| 28 | F | 0 | 0 | 9.0 | 10.3 | 130 | 151 | 1500 | 360† | NA | NA | 45.0 | 22.2 | Major | |
| 29 | F | 12 | 0 | 9.2 | 10.4 | 165 | 135 | 2440 | 252 | 24 | 15 | 69.0 | 26.1 | Major | |
| 30 | F | 2 | 0 | 8.2 | 10.2 | 274 | 170 | 2388 | 217 | 35 | 24 | 67.0 | 34.3 | Major | |
| 31 | F | No | 4 | 0 | 7.0 | 8.5 | 1940 | 230 | 1940 | 210 | 30 | 38 | 36.0 | 33.3 | Major |
| 32 | F | 1 | 0 | 7.2 | 8.9 | 120 | 20 | 1700 | 310 | 54 | 24 | 67.0 | 5.2 | Major | |
| 33 | F | 6 | 0 | 7.3 | 9.5 | 320 | 270 | 3436 | 479 | 16 | 35 | 92.0 | 45.7 | Major | |
| 35 | M | NA | 0 | 10.0 | 10.9 | 252 | 205 | 2300 | 290 | 45 | NA | 22.0 | 22.7 | Major | |
| 37 | F | 30 | 0 | 9.0 | 10.3 | 454 | 400 | 4191 | 269 | 45 | 29 | 96.0 | 60.4 | Major | |
| 1 | F | 15 | 6 | 7.3 | 8.7 | 200 | 160 | 2329 | 261 | 27 | 12.7 | 48.0 | 33.3 | Partial | |
| 2 | F | 17 | 10 | 9.1 | 9.9 | 220 | 290 | 1954 | 221 | 89 | 148 | 95.0 | 36.0 | Partial | |
| 11 | M | 36 | 13 | 8.5 | 6.3 | 420 | 400 | 6553 | 993† | 21 | 20 | 57.0 | 51.2 | Partial | |
| 18 | F | 16 | 4 | 7.0 | 9.5 | 266 | 230 | 1837 | 200 | 26.7 | 38.3 | 42.6 | 46.2 | Partial | |
| 38 | F | Yes | 30 | 18 | 6 | 9 | 256 | 401 | 1400 | 288 | 51 | 29 | 10 | 5.0 | Partial |
| 12* | F | 21 | 21 | 7.0 | 7.1 | 220 | 121 | NA | 309† | NA | NA | 12.0 | 4.2 | Minor | |
| 24* | F | 4 | 24 | 8.4 | 8.6 | 167 | 208 | 2000 | 331 | 31 | 40 | 50.7 | 15.8 | Minor | |
| 39* | M | NA | 17 | 6.0 | 7.0 | 180 | 190 | 1042 | 283 | 38 | 28 | 28.0 | 7.1 | Minor | |
| UPN . | Sex . | TE . | Tx/y . | Hb . | ARC . | LDH . | T Bil . | % PNH RBCs . | % C3+ PNH RBCs . | Hematologic response . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before . | During . | Before . | During . | Before . | During . | Before . | During . | Before . | During . | ||||||
| 6 | F | 2 | 0 | 9.6 | 13.0 | 100 | 120 | 743 | 239 | 14 | 19 | 55.2 | 3.3 | Optimal | |
| 7 | M | 4 | 0 | 5.0 | 14.0 | 150 | 130 | 2282 | 339 | 22 | 26 | 62.7 | 34.6 | Optimal | |
| 8 | M | BC | 12 | 0 | 9.1 | 11.8 | 90 | 90 | 1264 | 255 | 106 | 250 | 19.5 | 0.5 | Optimal |
| 9 | M | 6 | 0 | 8.0 | 13.2 | 212 | 312 | 1934 | 266 | 22 | 28 | 78.0 | 21.3 | Optimal | |
| 14 | M | BC | 12 | 0 | 11.0 | 13.3 | 42 | 201 | 489 | 170 | 47.4 | 54.1 | 82.0 | 0.6 | Optimal |
| 16 | M | 9 | 0 | 7.5 | 12.9 | 234 | 179 | 1216 | 192 | 10.6 | 36.7 | 98.0 | 34.1 | Optimal | |
| 19 | F | 9 | 0 | 6.5 | 12.0 | 94 | 147 | 1365 | 374 | 26.3 | 19.1 | 33.0 | 8.3 | Optimal | |
| 21 | M | 22 | 0 | 7.5 | 13.0 | 290 | 150 | 5311 | 200 | 180.8 | 129.4 | 96.1 | 55.2 | Optimal | |
| 23 | M | 10 | 0 | 8.5 | 11.5 | 155 | 130 | 1306 | 335† | 28 | 26 | 37.6 | 22.6 | Optimal | |
| 25 | M | 48 | 0 | 10.1 | 12.9 | 176 | 55 | 1524 | 201 | 33 | 14 | 12.0 | 1.7 | Optimal | |
| 26 | F | 12 | 0 | 8.5 | 11.5 | 126 | 126 | 1800 | 259 | 28 | 26 | 78.7 | 17.0 | Optimal | |
| 34 | F | DVT | 2 | 0 | 7.9 | 11.0 | 130 | 140 | 3915 | 529 | 11 | 16 | 76.0 | 15.8 | Optimal |
| 36 | M | CVA, PVT | 0 | 0 | 10.0 | 12.8 | 130 | 54 | 680 | 225 | 26 | 30 | 64.5 | 20.2 | Optimal |
| 40 | F | 3 | 0 | 7.0 | 11.0 | 97 | 120 | 1392 | 177 | 46 | 40 | 48.0 | 21.9 | Optimal | |
| 41 | M | 0 | 0 | 9.0 | 11.7 | 241 | 285 | 2100 | 250 | 29 | 19 | 54.0 | 59.3 | Optimal | |
| 3 | F | 11 | 0 | 8.0 | 10.6 | 240 | 310 | 2051 | 228 | 24 | 24 | 61.0 | 33.6 | Major | |
| 4 | F | CVA | 2 | 0 | 8.7 | 10.0 | 160 | 340 | 2145 | 460 | 25 | 21 | 65.0 | 21.5 | Major |
| 5 | F | 2 | 0 | 8.1 | 9.3 | 240 | 230 | 1438 | 969† | 15 | 27 | 68.0 | 10.3 | Major | |
| 10 | F | 11 | 0 | 7.0 | 10.5 | 60 | 130 | 2545 | 209 | 41 | 16 | 91.0 | 52.7 | Major | |
| 13 | M | 4 | 0 | 10.5 | 9.9 | 51 | 204 | 1986 | 232 | 25.2 | 41.9 | 88.0 | 61.4 | Major | |
| 15 | F | 10 | 0 | 7.8 | 10.9 | 202 | 274 | 1170 | 211 | 30.8 | 20.9 | 50.0 | 44.4 | Major | |
| 17 | M | 0 | 0 | 8.0 | 10.0 | 198 | 299 | 3968 | 860† | 57.4 | 73.5 | 88.7 | 16.6 | Major | |
| 20 | M | Yes | 48 | 0 | 7.5 | 10.0 | 150 | 226 | 7190 | 296 | 73.0 | 47.9 | 98.5 | 58.2 | Major |
| 22 | M | 0 | 0 | 7.5 | 10.0 | 140 | 185 | 1216 | 356† | 16 | 18 | 60.0 | 43.3 | Major | |
| 27 | F | 0 | 0 | 9.3 | 10.5 | 230 | 139 | 727 | 250 | 14 | 16 | 82.0 | 8.5 | Major | |
| 28 | F | 0 | 0 | 9.0 | 10.3 | 130 | 151 | 1500 | 360† | NA | NA | 45.0 | 22.2 | Major | |
| 29 | F | 12 | 0 | 9.2 | 10.4 | 165 | 135 | 2440 | 252 | 24 | 15 | 69.0 | 26.1 | Major | |
| 30 | F | 2 | 0 | 8.2 | 10.2 | 274 | 170 | 2388 | 217 | 35 | 24 | 67.0 | 34.3 | Major | |
| 31 | F | No | 4 | 0 | 7.0 | 8.5 | 1940 | 230 | 1940 | 210 | 30 | 38 | 36.0 | 33.3 | Major |
| 32 | F | 1 | 0 | 7.2 | 8.9 | 120 | 20 | 1700 | 310 | 54 | 24 | 67.0 | 5.2 | Major | |
| 33 | F | 6 | 0 | 7.3 | 9.5 | 320 | 270 | 3436 | 479 | 16 | 35 | 92.0 | 45.7 | Major | |
| 35 | M | NA | 0 | 10.0 | 10.9 | 252 | 205 | 2300 | 290 | 45 | NA | 22.0 | 22.7 | Major | |
| 37 | F | 30 | 0 | 9.0 | 10.3 | 454 | 400 | 4191 | 269 | 45 | 29 | 96.0 | 60.4 | Major | |
| 1 | F | 15 | 6 | 7.3 | 8.7 | 200 | 160 | 2329 | 261 | 27 | 12.7 | 48.0 | 33.3 | Partial | |
| 2 | F | 17 | 10 | 9.1 | 9.9 | 220 | 290 | 1954 | 221 | 89 | 148 | 95.0 | 36.0 | Partial | |
| 11 | M | 36 | 13 | 8.5 | 6.3 | 420 | 400 | 6553 | 993† | 21 | 20 | 57.0 | 51.2 | Partial | |
| 18 | F | 16 | 4 | 7.0 | 9.5 | 266 | 230 | 1837 | 200 | 26.7 | 38.3 | 42.6 | 46.2 | Partial | |
| 38 | F | Yes | 30 | 18 | 6 | 9 | 256 | 401 | 1400 | 288 | 51 | 29 | 10 | 5.0 | Partial |
| 12* | F | 21 | 21 | 7.0 | 7.1 | 220 | 121 | NA | 309† | NA | NA | 12.0 | 4.2 | Minor | |
| 24* | F | 4 | 24 | 8.4 | 8.6 | 167 | 208 | 2000 | 331 | 31 | 40 | 50.7 | 15.8 | Minor | |
| 39* | M | NA | 17 | 6.0 | 7.0 | 180 | 190 | 1042 | 283 | 38 | 28 | 28.0 | 7.1 | Minor | |
TE indicates history of thromboembolic events; Tx/y, number of packed red cell units transfused in the last year (before) or in 1 year during eculizumab treatment (after); ARC, absolute reticulocyte count (× 109/L); LDH, lactate dehydrogenase, IU/L (normal range, <223); T Bil, unfractionated bilirubin (μM/L; normal range, <17); hematologic response, hematologic response to eculizumab (see “Eculizumab treatment”); BC, Budd-Chiari syndrome; DVT, deep vein thrombosis; CVA, cerebrovascular accident; PVT, portal vein thrombosis; and NA, not available.
All 41 patients had hemolytic PNH at the time of starting eculizumab; 3 patients (marked by *) subsequently developed aplastic anemia and were excluded by the correlation analysis of C3 coating and hematologic response. Twenty-eight PNH patients were also analyzed free from eculizumab (of whom 15 were not listed in the table because they never started treatment), and in none of them did we find C3+ RBCs.
Patients with eculizumab breakthrough (defined as inefficacy in blocking complement in the last days before the next drug administration).
Eculizumab treatment
Eculizumab was administered according to the standard schedule15-17 (900 mg every 14 ± 2 days, after a loading phase of 600 mg every 7 ± 1 days for 4 doses). Most patients were initially registered either in the TRIUMPH (C04-001)15 or in the SHEPHERD (C04-002)16 trial, all eventually merging in the Extension trial (E05-001)17 at the time of our biologic study; the residual patients started the treatment according to the Italian Early Access Program. Fifteen patients had no indication to start the anticomplement treatment. Of the 41 PNH patients receiving eculizumab, only one had a serious adverse event requiring emergency admission (high pyrexia associated with hypotension: no organism was isolated, and the patient made a complete recovery). Three patients discontinued the treatment (for transplantation due to progression to severe aplastic anemia, spontaneous recovery from PNH, and pregnancy). Some patients presented minor adverse events, mainly headache and mild infections. All patients showed a dramatic reduction of intravascular hemolysis, as documented by the LDH level (Table 1). For the purpose of the study, we classified patients as follows: (a) optimal hematologic responders: patients achieving transfusion independence with Hb levels of 110 g/L or higher; (b) major responders: transfusion independence and Hb levels of 80 g/L or higher; (c) partial responders: reduction (at least 50%) without abrogation of blood transfusions; (d) minor responders: no significant change in blood transfusion requirement or Hb levels but with marked reduction of LDH levels. According to these categories, 15 patients (37%) achieved an optimal response; 18 (44%), a major response; 5 (12%), a partial response; and 3, a minor response (attributed to progression to aplastic anemia, Table 1).
Direct antiglobulin test
The Coombs test was performed by gel microcolumns (DiaMed Italiana Srl, Milan, Italy) after incubation with polyspecific and monospecific anti-IgG, anti-IgM, and anti-C3d sera (DiaMed Italiana Srl). In addition, washed RBCs incubated with anti-C3d antiserum were analyzed for agglutination by microscope.
Flow cytometry
After collection of peripheral blood samples in EDTA, RBCs were washed 3 times and resuspended in saline at the final concentration of 104/μL; CAD samples were preincubated for 30 minutes at 37°C to prevent possible autoagglutination. After titration experiments, optimal staining conditions were set as 50 μL RBCs incubated with 1 μL of either Ab4214 or Ab14396 (diluted 1:20), both commercially available FITC-conjugated anti-C3 polyclonal antibodies (Abcam, Cambridge, United Kingdom); these antibodies, in contrast with that used for direct antiglobulin test (DAT), do not contain any bridge leading to agglutination. In 2-color flow cytometry experiments, 5 μL of a PE-conjugated anti-CD59 monoclonal antibody (BD Pharmingen; no. 555764 [Becton Dickinson Italia, Milan, Italy] or no. 59PE [Valter Occhiena, Torino, Italy]) was added to identify PNH RBCs. Samples were incubated at room temperature for 1 hour, and then analyzed with a FACScan cytometer (Becton Dickinson Italia).
51Cr survival study
The radioisotopic RBC survival study was performed according to standard methods18 ; in brief, after collection by venipuncture, RBCs were labeled with 3.1 MBq sodium chromate (51Cr) and reinjected into the patients by intravenous infusion. Radioactivity was then serially assessed on blood samples (γ-counter LKB-Wallac 1282 COMPUGAMMA; Wallac Oy, Turku, Finland) and on anatomic sites, such as heart (background organ), liver, and spleen (scintillation probe ACN Monogamma; L'Accessorio Nucleare Srl, Milan, Italy). After normalization for background and isotopic decay, radioactivity data were plotted to establish RBC half-life, whereas counts on anatomic sites (corrected also for blood radioactivity; ie, heart counts) were plotted to evaluate possible site(s) of erythrocatheresis.
Statistical analysis
Standard descriptive statistic measures were used to analyze flow cytometry data. To increase conservativeness, nonparametric tests were used to analyze relationship among flow cytometry data and clinical variables: Kruskal-Wallis test, χ2, and Spearman rank order correlation, as appropriate.
Results
Preliminary evidence of C3 coating by direct antiglobulin test (DAT) and single-color flow cytometry
In a first series of 8 PNH patients, we performed a routine DAT by gel microcolumns: in all but one patients the test was negative before eculizumab and became positive for C3 during eculizumab treatment (4 were strongly positive and 4 showed a double band on the gel). The only exception was a female who, in addition to the C3d positivity developed after treatment, also had a pretreatment IgG positivity (which did not change after treatment; the patient had concomitant antinucleus antibody as a sign of subclinical immunologic disorder). On microscopic observation of RBCs incubated with anti-C3d, all samples showed evident agglutination. Then, we proceeded to flow cytometry studies on washed RBCs using anti-C3 polyclonal antibodies; physical parameters assessed by flow cytometry were used to exclude possible RBC autoagglutination. As a paradigmatic example of C3 binding, we used RBCs from 5 CAD patients: in all cases we observed a single RBC population with a substantial C3 binding (Figure 1A). In the 8 PNH patients on eculizumab, we found C3 on a portion of RBCs (Figure 1A); the percentage of these C3-bound RBCs directly correlated with the size of the PNH RBC population (P = .04).19 This finding, together with a bimodal distribution of C3 coating on RBCs from eculizumab-treated PNH patients (in contrast with the unimodal distribution observed in CAD patients), prompted us to identify which population of RBCs was binding C3.
C3 coating on RBCs by flow cytometry. (A) Single-color flow cytometry. (y-axis) Cell count; (x-axis) FITC-conjugated anti-C3 polyclonal antibody (logarithm of fluorescence). Each filled histogram represents a single case, with the corresponding isotypic control (empty histogram). CAD indicates cold agglutinin disease (positive control). In the PNH patients, a discrete population of RBCs coated by C3 appears under eculizumab treatment. (B) Double-color flow cytometry. (y-axis) PE-conjugated anti-CD59 monoclonal antibody (logarithm of fluorescence); (x-axis) FITC-conjugated anti-C3 polyclonal antibody (logarithm of fluorescence). Each dot plot represents a single case. The typical bimodal pattern (CD59+ and CD59−) of untreated PNH patient becomes trimodal under eculizumab treatment for the presence of CD59−/C3+ RBCs.
C3 coating on RBCs by flow cytometry. (A) Single-color flow cytometry. (y-axis) Cell count; (x-axis) FITC-conjugated anti-C3 polyclonal antibody (logarithm of fluorescence). Each filled histogram represents a single case, with the corresponding isotypic control (empty histogram). CAD indicates cold agglutinin disease (positive control). In the PNH patients, a discrete population of RBCs coated by C3 appears under eculizumab treatment. (B) Double-color flow cytometry. (y-axis) PE-conjugated anti-CD59 monoclonal antibody (logarithm of fluorescence); (x-axis) FITC-conjugated anti-C3 polyclonal antibody (logarithm of fluorescence). Each dot plot represents a single case. The typical bimodal pattern (CD59+ and CD59−) of untreated PNH patient becomes trimodal under eculizumab treatment for the presence of CD59−/C3+ RBCs.
Double-color flow cytometric analysis of C3 coating
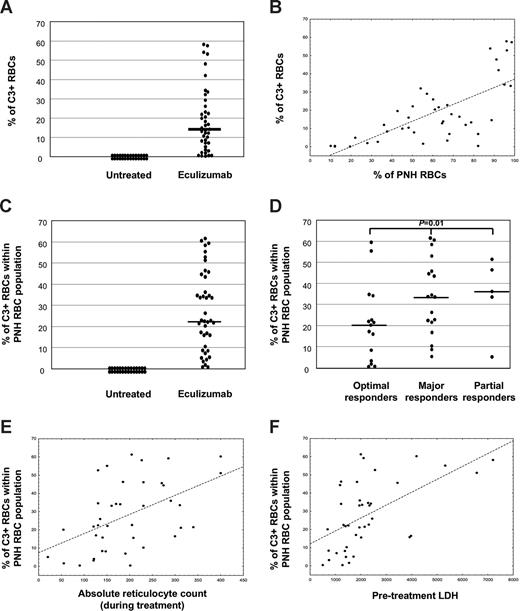
Because the coexistence in the peripheral blood of 2 biologically distinct RBC populations—PNH RBCs (CD59−) and normal RBCs (CD59+)—is a distinctive characteristic of PNH patients, it was important to establish whether only one or both were involved in C3 binding. For this purpose, we designed a 2-color flow cytometry protocol for testing all the Italian patients receiving eculizumab, by combining a FITC-conjugated anti-C3 polyclonal antibody with a PE-conjugated anti-CD59 monoclonal antibody. As expected, all RBCs from healthy controls were CD59+/C3−; by contrast, most RBCs from patients with cold agglutinin disease were CD59+/C3+ (Figure 1B). Untreated PNH patients had both CD59+/C3− and CD59−/C3− RBCs, and no C3+ RBCs (Figure 1B). By contrast, all the 41 PNH patients receiving eculizumab were characterized by the presence of 3 distinct RBC populations: (1) CD59+/C3− (normal RBCs); (2) CD59−/C3− (PNH RBCs, without C3); and (3) CD59−/C3+ (PNH RBCs, with C3; Figure 1B). Thus, C3 binding was restricted to the PNH cells. In 13 of the PNH patients, C3 binding was investigated before and during eculizumab treatment: C3+ PNH RBCs were constantly detectable during eculizumab treatment and never before. In keeping with these findings, in a single patient in whom eculizumab was discontinued because of pregnancy, the C3+ PNH RBCs gradually decreased, and finally disappeared within 6 weeks from drug withdrawal. The binding of C3 on RBCs was demonstrated only during eculizumab treatment and it was restricted to PNH RBCs having either total (type III PNH cells) or partial (type II PNH cells) deficiency of GPI-anchored proteins (Figure 1B). However, because patients with type II RBCs were rare in our series, possible differential C3 binding on the 2 types of PNH red cells has not been investigated. The percentage of C3+ RBCs calculated on the total RBC mass (regardless of their normal or PNH phenotype) was greatly variable among patients (Figure 2A) and correlated with the percentage of RBCs with a PNH phenotype (P < .001; Figure 2B). Given that only PNH RBCs were subjected to C3 binding, we decided to use the percentage of C3+ cells within the PNH RBC population as a more reliable measure of C3 binding. Even the percentage of C3+ PNH RBCs was highly variable among individual patients, ranging between 0.5% and 61.3% (median, 22.6%; Figure 2C).
C3 coating in PNH patients. (A) Absolute percentage of C3+ RBCs in untreated (n = 28) and eculizumab-treated (n = 41) PNH patients. Each dot represents a single case; bar represents median value. (B) Linear correlation between percentage of C3+ RBCs (y-axis) and PNH RBC clone size (in percentage; x-axis). Each dot represents a single case, with correlation line. Spearman rank order correlation, r = 0.70, P < .001. (C) Percentage of C3+ RBCs within the PNH RBC population in untreated (n = 28) and eculizumab-treated (n = 41) PNH patients. Each dot represents a single case; bar represents median value. (D) Percentage of C3+ RBCs within the PNH RBC population in eculizumab-treated PNH patients achieving optimal (n = 15), major (n = 18), or partial (n = 5) hematologic response. Each dot represents a single case; bars represent median values. Kruskal-Wallis U test; P = .01. (E) Linear correlation between percentage of C3+ PNH RBCs (y-axis) and absolute reticulocyte count (cells × 109/L) during eculizumab treatment. Each dot represents a single case, with correlation line. Spearman rank order correlation, r = 0.39, P = .001. (F) Linear correlation between percentage of C3+ PNH RBCs (y-axis) and pretreatment LDH level (IU/L; normal range, < 223). Each dot represents a single case, with correlation line. Spearman rank order correlation, r = 0.54, P < .001.
C3 coating in PNH patients. (A) Absolute percentage of C3+ RBCs in untreated (n = 28) and eculizumab-treated (n = 41) PNH patients. Each dot represents a single case; bar represents median value. (B) Linear correlation between percentage of C3+ RBCs (y-axis) and PNH RBC clone size (in percentage; x-axis). Each dot represents a single case, with correlation line. Spearman rank order correlation, r = 0.70, P < .001. (C) Percentage of C3+ RBCs within the PNH RBC population in untreated (n = 28) and eculizumab-treated (n = 41) PNH patients. Each dot represents a single case; bar represents median value. (D) Percentage of C3+ RBCs within the PNH RBC population in eculizumab-treated PNH patients achieving optimal (n = 15), major (n = 18), or partial (n = 5) hematologic response. Each dot represents a single case; bars represent median values. Kruskal-Wallis U test; P = .01. (E) Linear correlation between percentage of C3+ PNH RBCs (y-axis) and absolute reticulocyte count (cells × 109/L) during eculizumab treatment. Each dot represents a single case, with correlation line. Spearman rank order correlation, r = 0.39, P = .001. (F) Linear correlation between percentage of C3+ PNH RBCs (y-axis) and pretreatment LDH level (IU/L; normal range, < 223). Each dot represents a single case, with correlation line. Spearman rank order correlation, r = 0.54, P < .001.
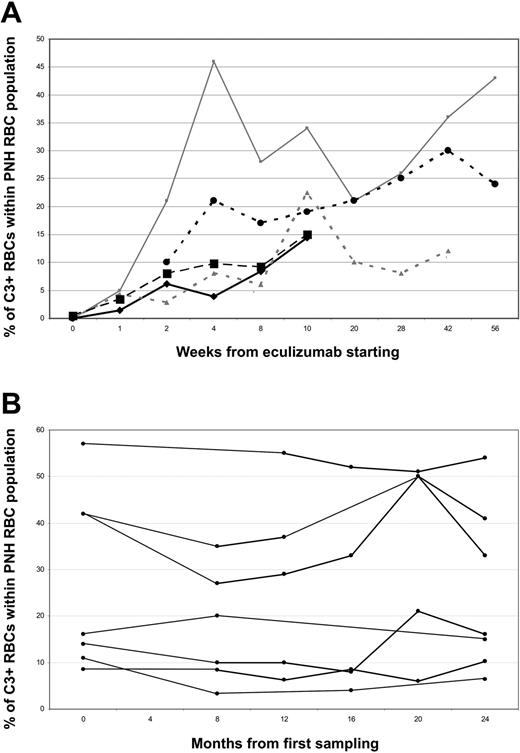
In 5 patients, the kinetics of C3 opsonization was analyzed longitudinally with weekly blood sampling soon after the start of treatment: in all of them C3 coating became apparent after the first week, and progressively increased during the following 4 to 8 weeks (Figure 3A). In 7 additional patients, the periodic analysis of the percentage of C3+ PNH RBCs during a treatment follow up of 24 months showed that this percentage was relatively stable in most of the patients (Figure 3B). Only 2 patients showed a drop in C3+ PNH RBCs, which was associated with hemoglobinuria, rising LDH, and reduced Hb level; it was attributed to eculizumab breakthrough.
Kinetics of C3 coating on RBCs in PNH patients receiving eculizumab. (A) Each line represents 1 of 5 newly diagnosed PNH patients starting treatment by eculizumab and followed longitudinally. (y-axis) Percentage of C3+ RBCs within PNH erythrocytes; (x-axis) weeks from start of treatment. (B) Each line represents 1 of 7 PNH patients longitudinally analyzed while on eculizumab treatment with a 2-year follow-up. (y-axis) Percentage of C3+ RBCs within PNH erythrocytes; (x-axis) months from start of treatment.
Kinetics of C3 coating on RBCs in PNH patients receiving eculizumab. (A) Each line represents 1 of 5 newly diagnosed PNH patients starting treatment by eculizumab and followed longitudinally. (y-axis) Percentage of C3+ RBCs within PNH erythrocytes; (x-axis) weeks from start of treatment. (B) Each line represents 1 of 7 PNH patients longitudinally analyzed while on eculizumab treatment with a 2-year follow-up. (y-axis) Percentage of C3+ RBCs within PNH erythrocytes; (x-axis) months from start of treatment.
C3 binding and hematologic response to eculizumab
To assess the biologic relevance of C3 binding to RBCs, namely its relationship to increase in Hb levels (“Methods”), we grouped patients according to their hematologic response to eculizumab. Three patients who developed aplastic anemia while on eculizumab (classified as minor responders in Table 1) were excluded from this analysis. The percentage of C3+ PNH RBCs resulted in differences among the groups (P = .01, Figure 2D), with optimal responders showing the lowest level of C3-coated RBCs. Given that major and partial responders showed comparable level of C3 binding, we limited our analysis to only 2 groups: optimal responders versus all others. The size of the PNH RBC population (both before starting eculizumab and at the time of the study) was not significantly different between the 2 groups. By contrast, the percentage of C3+ PNH RBCs was lower in optimal responders than in all others (21.0 ± 18.3 vs 32.6 ± 18.1; P = .04); however, even among optimal responders a few had a high percentage of C3+ PNH RBCs. Finally, compared with other variables related to hemolysis (Hb, reticulocytes, bilirubin, LDH), the percentage of C3+ PNH RBCs correlated with the absolute reticulocyte count at the time of the study (P = .001; Figure 2E) and with pretreatment level of LDH (P < .001; Figure 2F).
In vivo RBC survival study
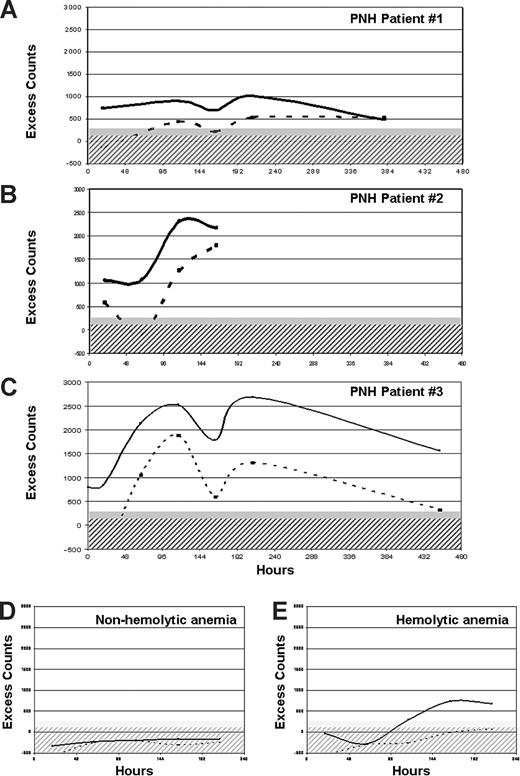
In 3 patients with suboptimal hematologic response and massive C3 RBC binding (UPNs 1, 2, and 3 in Table 1), we measured RBC survival in vivo by 51Cr labeling. Even though the patients were on eculizumab and had normal LDH, all showed markedly reduced RBC half-life (10, 11, and 13 days, respectively; normal range, 25-35 days), with excess counts on spleen and liver (Figure 4), confirming that extravascular hemolysis was taking place in vivo. One of these patients underwent videolaparoscopic splenectomy; this procedure was clearly successful, as the patient became transfusion-independent and had a significant increase in Hb level.20
Results of in vivo RBC survival study. (A-C) Three PNH patients (UPNs 1, 2, and 3 in Table 1) with suboptimal response to eculizumab studied by 51Cr RBC labeling. Excess counts on spleen (continuous line) and liver (dashed line) are plotted after correction for background, radioactive decay, and blood radioactivity (ie, heart counts), in function of time. Hatched and filled gray areas represent normal range for liver and spleen excess counts, respectively. Increased entrapment of RBCs in spleen and liver was observed in all the 3 PNH patients. (D) A representative example of a patient with a hyporegenerative (nonhemolytic) anemia, showing no liver or spleen excess count (RBC half-life, 35 days). (E) A representative example of a patient with hemolytic anemia due to extravascular hemolysis, showing liver and especially spleen excess counts (RBC half-life, 8 days).
Results of in vivo RBC survival study. (A-C) Three PNH patients (UPNs 1, 2, and 3 in Table 1) with suboptimal response to eculizumab studied by 51Cr RBC labeling. Excess counts on spleen (continuous line) and liver (dashed line) are plotted after correction for background, radioactive decay, and blood radioactivity (ie, heart counts), in function of time. Hatched and filled gray areas represent normal range for liver and spleen excess counts, respectively. Increased entrapment of RBCs in spleen and liver was observed in all the 3 PNH patients. (D) A representative example of a patient with a hyporegenerative (nonhemolytic) anemia, showing no liver or spleen excess count (RBC half-life, 35 days). (E) A representative example of a patient with hemolytic anemia due to extravascular hemolysis, showing liver and especially spleen excess counts (RBC half-life, 8 days).
Discussion
Eculizumab is the first agent that has proven specifically effective in the control of one of the cardinal manifestations of PNH, intravascular hemolysis.13-17 Given its mechanism of action, namely the blockade of terminal complement activation, which in turn is responsible for the intravascular lysis of PNH RBCs, eculizumab is a good example of targeted therapy.12
Although eculizumab effectively abolishes intravascular hemolysis in all PNH patients, as demonstrated by prompt and sustained reduction of the LDH level,13-16 the overall clinical benefit is not uniform. We are not considering here patients with a substantial component of bone marrow failure, in whom eculizumab may not be even indicated. All patients included in this study satisfied the criteria for florid hemolytic PNH, and in all of them we have evidenced that on eculizumab their intravascular hemolysis was abrogated. Among these, a majority became transfusion independent and had a substantial increase in steady-state hemoglobin, but only a fraction achieved hemoglobin values near to the normal range. In addition, most patients had persistent reticulocytosis and raised unconjugated bilirubin, suggesting that they had persistent hemolysis. In this paper, we provide evidence for extravascular hemolysis as a novel mechanism of red cell destruction in PNH patients on eculizumab.
Our main finding is that in patients receiving eculizumab a substantial proportion of red cells have bound C3 on their surface; this is not the case for untreated PNH patients. C3 binding is strictly confined to GPI-negative red cells (regardless of PNH III or PNH II, as identified by Rosse and Dacie21 ). The most obvious explanation for this finding is based on the consideration that PNH cells are deficient in decay accelerating factor (CD55),10,22 a regulator of C3 convertase.23 Because eculizumab blocks the complement pathway at level of C5, the earlier steps of the complement cascade, including activation, deposition, and proteolytic cleavage of C3 to C3b and further split products, are not affected by eculizumab. Thus, CD55-deficient PNH red cells may become overloaded with C3 fragments: this phenomenon is not detectable in untreated PNH patients, presumably because these cells are rapidly destroyed due to activation of the complement cascade progressing toward MAC formation. C3+ RBCs are apt to be recognized by macrophages that bear complement receptors (CRs) in the spleen, in the liver, and elsewhere, and this may explain persisting hemolysis, but of extravascular origin, in PNH patients on eculizumab. We cannot yet say whether this pathophysiologic process is activated de novo by eculizumab (although direct activation of the complement cascade is not expected from an antibody in which the Fc portion is of the IgG4 subclass),12 or whether eculizumab brings to the fore a process of extravascular hemolysis that already existed but was of lesser degree and difficult to detect in the absence of C5 blockade.
We need to understand why we see extravascular hemolysis in our patients on eculizumab, whereas this is not seen in subjects who are genetically deficient in CD55 (the so-called Inab phenotype). It has been shown that the CD55− RBCs of Inab subjects do bind C3 in vitro, and this binding is markedly enhanced when CD59 is blocked by an anti-CD59 antibody.24 Eculizumab-treated PNH patients are a close mimic of that situation: they are CD55− and, because they are also CD59−, they have in fact a natural CD59 “block” as well; therefore, they bind C3 in large amounts in vivo, unlike the Inab subjects, and this can be detected if the MAC lytic action is suppressed by eculizumab.
Interestingly, it has been suggested that C3 binding is involved in the physiologic clearance of senescent red cells.25 In this process, different C3 fragments (C3b, iC3b, and C3d) may play different roles,26,27 considering also that these have different affinities for complement receptors on macrophages. Macrophage-mediated extravascular hemolysis has been previously demonstrated in a PNH mouse model; however, in that case the process was considered complement independent.28
The fact that C3 binding mediates extravascular hemolysis is supported by a positive correlation between the proportion of C3-coated cells and reticulocyte count (Figure 2E). In addition, there was a higher proportion of coated cells in suboptimal responders versus optimal responders, although we observed overlap between the 2 groups (Figure 2D). Even full responders have persistently increased reticulocyte counts and elevated bilirubin (Table 1), suggesting that low-level extravascular hemolysis is the rule rather than the exception.
We have firmly established that only PNH RBCs are susceptible to C3 binding. We do not have a clear explanation of why only a discrete fraction of PNH RBCs of our eculizumab-treated patients are C3 positive. One possibility is that only a fraction of RBCs have been exposed in vivo in some part of the body to a high-level complement activation, such to produce a high level of C3 binding only on those cells. Paroxysmal hemolytic attacks typical of PNH in patients without C5 blockade are thought to result from paroxysms of complement activation; similarly, they may produce C3-mediated extravascular hemolysis during eculizumab treatment.
In addition, we do not yet know the basis for the variability in C3 coating among PNH patients, and why some patients have an optimal clinical response despite a substantial proportion of C3-coated red cells. One possibility is that the C3 processing on red cells may be different in individual patients. Activated C3 is bound to PNH RBC surface through glycophorin (GPA)29 ; the C3 convertase activity and the further processing of glycophorin A–bound C3 to iC3b and C3dg on red cell surface is strictly modulated by different complement regulators such as factor H (FH),30,31 factor I (FI),32 complement receptor 1 (CR1),33 and even GPA itself.34 Allelic variants of GPA and CR1 are known, and their possible functional changes have not been fully investigated; polymorphic mutations of FH or FI35 (as well as of membrane cofactor protein and C3 itself)36 have been found associated with atypical hemolytic uremic syndrome. Such genetic variability may account for differences in C3 binding rate among PNH patients. In addition, C3 binding on RBCs may be balanced by the active removal of C3 particles; for instance, C3 receptors on circulating white cells may work as a scavenging pathway, thus avoiding RBCs entrapping in the reticuloendothelial system.37 The clearance of C3+ RBCs may also be regulated by patient-specific factors interfering with the function of the reticuloendothelial system; this would not be surprising, in agreement with what occurs in some other extravascular hemolytic anemias.38 Finally, decreased membrane deformability subsequent to C3 deposition may also play a role, independently from complement receptors.39,40 Considering such a multitude of mechanisms, one may hypothesize that suboptimal hematologic responders might benefit from additional therapeutic strategies targeting extravascular hemolysis (eg, splenectomy20 or low-dose steroids), even though these strategies were ineffective in the pre-eculizumab era. We must of course consider that splenectomy may further increase the eculizumab-related risk of serious infection, especially from Neisseria and other capsulated bacteria; even though previous splenectomy is not considered a contraindication to eculizumab and no infectious complications were observed in one patient submitted to splenectomy20 and in a few patients who had been previously splenectomized (A.M.R. and B.R., unpublished data, 2008).
In conclusion, we have shown that eculizumab treatment is associated with binding of C3 on a significant fraction of PNH red cells, and this may lead to complement-mediated extravascular hemolysis, which in some cases becomes limiting for the blood transfusion–sparing efficacy of eculizumab. It is important to note that these findings do not detract from the remarkable clinical efficacy of eculizumab in patients with florid hemolytic PNH. Even in patients with suboptimal response, eculizumab is beneficial in abolishing or reducing transfusion requirement, abrogating hemoglobinuria, relieving symptoms such as abdominal pain, and reducing the risk of thrombosis. A more thorough understanding of eculizumab-driven changes in PNH biology and especially of the process of extravascular hemolysis that we have here reported may help to identify patients who are likely to have suboptimal response to eculizumab, and possibly to find ways to overcome this problem.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank all the patients and the colleagues from other institutions who contributed to the study. The colleagues are listed below in alphabetical order: Elisabetta Antonioli (Florence), Wilma Barcellini (Milan), Silvana Bonfigli (Sassari), Silvana Capalbo (Foggia), Angelo Michele Carella (S Giovanni Rotondo, Foggia), Francesco Fabbiano (Palermo), Giuseppe Fioritoni (Pescara), Franco Iuliano (Rossano Calabro), Francesco Mannelli (Florence), Antonio Marino (Reggio Calabria), Filippo Milano (Rome), Francesco Pietrogrande (Padova), Stefano Pulini (Pescara). We thank Russell Rother (Alexion) for critical discussion of the results described in this paper.
We also thank Alexion Pharmaceuticals for sponsoring the clinical trials and partially supporting the study with a research grant.
Authorship
Contribution: A.M.R. conceived the study with B.R. and performed the experimental work; A.M.R. discussed and interpreted the data with C.S., R.N., L.L., and B.R.; B.S., P.R., L.M., and D.R. contributed to the experimental work; G.F. performed the Coombs tests; F.B. performed the RBC survival study; all of the authors, with the exception of G.F., D.R., and F.B., were in charge of the clinical management of the PNH patients included in the study; the paper was written by A.M.R., R.N., L.L., and B.R., who also supervised the clinical and laboratory work; and all the authors critically revised the paper and contributed to the preparation of the final version.
Conflict-of-interest disclosure: A.M.R. and B.R. have received lecture fees and a research grant (together with R.N.), and have served as consultants on the Advisory Committee for Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Antonio M. Risitano, Hematology, Department of Biochemistry and Medical Biotechnologies, Federico II University of Naples, Via Pansini 5, 80131 Naples, Italy; e-mail: amrisita@unina.it.